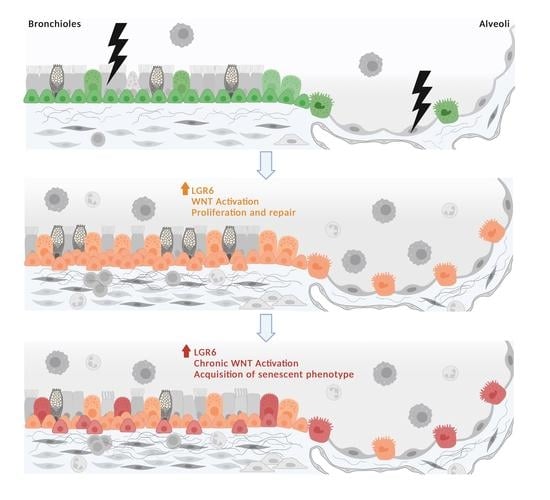
Increased LGR6 Expression Sustains Long-Term Wnt Activation and Acquisition of Senescence in Epithelial Progenitors in Chronic Lung Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Tissue Dissociation
2.3. Immunohistochemical Stainings
2.4. Immunofluorescence Analyses
2.5. SA-β-Galactosidase Stainings
2.6. Flow Cytometry Analyses
2.7. TUNEL Assay
3. Results
3.1. LGR6 Expression Is Increased in Fibrotic and Inflated Lesions and in Areas of Bronchiolization in COPD and IPF Samples
3.2. LGR6 Is Highly Expressed in Basal, Club and Alveolar Type II Progenitors Localized in Damaged Bronchioles and Fibrotic Alveoli
3.3. In Fibrotic and Inflated Areas, Senescent Progenitor Cells Show Increased LGR6 Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Bowdish, D.M.E. The aging lung: Is lung health good health for older adults? Chest 2019, 155, 391–400. [Google Scholar] [CrossRef]
- Brandsma, C.A.; de Vries, M.; Costa, R.; Woldhuis, R.R.; Königshoff, M.; Timens, W. Lung ageing and COPD: Is there a role for ageing in abnormal tissue repair? Eur. Respir. Rev. 2017, 26, 170073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Liu, X.; Cao, X.; Guo, M.; Li, X. Trends in prevalence and incidence of chronic respiratory diseases from 1990 to 2017. Respir. Res. 2020, 21, 49. [Google Scholar]
- Nalysnyk, L.; Cid-Ruzafa, J.; Rotella, P.; Esser, D. Incidence and prevalence of idiopathic pulmonary fibrosis: Review of the literature. Eur. Respir. Rev. 2012, 21, 355–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization—WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 7 October 2021).
- Boucherat, O.; Morissette, M.C.; Provencher, S.; Bonnet, S.; Maltais, F. Bridging lung development with Chronic Obstructive Pulmonary Disease. Relevance of developmental pathways in Chronic Obstructive Pulmonary Disease pathogenesis. Am. J. Respir. Crit. Care Med. 2016, 193, 362–375. [Google Scholar] [CrossRef]
- Chanda, D.; Otoupalova, E.; Smith, S.R.; Volckaert, T.; De Langhe, S.P.; Thannickal, V.J. Developmental pathways in the pathogenesis of lung fibrosis. Mol. Asp. Med. 2019, 65, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; Driscoll, B. Regeneration of the aging lung: A mini-review. Gerontology 2017, 63, 270–280. [Google Scholar] [CrossRef]
- Königshoff, M.; Balsara, N.; Pfaff, E.M.; Kramer, M.; Chrobak, I.; Seeger, W.; Eickelberg, O. Functional Wnt signaling is increased in Idiopathic Pulmonary Fibrosis. PLoS ONE 2008, 3, e2142. [Google Scholar] [CrossRef] [Green Version]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef]
- Carlier, F.M.; Dupasquier, S.; Ambroise, J.; Detry, B.; Lecocq, M.; Biétry-Claudet, C.; Boukala, Y.; Gala, J.L.; Bouzin, C.; Verleden, S.E.; et al. Canonical Wnt pathway is activated in the airway epithelium in Chronic Obstructive Pulmonary Disease. EBioMedicine 2020, 61, 103034. [Google Scholar] [CrossRef] [PubMed]
