A Novel Boron Lipid to Modify Liposomal Surfaces for Boron Neutron Capture Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Lipids
2.2. Synthesis of PBL
2.3. Preparation of Liposome-Modified PBL
2.4. Physical Properties of Liposomes
3. Results
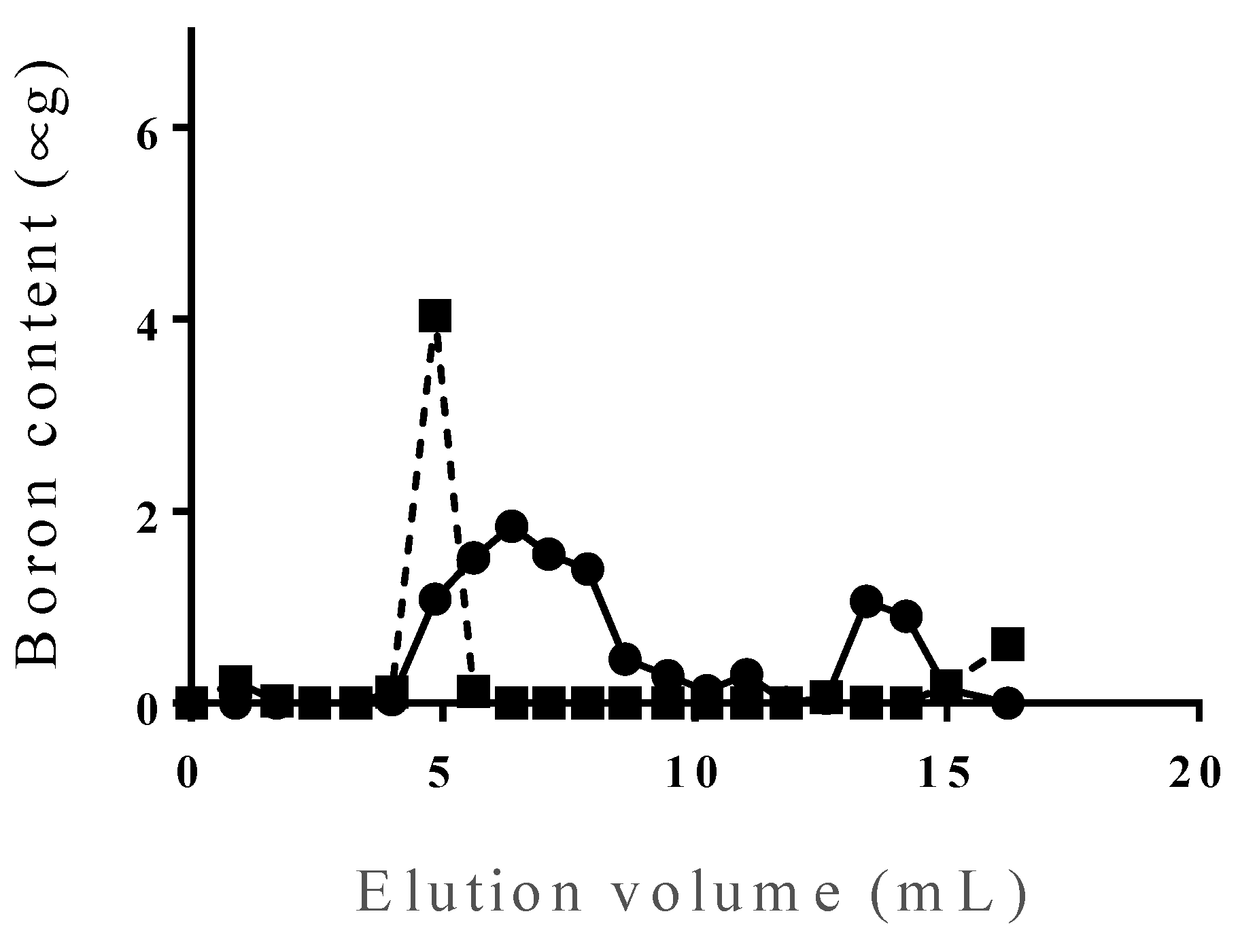
3.1. Identification of PBL
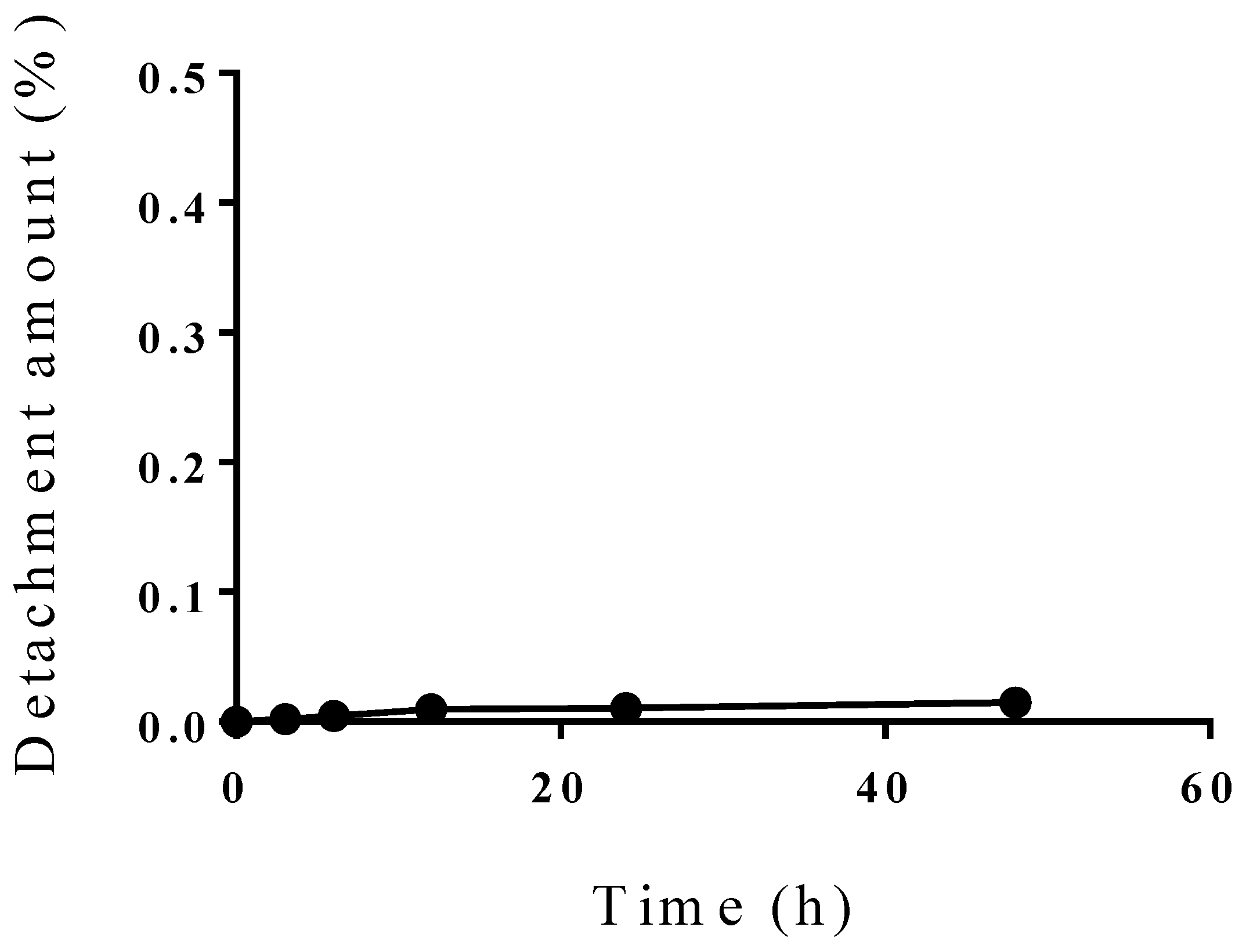
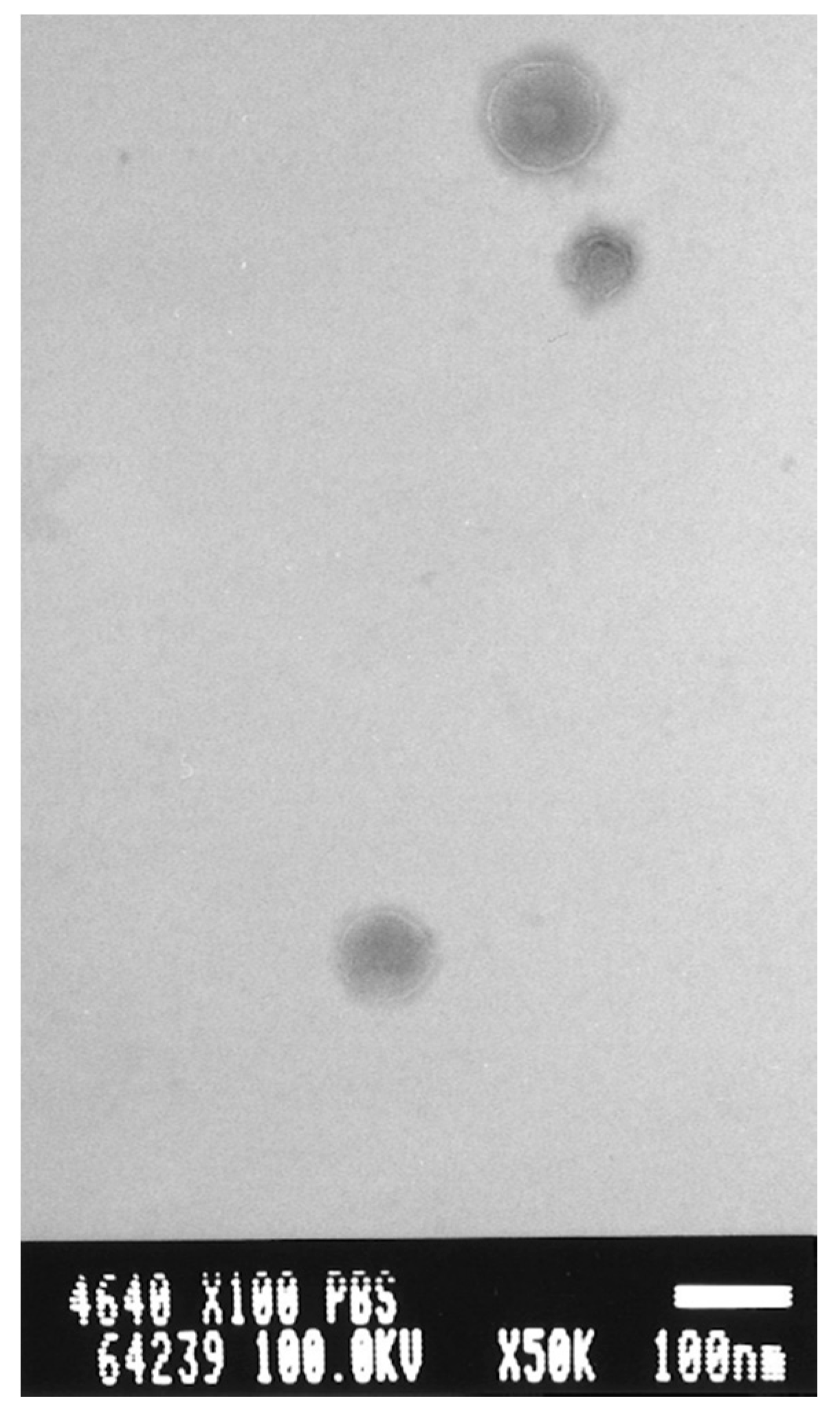
3.2. Incorporation Efficiency of PBL and Characterization of PBL Liposomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Nakamura, H. Liposomal boron delivery system for neutron capture therapy. Yakugaku. Zasshi. 2008, 128, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Yanagie, H.; Tomita, T.; Kobayashi, H.; Fujii, Y.; Takahashi, T.; Hasumi, K.; Nariuchi, H.; Sekiguchi, M. Application of boronated anti-CEA immunoliposome to tumour cell growth inhibition in in vitro boron neutron capture therapy model. Br. J. Cancer. 1991, 63, 522–526. [Google Scholar] [CrossRef]
- Yanagië, H.; Tomita, T.; Kobayashi, H.; Fujii, Y.; Nonaka, Y.; Saegusa, Y.; Hasumi, K.; Eriguchi, M.; Kobayashi, T.; Ono, K. Inhibition of human pancreatic cancer growth in nude mice by boron neutron capture therapy. Br. J. Cancer. 1997, 75, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Shelly. K.; Feakes, D.A.; Hawthorne, M.F.; Schmidt, P.G.; Krisch, T.A.; Bauer, W.F. Model studies directed toward the boron neutron-capture therapy of cancer: Boron delivery to murine tumors with liposomes. Proc. Natl. Acad. Sci. USA. 1992, 89, 9039–9043. [Google Scholar] [CrossRef] [Green Version]
