Homeostatic Regulation of Glucocorticoid Receptor Activity by Hypoxia-Inducible Factor 1: From Physiology to Clinic
Abstract
:1. Introduction
2. Glucocorticoids
2.1. Biosynthesis, Secretion and Availability
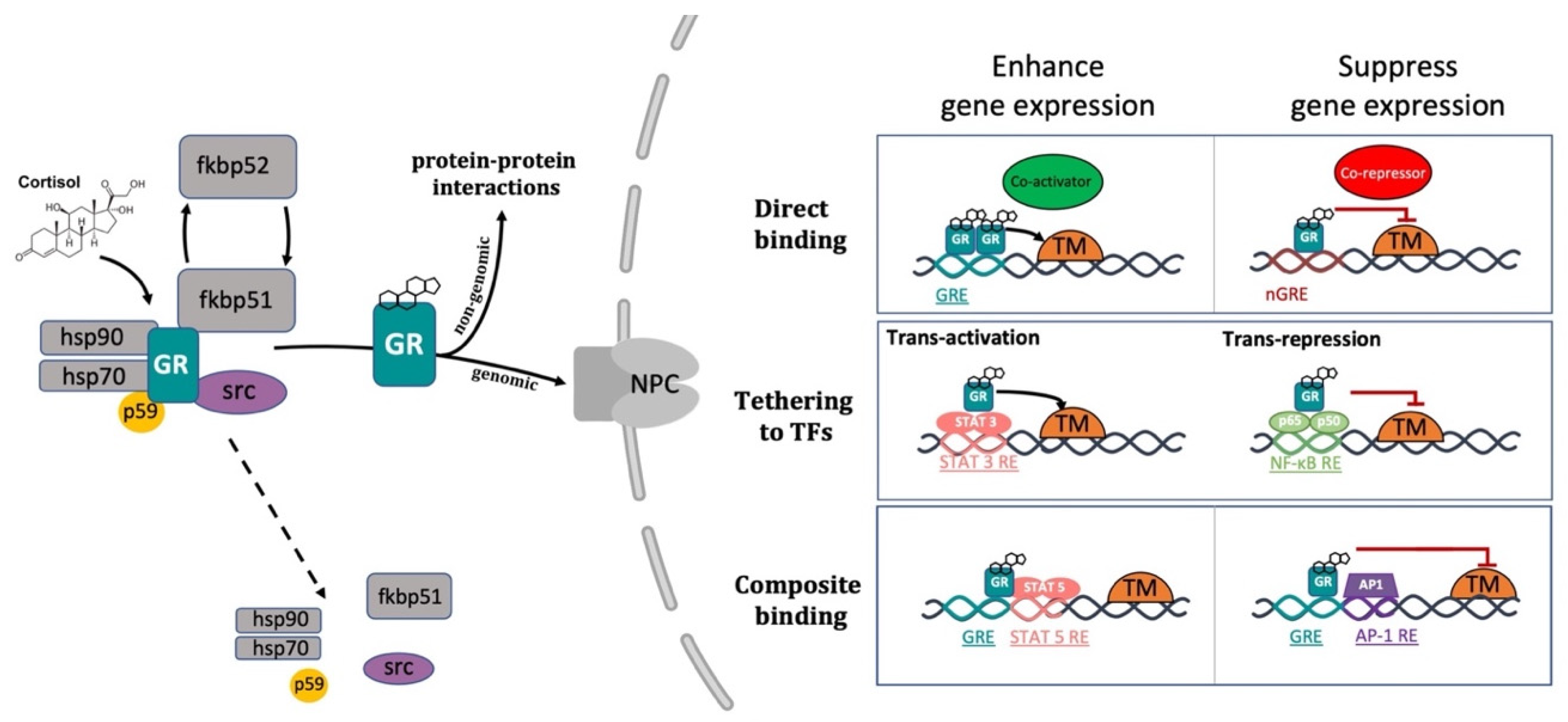
2.2. The Glucocorticoid Receptor: Structure and Functions
2.3. GCs Mechanisms of Action
2.4. The Mineralocorticoid Receptor: Structure and Functions
2.5. The Role of Glucocorticoids in Inflammation
3. The HIF Signalling Pathway
The Role of Hypoxia in Inflammation
4. HIF-GC-Crosstalk: Previous Insights
5. HIF-GC-Crosstalk: HIF as Negative Regulator of GC Biosynthesis and Responsiveness
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alsop, D.; Vijayan, M. The zebrafish stress axis: Molecular fallout from the teleost-specific genome duplication event. Gen. Comp. Endocrinol. 2009, 161, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, B.B.; Schoonheim, P.J.; Ziv, L.; Voelker, L.; Baier, H.; Gahtan, E. A zebrafish model of glucocorticoid resistance shows serotonergic modulation of the stress response. Front. Behav. Neurosci. 2012, 6, 68. [Google Scholar] [CrossRef] [Green Version]
- Tokarz, J.; Möller, G.; de Angelis, M.H.; Adamski, J. Zebrafish and steroids: What do we know and what do we need to know? J. Steroid Biochem. Mol. Biol. 2013, 137, 165–173. [Google Scholar] [CrossRef]
- Faught, E.; Vijayan, M.M. The mineralocorticoid receptor is essential for stress axis regulation in zebrafish larvae. Sci. Rep. 2018, 8, 18081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munck, A.; Guyre, P.M.; Holbrook, N.J. Physiological Functions of Glucocorticoids in Stress and Their Relation to Pharmacological Actions. Endocr. Rev. 1984, 5, 25–44. [Google Scholar] [CrossRef]
- Macfarlane, D.P.; Forbes, S.; Walker, B.R. Glucocorticoids and fatty acid metabolism in humans: Fuelling fat redistribution in the metabolic syndrome. J. Endocrinol. 2008, 197, 189–204. [Google Scholar] [CrossRef] [Green Version]
- Kuo, T.; McQueen, A.; Chen, T.-C.; Wang, J.-C. Regulation of Glucose Homeostasis by Glucocorticoids. In Glucocorticoid Signaling: From Molecules to Mice to Man; Wang, J.-C., Harris, C., Eds.; Springer: New York, NY, USA, 2015; pp. 99–126. [Google Scholar] [CrossRef]
- Facchinello, N.; Skobo, T.; Meneghetti, G.; Colletti, E.; Dinarello, A.; Tiso, N.; Costa, R.; Gioacchini, G.; Carnevali, O.; Argenton, F.; et al. nr3c1 null mutant zebrafish are viable and reveal DNA-binding-independent activities of the glucocorticoid receptor. Sci. Rep. 2017, 7, 4371. [Google Scholar] [CrossRef] [Green Version]