- Heijink, I.H.; de Bruin, H.G.; Dennebos, R.; Jonker, M.R.; Noordhoek, J.A.; Brandsma, C.A.; van den Berge, M.; Postma, D.S. Cigarette smoke-induced epithelial expression of WNT-5B: Implications for COPD. Eur. Respir. J. 2016, 48, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Skronska-Wasek, W.; Gosens, R.; Königshoff, M.; Baarsma, H.A. WNT receptor signalling in lung physiology and pathology. Pharmacol. Ther. 2018, 187, 150–166. [Google Scholar] [CrossRef]
- Chilosi, M.; Poletti, V.; Zamò, A.; Lestani, M.; Montagna, L.; Piccoli, P.; Pedron, S.; Bertaso, M.; Scarpa, A.; Murer, B.; et al. Aberrant Wnt/β-catenin pathway activation in Idiopathic Pulmonary Fibrosis. Am. J. Pathol. 2003, 162, 1495–1502. [Google Scholar] [CrossRef]
- Hu, H.H.; Cao, G.; Wu, X.Q.; Vaziri, N.D.; Zhao, Y.Y. Wnt signaling pathway in aging-related tissue fibrosis and therapies. Ageing Res. Rev. 2020, 60, 101063. [Google Scholar] [CrossRef]
- Shi, J.; Li, F.; Luo, M.; Wei, J.; Liu, X. Distinct roles of Wnt/β-Catenin signaling in the pathogenesis of Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis. Mediat. Inflamm. 2017, 2017, 3520581. [Google Scholar] [CrossRef] [Green Version]
- Baarsma, H.A.; Skronska-Wasek, W.; Mutze, K.; Ciolek, F.; Wagner, D.E.; John-Schuster, G.; Heinzelmann, K.; Günther, A.; Bracke, K.R.; Dagouassat, M.; et al. Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J. Exp. Med. 2017, 214, 143–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, M.; Gómez Vázquez, J.L.; Sun, D.I.; Tran, H.T.; Brislinger, M.; Tasca, A.; Shomroni, O.; Vleminckx, K.; Walentek, P. ΔN-Tp63 mediates Wnt/β-Catenin-Induced inhibition of differentiation in basal stem cells of mucociliary epithelia. Cell Rep. 2019, 28, 3338–3352.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basil, M.C.; Katzen, J.; Engler, A.E.; Guo, M.; Herriges, M.J.; Kathiriya, J.J.; Windmueller, R.; Ysasi, A.B.; Zacharias, W.J.; Chapman, H.A.; et al. The cellular and physiological basis for lung repair and regeneration: Past, present, and future. Cell Stem Cell 2020, 26, 482–502. [Google Scholar] [CrossRef]
- Ganesan, S.; Sajjan, U.S. Repair and remodeling of airway epithelium after injury in Chronic Obstructive Pulmonary Disease. Curr. Respir. Care Rep. 2013, 2, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Meiners, S.; Eickelberg, O.; Königshoff, M. Hallmarks of the ageing lung. Eur. Respir. J. 2015, 45, 807–827. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.M.; Vijg, J.; Van Steeg, H.; Dollé, M.E.; et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, M.; Hu, Q.; Hu, Y.; Hafner, K.; Costa, R.; van den Berg, A.; Königshoff, M. Chronic Wnt/β-catenin signaling induces cellular senescence in lung epithelial cells. Cell Signal. 2020, 70, 109588. [Google Scholar] [CrossRef]
