P2Y12 Inhibition in Murine Myocarditis Results in Reduced Platelet Infiltration and Preserved Ejection Fraction
Abstract
:1. Introduction
2. Material and Methods
2.1. Animal Model
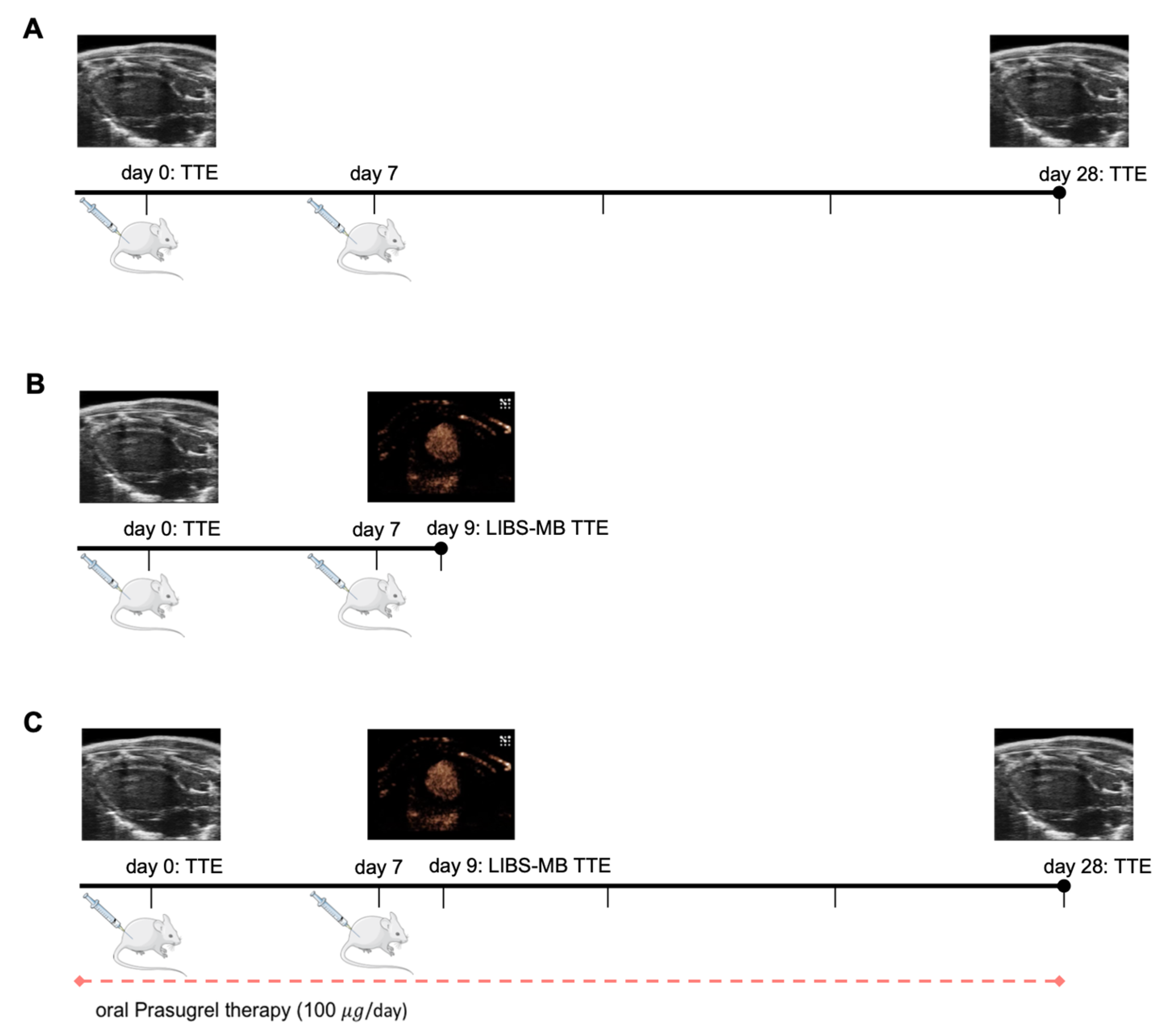
2.1.1. Myocarditis Induction
2.1.2. Prasugrel Therapy
2.2. Contrast Agent Directed against Activated Platelets (LIBS-MB)
2.3. Echocardiography
2.3.1. Evaluation of Ejection Fraction
2.3.2. Evaluation of LIBS-MB Binding by Ultrasound
2.4. Histology
2.4.1. Inflammation and Necrosis
2.4.2. Platelet Count
2.4.3. Evaluation of LIBS-MB Binding by Histology
2.5. Statistical Analysis
3. Results
3.1. LIBS-MB Is Not Suitable for Specific Detection of Early Myocarditis by Ultrasound in Mice
3.2. Platelet Infiltration of the Myocardium Occurs before Inflammatory Infiltrates and Reduction of Ejection Fraction in Murine Autoimmune Myocarditis
3.3. P2Y12 Receptor Inhibition by Prasugrel Limits Heart Failure and Platelet Aggregates in the Myocardium in Murine Myocarditis
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calabrese, F.; Thiene, G. Myocarditis and Inflammatory Cardiomyopathy: Microbiological and Molecular Biological Aspects. Cardiovasc. Res. 2003, 60, 11–25. [Google Scholar] [CrossRef]
- Basso, C.; Calabrese, F.; Corrado, D.; Thiene, G. Postmortem Diagnosis in Sudden Cardiac Death Victims: Macroscopic, Microscopic and Molecular Findings. Cardiovasc. Res. 2001, 50, 290–300. [Google Scholar] [CrossRef]
- Phillips, M.; Robinowitz, M.; Higgins, J.R.; Boran, K.J.; Reed, T.; Virmani, R. Sudden Cardiac Death in Air Force Recruits: A 20-Year Review. JAMA 1986, 256, 2696–2699. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Calabrese, F.; Angelini, A.; Tona, F.; Vinci, A.; Bottaro, S.; Ramondo, A.; Carturan, E.; Iliceto, S.; Thiene, G.; et al. A Prospective Study of Biopsy-Proven Myocarditis: Prognostic Relevance of Clinical and Aetiopathogenetic Features at Diagnosis. Eur. Heart, J. 2007, 28, 1326–1333. [Google Scholar] [CrossRef]
- Felker, G.M.; Hu, W.; Hare, J.M.; Hruban, R.H.; Baughman, K.L.; Kasper, E.K. The Spectrum of Dilated Cardiomyopathy. The Johns Hopkins Experience with 1278 Patients. Medicine 1999, 78, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Kühl, U.; Pauschinger, M.; Noutsias, M.; Seeberg, B.; Bock, T.; Lassner, D.; Poller, W.; Kandolf, R.; Schultheiss, H.-P. High Prevalence of Viral Genomes and Multiple Viral Infections in the Myocardium of Adults with “Idiopathic” Left Ventricular Dysfunction. Circulation 2005, 111, 887–893. [Google Scholar] [CrossRef]