- Feakes, D.A.; Shelly, K.; Knobler, C.B.; Hawthorne, M.F. Na3[B20H17NH3]: Synthesis and liposomal delivery to murine tumors. Proc. Natl. Acad. Sci. USA. 1994, 91, 3029–3033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, K.; Ishida, O.; Kasaoka, S.; Takizawa, T.; Utoguchi, N.; Shinohara, A.; Chiba, M.; Kobayashi, H.; Eriguchi, M.; Yanagie, H. Intracellular targeting of sodium mercaptoundecahydrododecaborate (BSH) to solid tumors by transferrin-PEG liposomes, for boron neutron-capture therapy (BNCT). J. Control. Release. 2004, 98, 195–207. [Google Scholar] [CrossRef]
- Feakes, D.A.; Shelly, K.; Hawthorne, M.F. Selective boron delivery to murine tumors by lipophilic species incorporated in the membranes of unilamellar liposomes. Proc. Natl. Acad. Sci. U S A 1995, 92, 1367–1370. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, H.; Miyajima, Y.; Takei, T.; Kasaoka, S.; Maruyama, K. Synthesis and vesicle formation of a nido-carborane cluster lipid for boron neutron capture therapy. Chem. Commun (Camb). 2004, 17, 1910–1911. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Ueno, M.; Miyajima, Y.; Nakamura, H. Synthesis of boron cluster lipids: Closo-dodecaborate as an alternative hydrophilic function of boronated liposomes for neutron capture therapy. Org. Lett. 2007, 9, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Uster, P.S.; Allen, T.M.; Daniel, B.E.; Mendez, C.J.; Newman, M.S.; Zhu, G.Z. Insertion of poly(ethylene glycol) derivatized phospholipid into pre-formed liposomes results in prolonged in vivo circulation time. FEBS Lett. 1996, 386, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Shirakawa, M.; Yamamto, T.; Nakai, K.; Aburai, K.; Kawatobi, S.; Tsurubuchi, T.; Yamamoto, Y.; Yokoyama, Y.; Okuno, H.; Matsumura, A. Synthesis and evaluation of a novel liposome containing BPA-peptide conjugate for BNCT. Appl. Radiat. Isot. 2009, 67, S88–90. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Mason, J.T. Geometric packing constraints in egg phosphatidylcholine vesicles. Proc. Natl. Acad. Sci. USA 1978, 75, 308–310. [Google Scholar] [CrossRef] [Green Version]
- Hawthorne, M.F.; Shelly, K. Liposomes as drug delivery vehicles for boron agents. J. Neurooncol. 1997, 33, 53–58. [Google Scholar] [CrossRef]
- Nakamura, H. Liposomal boron delivery for neutron capture therapy. Methods Enzym. 2009, 465, 179–208. [Google Scholar] [CrossRef]
- Kueffer, P.J.; Maitz, C.A.; Khan, A.A.; Schuster, S.A.; Shlyakhtina, N.I.; Jalisatgi, S.S.; Brockman, J.D.; Nigg, D.W.; Hawthorne, M.F. Boron neutron capture therapy demonstrated in mice bearing EMT6 tumors following selective delivery of boron by rationally designed liposomes. Proc. Natl. Acad. Sci. USA 2013, 110, 6512–6517. [Google Scholar] [CrossRef] [Green Version]