- Oeztuerk-Winder, F.; Guinot, A.; Ochalek, A.; Ventura, J.J. Regulation of human lung alveolar multipotent cells by a novel p38α MAPK/miR-17-92 axis. EMBO J. 2012, 31, 3431–3441. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Carmon, K.S.; Lin, Q.; Thomas, A.; Yi, J.; Liu, Q. LGR6 is a high affinity receptor of R-spondins and potentially functions as a tumor suppressor. PLoS ONE 2012, 7, e37137. [Google Scholar] [CrossRef]
- Snippert, H.J.; Haegebarth, A.; Kasper, M.; Jaks, V.; van Es, J.H.; Barker, N.; van de Wetering, M.; van den Born, M.; Begthel, H.; Vries, R.G.; et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 2010, 327, 1385–1389. [Google Scholar] [CrossRef] [Green Version]
- Blaas, L.; Pucci, F.; Messal, H.A.; Ruiz, E.J.; Gerling, M.; Douagi, I.; Spencer-Dene, B.; Musch, A.; Mitter, R.; Bhaw, L.; et al. Lgr6 labels a rare population of mammary gland progenitor cells that are able to originate luminal mammary tumours. Nat. Cell Biol. 2016, 18, 1346–1356. [Google Scholar] [CrossRef]
- Kong, Y.; Ou, X.; Li, X.; Zeng, Y.; Gao, G.; Lyu, N.; Liu, P. LGR6 promotes tumor proliferation and metastasis through Wnt/β-Catenin signaling in Triple-Negative Breast Cancer. Mol. Ther. Oncolytics 2020, 18, 351–359. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Ni, W.; Guo, L.; Lu, X.; Liu, L.; Li, W.; Sun, S.; Wang, L.; Li, H. Dynamic expression of Lgr6 in the developing and mature mouse cochleas. Front. Cell. Neurosci. 2015, 9, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Füllgrabe, A.; Joost, S.; Are, A.; Jacob, T.; Sivan, U.; Haegebarth, A.; Linnarsson, S.; Simons, B.D.; Clevers, H.; Toftgård, R.; et al. Dynamics of Lgr6+ progenitor cells in the hair follicle, sebaceous gland, and interfollicular epidermidis. Stem Cell Rep. 2015, 5, 843–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Kuri, P.; Aubert, Y.; Brewster, M.; Li, N.; Farrelly, O.; Rice, G.; Bae, H.; Prouty, S.; Dentchev, T.; et al. Lgr6 marks epidermal stem cells with a nerve-dependet role in wound re-epithelization. Cell Stem Cell 2021, 28, 1582–1596.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Zhou, Y.M.; Tang, D.B.; Zhou, N.; Zheng, W.W.; Tang, Z.H.; Duan, C.W.; Zheng, L.; Chen, J. LGR6 promotes osteogenesis by activating the Wnt/β-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2019, 519, 1–7. [Google Scholar] [CrossRef]
- Ruiz, E.J.; Oeztuerk-Winder, F.; Ventura, J.J. A paracrine network regulates the cross-talk between human lung stem cells and the stroma. Nat. Commun. 2014, 5, 3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinot, A.; Oeztuerk-Winder, F.; Ventura, J.J. mir-17-92/p38α dysregulation enhances Wnt signaling and selects Lgr6+ cancer stem-like cells during lung adenocarcinoma progression. Cancer Res. 2016, 76, 4012–4022. [Google Scholar] [CrossRef] [Green Version]
- GOLD. The Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD. 2019. Available online: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf (accessed on 11 October 2021).