- Kühl Uwe; Pauschinger Matthias; Seeberg Bettina; Lassner Dirk; Noutsias Michel; Poller Wolfgang; Schultheiss Heinz-Peter Viral Persistence in the Myocardium Is Associated with Progressive Cardiac Dysfunction. Circulation 2005, 112, 1965–1970. [CrossRef]
- Bock, C.-T.; Klingel, K.; Kandolf, R. Human Parvovirus B19-Associated Myocarditis. N. Engl. J. Med. 2010, 362, 1248–1249. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current State of Knowledge on Aetiology, Diagnosis, Management, and Therapy of Myocarditis: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Maier, A.; Braig, M.; Jakob, K.; Bienert, T.; Schäper, M.; Merkle, A.; Wadle, C.; Menza, M.; Neudorfer, I.; Bojti, I.; et al. Molecular Magnetic Resonance Imaging of Activated Platelets Allows Noninvasive Detection of Early Myocarditis in Mice. Sci. Rep. 2020, 10, 13211. [Google Scholar] [CrossRef]
- Henn, V.; Slupsky, J.R.; Gräfe, M.; Anagnostopoulos, I.; Förster, R.; Müller-Berghaus, G.; Kroczek, R.A. CD40 Ligand on Activated Platelets Triggers an Inflammatory Reaction of Endothelial Cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Martins, P.; van den Berk, N.; Ulfman Laurien, H.; Koenderman, L.; Hordijk, P.L.; Zwaginga, J.J. Platelet-Monocyte Complexes Support Monocyte Adhesion to Endothelium by Enhancing Secondary Tethering and Cluster Formation. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 193–199. [Google Scholar] [CrossRef]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation and Thrombosis. Circ Res. 2018, 122, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J.; Ni, H. Crosstalk Between Platelets and Microbial Pathogens. Front. Immunol. 2020, 11, 1962. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, M.; Kaplan, Z.S.; Alwis, I.; Schoenwaelder, S.M.; Ashworth, K.J.; Westein, E.; Hosseini, E.; Salem, H.H.; Slattery, R.; McColl, S.R.; et al. The CXCR1/2 Ligand NAP-2 Promotes Directed Intravascular Leukocyte Migration through Platelet Thrombi. Blood 2013, 121, 4555–4566. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 Activates Neutrophil Extracellular Traps to Ensnare Bacteria in Septic Blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Krijgsveld, J.; Zaat, S.A.J.; Meeldijk, J.; Veelen, P.A.; van Fang, G.; Poolman, B.; Brandt, E.; Ehlert, J.E.; Kuijpers, A.J.; Engbers, G.H.M.; et al. Thrombocidins, Microbicidal Proteins from Human Blood Platelets, Are C-Terminal Deletion Products of CXC Chemokines. J. Biol. Chem. 2000, 275, 20374–20381. [Google Scholar] [CrossRef]