- Heber, E.M.; Hawthorne, M.F.; Kueffer, P.J.; Garabalino, M.A.; Thorp, S.I.; Pozzi, E.C.; Monti Hughes, A.; Maitz, C.A.; Jalisatgi, S.S.; Nigg, D.W.; et al. Therapeutic efficacy of boron neutron capture therapy mediated by boron-rich liposomes for oral cancer in the hamster cheek pouch model. Proc. Natl. Acad. Sci. USA 2014, 111, 16077–16081. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, I.; Kanno, Y.; Uchiro, H.; Makino, K. Polyborane-encapsulated PEGylated liposomes prepared using post-insertion technique for boron neutron capture therapy. J. Oleo. Sci. 2019, 68, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Zavjalov, E.; Zaboronok, A.; Kanygin, V.; Kasatova, A.; Kichigin, A.; Mukhamadiyarov, R.; Razumov, I.; Sycheva, T.; Mathis, B.J.; Maezono, S.E.B.; et al. Accelerator-based boron neutron capture therapy for malignant glioma: A pilot neutron irradiation study using boron phenylalanine, sodium borocaptate and liposomal borocaptate with a heterotopic U87 glioblastoma model in SCID mice. Int, J. Radiat. Biol. 2020, 96, 868–878. [Google Scholar] [CrossRef]