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef]
- Liu, H.; Fergusson, M.M.; Castilho, R.M.; Liu, J.; Cao, L.; Chen, J. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 2007, 317, 803–806. [Google Scholar] [CrossRef] [Green Version]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Chilosi, M.; Carloni, A.; Rossi, A.; Poletti, V. Premature lung aging and cellular senescence in the pathogenesis of Idiopathic Pulmonary Fibrosis and COPD/emphysema. Transl. Res. 2013, 162, 156–173. [Google Scholar] [CrossRef]
- Kiyokawa, H.; Morimoto, M. Notch signaling in the mammalian respiratory system, specifically the trachea and lungs, in development, homeostasis, regeneration, and disease. Dev. Growth Differ. 2020, 62, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Kusko, R.L.; Brothers, J.F.; Tedrow, J.; Pandit, K.; Huleihel, L.; Perdomo, C.; Liu, G.; Juan-Guardela, B.; Kass, D.; Zhang, S.; et al. Integrated genomics reveals convergent transcriptomic networks underlying Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2016, 194, 948–960. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, I.E.; Eickelberg, O. The impact of TGF-β on lung fibrosis: From targeting to biomarkers. Proc. Am. Thorac. Soc. 2012, 9, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.V.; Lee, J.H. Alveolar wars: The rise of in vitro models to understand human lung alveolar maintenance, regeneration, and disease. Stem Cells Transl. Med. 2020, 9, 867–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynaud, P.; Ahmed, E.; Serre, I.; Knabe, L.; Bommart, S.; Suehs, C.; Vachier, I.; Berthet, J.P.; Romagnoli, M.; Vernisse, C.; et al. Club cell loss as a feature of bronchiolization in ILD. Front. Immunol. 2021, 12, 630096. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhou, S.; Zhou, Z.; Liu, Y.; Yang, L.; Liu, J.; Zhang, Y.; Li, H.; Liu, Y.; Hou, F.F.; et al. Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J. Am. Soc. Nephrol. 2018, 29, 1238–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Xiong, Y.; Chen, W.; Wu, L. Wnt/β-catenin signaling may induce senescence of chondrocytes in osteoarthritis. Exp. Ther. Med. 2020, 20, 2631–2638. [Google Scholar] [CrossRef]
- Munguía-Reyes, A.; Balderas-Martínez, Y.I.; Becerril, C.; Checa, M.; Ramírez, R.; Ortiz, B.; Meléndez-Zajgla, J.; Pardo, A.; Selman, M. R-spondin 2 is upregulated in Idiopathic Pulmonary Fibrosis and affects fibroblast behavior. Am. J. Respir. Cell Mol. Biol. 2018, 59, 65–76. [Google Scholar] [CrossRef]
- Zhang, M.; Haughey, M.; Wang, N.Y.; Blease, K.; Kapoun, A.M.; Couto, S.; Belka, I.; Hoey, T.; Groza, M.; Hartke, J.; et al. Targeting the Wnt signaling pathway through R-spondin 3 identifies an anti-fibrosis treatment strategy for multiple organs. PLoS ONE 2020, 15, e0229445. [Google Scholar] [CrossRef] [Green Version]
- Hiemstra, P.S. Altered macrophage function in Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2013, 10, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Janssen, W.J.; Terada, M. Defective efferocytosis by alveolar macrophages in IPF patients. Respir. Med. 2012, 106, 1800–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, N.; Libreros, S.; Norris, P.C.; de la Rosa, X.; Serhan, C.N. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J. Clin. Investig. 2019, 129, 5294–5311. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortesi, E.E.; Meeusen, B.; Vanstapel, A.; Verleden, S.E.; Vanaudenaerde, B.M.; Wuyts, W.A.; Janssens, W.; Janssens, V.; Roskams, T.; Ventura, J.-J. Increased LGR6 Expression Sustains Long-Term Wnt Activation and Acquisition of Senescence in Epithelial Progenitors in Chronic Lung Diseases. Cells 2021, 10, 3437. https://doi.org/10.3390/cells10123437
Cortesi EE, Meeusen B, Vanstapel A, Verleden SE, Vanaudenaerde BM, Wuyts WA, Janssens W, Janssens V, Roskams T, Ventura J-J. Increased LGR6 Expression Sustains Long-Term Wnt Activation and Acquisition of Senescence in Epithelial Progenitors in Chronic Lung Diseases. Cells. 2021; 10(12):3437. https://doi.org/10.3390/cells10123437
Chicago/Turabian StyleCortesi, Emanuela E., Bob Meeusen, Arno Vanstapel, Stijn E. Verleden, Bart M. Vanaudenaerde, Wim A. Wuyts, Wim Janssens, Veerle Janssens, Tania Roskams, and Juan-José Ventura. 2021. "Increased LGR6 Expression Sustains Long-Term Wnt Activation and Acquisition of Senescence in Epithelial Progenitors in Chronic Lung Diseases" Cells 10, no. 12: 3437. https://doi.org/10.3390/cells10123437