- Verschoor, A.; Neuenhahn, M.; Navarini, A.A.; Graef, P.; Plaumann, A.; Seidlmeier, A.; Nieswandt, B.; Massberg, S.; Zinkernagel, R.M.; Hengartner, H.; et al. A Platelet-Mediated System for Shuttling Blood-Borne Bacteria to CD8α + Dendritic Cells Depends on Glycoprotein GPIb and Complement C3. Nat. Immunol. 2011, 12, 1194–1201. [Google Scholar] [CrossRef]
- Eisinger, F.; Patzelt, J.; Langer, H.F. The Platelet Response to Tissue Injury. Front. Med. 2018, 5, 317. [Google Scholar] [CrossRef]
- Hashimoto, M.; Sugidachi, A.; Isobe, T.; Niitsu, Y.; Ogawa, T.; Jakubowski, J.A.; Asai, F. The Influence of P2Y12 Receptor Deficiency on the Platelet Inhibitory Activities of Prasugrel in a Mouse Model: Evidence for Specific Inhibition of P2Y12 Receptors by Prasugrel. Biochem. Pharm. 2007, 74, 1003–1009. [Google Scholar] [CrossRef]
- Schwarz, M.; Röttgen, P.; Takada, Y.; Gall, F.L.; Knackmuss, S.; Bassler, N.; Büttner, C.; Little, M.; Bode, C.; Peter, K. Single-Chain Antibodies for the Conformation-Specific Blockade of Activated Platelet Integrin AIIbβ3 Designed by Subtractive Selection from Naïve Human Phage Libraries. FASEB. J. 2004, 18, 1704–1706. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.A.; Carr, C.L.; Belcik, T.; Xie, A.; Kron, B.; Yue, Q.; Lindner, J.R. Effect of Acoustic Power on in Vivo Molecular Imaging with Targeted Microbubbles: Implications for Low-Mechanical Index Real-Time Imaging. J. Am. Soc. Echocardiogr 2010, 23, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Von Elverfeldt, D.; Maier, A.; Duerschmied, D.; Braig, M.; Witsch, T.; Wang, X.; Mauler, M.; Neudorfer, I.; Menza, M.; Idzko, M.; et al. Dual-Contrast Molecular Imaging Allows Noninvasive Characterization of Myocardial Ischemia/Reperfusion Injury after Coronary Vessel Occlusion in Mice by Magnetic Resonance Imaging. Circulation 2014, 130, 676–687. [Google Scholar] [CrossRef]
- del Conde, I.; Crúz, M.A.; Zhang, H.; López, J.A.; Afshar-Kharghan, V. Platelet Activation Leads to Activation and Propagation of the Complement System. J. Exp. Med. 2005, 201, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Déchanet, J.; Grosset, C.; Taupin, J.L.; Merville, P.; Banchereau, J.; Ripoche, J.; Moreau, J.F. CD40 Ligand Stimulates Proinflammatory Cytokine Production by Human Endothelial Cells. J. Immunol. 1997, 159, 5640–5647. [Google Scholar] [PubMed]
- Palabrica, T.; Lobb, R.; Furie, B.C.; Aronovitz, M.; Benjamin, C.; Hsu, Y.-M.; Sajer, S.A.; Furie, B. Leukocyte Accumulation Promoting Fibrin Deposition Is Mediated in Vivo by P-Selectin on Adherent Platelets. Nature 1992, 359, 848–851. [Google Scholar] [CrossRef]