- Lee, W.; Sarkar, S.; Ahn, H.; Kim, J.Y.; Lee, Y.J.; Chang, Y.; Yoo, J. PEGylated liposome encapsulating nido-carborane showed significant tumor suppression in boron neutron capture therapy (BNCT). Biochem. Biophys. Res. Commun. 2020, 522, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Doi, A.; Kawabata, S.; Iida, K.; Yokoyama, K.; Kajimoto, Y.; Kuroiwa, T.; Shirakawa, T.; Kirihata, M.; Kasaoka, S.; Maruyama, K.; et al. Tumor-specific targeting of sodium borocaptate (BSH) to malignant glioma by transferrin-PEG liposomes: A modality for boron neutron capture therapy. J. Neurooncol. 2008, 87, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Ueda, N.; Ban, H.S.; Ueno, M.; Tachikawa, S. Design and synthesis of fluorescence-labeled closo-dodecaborate lipid: Its liposome formation and in vivo imaging targeting of tumors for boron neutron capture therapy. Org. Biomol Chem. 2012, 10, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Koganei, H.; Ueno, M.; Tachikawa, S.; Tasaki, L.; Ban, H.S.; Suzuki, M.; Shiraishi, K.; Kawano, K.; Yokoyama, M.; Maitani, Y.; et al. Development of high boron content liposomes and their promising antitumor effect for neutron capture therapy of cancers. Bioconjug. Chem. 2013, 24, 124–132. [Google Scholar] [CrossRef] [PubMed]




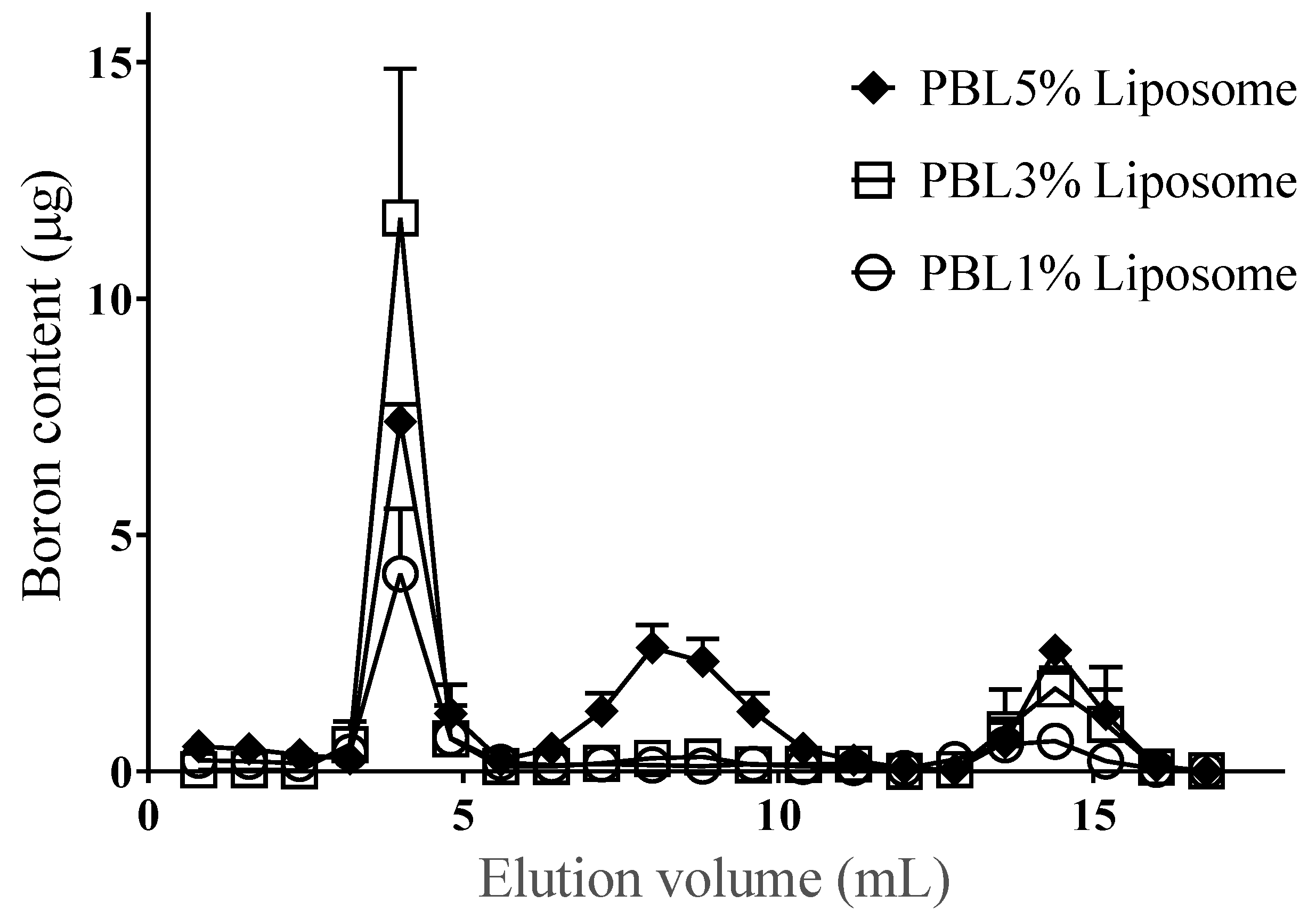
| Constituent PBL (%) | 1 | 3 | 5 |
|---|---|---|---|
| Incorporation efficiency (% ± SD) | 65.6 ± 7.4 | 70.5 ± 1.7 | 36.4 ± 2.4 |
| Loading content of PBL (µmol ± SD) | 0.041 ± 0.01 | 0.107 ± 0.025 | 0.072 ± 0.039 |
| Particle size (nm ± SD) | 149.3 ± 5.9 | 173.8 ± 25.2 | 144.1 ± 3.2 |
| Zeta potential (mV ± SD) | −27.0 ± 3.3 | −46.5 ± 7.6 | −45.4 ± 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirakawa, M.; Zaboronok, A.; Nakai, K.; Sato, Y.; Kayaki, S.; Sakai, T.; Tsurubuchi, T.; Yoshida, F.; Nishiyama, T.; Suzuki, M.; et al. A Novel Boron Lipid to Modify Liposomal Surfaces for Boron Neutron Capture Therapy. Cells 2021, 10, 3421. https://doi.org/10.3390/cells10123421
Shirakawa M, Zaboronok A, Nakai K, Sato Y, Kayaki S, Sakai T, Tsurubuchi T, Yoshida F, Nishiyama T, Suzuki M, et al. A Novel Boron Lipid to Modify Liposomal Surfaces for Boron Neutron Capture Therapy. Cells. 2021; 10(12):3421. https://doi.org/10.3390/cells10123421
Chicago/Turabian StyleShirakawa, Makoto, Alexander Zaboronok, Kei Nakai, Yuhki Sato, Sho Kayaki, Tomonori Sakai, Takao Tsurubuchi, Fumiyo Yoshida, Takashi Nishiyama, Minoru Suzuki, and et al. 2021. "A Novel Boron Lipid to Modify Liposomal Surfaces for Boron Neutron Capture Therapy" Cells 10, no. 12: 3421. https://doi.org/10.3390/cells10123421
APA StyleShirakawa, M., Zaboronok, A., Nakai, K., Sato, Y., Kayaki, S., Sakai, T., Tsurubuchi, T., Yoshida, F., Nishiyama, T., Suzuki, M., Tomida, H., & Matsumura, A. (2021). A Novel Boron Lipid to Modify Liposomal Surfaces for Boron Neutron Capture Therapy. Cells, 10(12), 3421. https://doi.org/10.3390/cells10123421