- Klinkhardt, U.; Bauersachs, R.; Adams, J.; Graff, J.; Lindhoff-Last, E.; Harder, S. Clopidogrel but Not Aspirin Reduces P-Selectin Expression and Formation of Platelet-Leukocyte Aggregates in Patients with Atherosclerotic Vascular Disease. Clin. Pharmacol. Ther. 2003, 73, 232–241. [Google Scholar] [CrossRef]
- Storey, R.; Judge, H.; Wilcox, R.; Heptinstall, S. Inhibition of ADP-Induced P-Selectin Expression and Platelet-Leukocyte Conjugate Formation by Clopidogrel and the P2Y12 Receptor Antagonist AR-C69931MX but Not Aspirin. Thromb. Haemost. 2002, 88, 488–494. [Google Scholar] [CrossRef]
- Judge, H.M.; Buckland, R.J.; Holgate, C.E.; Storey, R.F. Glycoprotein IIb/IIIa and P2Y12 Receptor Antagonists Yield Additive Inhibition of Platelet Aggregation, Granule Secretion, Soluble CD40L Release and Procoagulant Responses. Platelets 2009, 16, 398–407. [Google Scholar] [CrossRef]
- Negrotto, S.; Giusti, C.J.; de Rivadeneyra, L.; Ure, A.E.; Mena, H.A.; Schattner, M.; Gomez, R.M. Platelets Interact with Coxsackieviruses B and Have a Critical Role in the Pathogenesis of Virus-Induced Myocarditis. J. Thromb. Haemost. 2015, 13, 271–282. [Google Scholar] [CrossRef]
- Mansour, A.; Bachelot-Loza, C.; Nesseler, N.; Gaussem, P.; Gouin-Thibault, I. P2Y12 Inhibition beyond Thrombosis: Effects on Inflammation. Int. J. Mol. Sci. 2020, 21, 1391. [Google Scholar] [CrossRef]
- Siegel, P.M.; Sander, L.; Fricke, A.; Stamm, J.; Wang, X.; Sharma, P.; Bassler, N.; Ying, Y.-L.; Olivier, C.B.; Eisenhardt, S.U.; et al. P2Y12 Receptor Blockers Are Anti-Inflammatory Drugs Inhibiting Both Circulating Monocytes and Macrophages Including THP-1 Cells. Sci. Rep. 2021, 11, 17459. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Plaza-Heck, P.; Meixner, F.; Guenther, F.; Kaufmann, B.A.; Kramer, M.; Heidt, T.; Zirlik, A.; Hilgendorf, I.; Reinöhl, J.; et al. A Molecular Intravascular Ultrasound Contrast Agent Allows Detection of Activated Platelets on the Surface of Symptomatic Human Plaques. Atherosclerosis 2017, 267, 68–77. [Google Scholar] [CrossRef]
- Wang, X.; Hagemeyer, C.E.; Hohmann, J.D.; Leitner, E.; Armstrong, P.C.; Jia, F.; Olschewski, M.; Needles, A.; Peter, K.; Ahrens, I. Novel Single-Chain Antibody-Targeted Microbubbles for Molecular Ultrasound Imaging of Thrombosis: Validation of a Unique Noninvasive Method for Rapid and Sensitive Detection of Thrombi and Monitoring of Success or Failure of Thrombolysis in Mice. Circulation 2012, 125, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Von zur Muhlen, C.; von Elverfeldt, D.; Moeller, J.A.; Choudhury, R.P.; Paul, D.; Hagemeyer, C.E.; Olschewski, M.; Becker, A.; Neudorfer, I.; Bassler, N.; et al. Magnetic Resonance Imaging Contrast Agent Targeted toward Activated Platelets Allows in vivo Detection of Thrombosis and Monitoring of Thrombolysis. Circulation 2008, 118, 258–267. [Google Scholar] [CrossRef]
- Christiansen, J.P.; Lindner, J.R. Molecular and Cellular Imaging with Targeted Contrast Ultrasound. Proc. IEEE 2005, 93, 809–818. [Google Scholar] [CrossRef]
- Lux, J.; Sherry, A.D. Advances in Gadolinium-Based MRI Contrast Agent Designs for Monitoring Biological Processes in Vivo. Curr. Opin. Chem. Biol. 2018, 45, 121–130. [Google Scholar] [CrossRef]
- Pummerer, C.L.; Luze, K.; Grässl, G.; Bachmaier, K.; Offner, F.; Burrell, S.K.; Lenz, D.M.; Zamborelli, T.J.; Penninger, J.M.; Neu, N. Identification of Cardiac Myosin Peptides Capable of Inducing Autoimmune Myocarditis in BALB/c Mice. Available online: https://www.jci.org/articles/view/118642/pdf (accessed on 21 December 2020).
- Muller, A.-M.; Fischer, A.; Katus, H.A.; Kaya, Z. Mouse Models of Autoimmune Diseases-Autoimmune Myocarditis. Curr. Pharm. Des. 2015, 21, 2498–2512. [Google Scholar] [CrossRef]
- Baughman, K.L. Diagnosis of Myocarditis: Death of Dallas Criteria. Circulation 2006, 113, 593–595. [Google Scholar] [CrossRef]
- Chow, L.H.; Radio, S.J.; Sears, T.D.; Mcmanus, B.M. Insensitivity of Right Ventricular Endomyocardial Biopsy in the Diagnosis of Myocarditis. J. Am. Coll. Cardiol. 1989, 14, 915–920. [Google Scholar] [CrossRef]
- Hauck, A.J.; Kearney, D.L.; Edwards, W.D. Evaluation of Postmortem Endomyocardial Biopsy Specimens from 38 Patients with Lymphocytic Myocarditis: Implications for Role of Sampling Error. Mayo Clin. Proc. 1989, 64, 1235–1245. [Google Scholar] [CrossRef]
- Blyszczuk, P.; Behnke, S.; Lüscher, T.F.; Eriksson, U.; Kania, G. GM-CSF Promotes Inflammatory Dendritic Cell Formation but Does Not Contribute to Disease Progression in Experimental Autoimmune Myocarditis. Biochim. Et. Biophys. Acta. (BBA)-Mol. Cell. Res. 2013, 1833, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Blyszczuk, P.; Berthonneche, C.; Behnke, S.; Glönkler, M.; Moch, H.; Pedrazzini, T.; Lüscher, T.F.; Eriksson, U.; Kania, G. Nitric Oxide Synthase 2 Is Required for Conversion of Pro-Fibrogenic Inflammatory CD133+ Progenitors into F4/80+ Macrophages in Experimental Autoimmune Myocarditis. Cardiovasc. Res. 2013, 97, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Blyszczuk, P.; Kania, G.; Dieterle, T.; Marty, R.R.; Valaperti, A.; Berthonneche, C.; Pedrazzini, T.; Berger, C.T.; Dirnhofer, S.; Matter, C.M.; et al. Myeloid Differentiation Factor-88/Interleukin-1 Signaling Controls Cardiac Fibrosis and Heart Failure Progression in Inflammatory Dilated Cardiomyopathy. Circ. Res. 2009, 105, 912–920. [Google Scholar] [CrossRef]
- Blanco-Domínguez, R.; Sánchez-Díaz, R.; Fuente, H.; de la Jiménez-Borreguero, L.J.; Matesanz-Marín, A.; Relaño, M.; Jiménez-Alejandre, R.; Linillos-Pradillo, B.; Tsilingiri, K.; Martín-Mariscal, M.L.; et al. A Novel Circulating MicroRNA for the Detection of Acute Myocarditis. New. Engl. J. Med. 2021, 384, 2014–2027. [Google Scholar] [CrossRef]
- Kazama, T.; Ikeda, K. The Comparative Cardiovascular Effects of Sevoflurane with Halothane and Isoflurane. J. Anesth 1988, 2, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-P.; Liu, Y.-H.; Rhaleb, N.-E.; Kurihara, N.; Kim, H.E.; Carretero, O.A. Echocardiographic Assessment of Cardiac Function in Conscious and Anesthetized Mice. Am. J. Physiol. -Heart Circ. Physiol. 1999, 277, H1967–H1974. [Google Scholar] [CrossRef] [PubMed]
- Pachon, R.E.; Scharf, B.A.; Vatner, D.E.; Vatner, S.F. Best Anesthetics for Assessing Left Ventricular Systolic Function by Echocardiography in Mice. Am. J. Physiol. -Heart Circ. Physiol. 2015, 308, H1525–H1529. [Google Scholar] [CrossRef]
- Ter Haar, G. Safety and Bio-Effects of Ultrasound Contrast Agents. Med. Biol. Eng. Comput. 2009, 47, 893–900. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, S.N.; Reichardt, W.; Kaufmann, B.A.; Wadle, C.; von Elverfeldt, D.; Stachon, P.; Hilgendorf, I.; Wolf, D.; Heidt, T.; Duerschmied, D.; et al. P2Y12 Inhibition in Murine Myocarditis Results in Reduced Platelet Infiltration and Preserved Ejection Fraction. Cells 2021, 10, 3414. https://doi.org/10.3390/cells10123414
Schmidt SN, Reichardt W, Kaufmann BA, Wadle C, von Elverfeldt D, Stachon P, Hilgendorf I, Wolf D, Heidt T, Duerschmied D, et al. P2Y12 Inhibition in Murine Myocarditis Results in Reduced Platelet Infiltration and Preserved Ejection Fraction. Cells. 2021; 10(12):3414. https://doi.org/10.3390/cells10123414
Chicago/Turabian StyleSchmidt, Sarah Nasreen, Wilfried Reichardt, Beat A. Kaufmann, Carolin Wadle, Dominik von Elverfeldt, Peter Stachon, Ingo Hilgendorf, Dennis Wolf, Timo Heidt, Daniel Duerschmied, and et al. 2021. "P2Y12 Inhibition in Murine Myocarditis Results in Reduced Platelet Infiltration and Preserved Ejection Fraction" Cells 10, no. 12: 3414. https://doi.org/10.3390/cells10123414