- Hench, P.S.; Kendall, E.C.; Slocumb, C.H.; Polley, H.F. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: Compound E) and of pituitary Adrenocortical Hormone in Arthritis: Preliminary Report. Ann. Rheum. Dis. 1949, 8, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Perretti, M.; Ahluwalia, A. The microcirculation and inflammation: Site of action for glucocorticoids. Microcirculation 2000, 7, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Smoak, K.A.; Cidlowski, J.A. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech. Ageing Dev. 2004, 125, 697–706. [Google Scholar] [CrossRef]
- Newton, R.; Holden, N. Separating Transrepression and Transactivation: A Distressing Divorce for the Glucocorticoid Receptor? Mol. Pharmacol. 2007, 72, 799–809. [Google Scholar] [CrossRef]
- Necela, B.M.; Cidlowski, J.A. Crystallization of the human glucocorticoid receptor ligand binding domain: A step towards selective glucocorticoids. Trends Pharmacol. Sci. 2003, 24, 58–61. [Google Scholar] [CrossRef]
- Rhen, T.; Cidlowski, J. Antiinflammatory Action of Glucocorticoids—New Mechanisms for Old Drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [Green Version]
- Yeager, M.P.; Guyre, P.M.; Munck, A.U. Glucocorticoid regulation of the inflammatory response to injury. Acta Anaesthesiol. Scand. 2004, 48, 799–813. [Google Scholar] [CrossRef]
- Kleiman, A.; Tuckermann, J.P. Glucocorticoid receptor action in beneficial and side effects of steroid therapy: Lessons from conditional knockout mice. Mol. Cell. Endocrinol. 2007, 275, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Newton, R.; Shah, S.; Altonsy, M.O.; Gerber, A.N. Glucocorticoid and cytokine crosstalk: Feedback, feedforward, and co-regulatory interactions determine repression or resistance. J. Biol. Chem. 2017, 292, 7163–7172. [Google Scholar] [CrossRef] [Green Version]
- De Bosscher, K. Selective Glucocorticoid Receptor modulators. J. Steroid Biochem. Mol. Biol. 2010, 120, 96–104. [Google Scholar] [CrossRef]
- Chatzopoulou, A.; Schoonheim, P.J.; Torraca, V.; Meijer, A.H.; Spaink, H.; Schaaf, M.J. Functional analysis reveals no transcriptional role for the glucocorticoid receptor β-isoform in zebrafish. Mol. Cell. Endocrinol. 2017, 447, 61–70. [Google Scholar] [CrossRef]
- Schaaf, M.J.M.; Champagne, D.; van Laanen, I.H.C.; van Wijk, D.C.W.A.; Meijer, A.H.; Meijer, O.C.; Spaink, H.P.; Richardson, M.K. Discovery of a Functional Glucocorticoid Receptor β-Isoform in Zebrafish. Endocrinology 2007, 149, 1591–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, M.T.; Hillhouse, E.W.; Burden, J.L. Dynamics and mechanics of corticosteroid feedback at the hypothalamus and anterior pituitary gland. J. Endocrinol. 1977, 73, 405–417. [Google Scholar] [CrossRef]
- Dallman, M.F.; Akana, S.F.; Jacobson, L.; Levin, N.; Cascio, C.S.; Shinsako, J. Characterization of Corticosterone Feedback Regulation of ACTH Secretion. Ann. N. Y. Acad. Sci. 1987, 512, 402–414. [Google Scholar] [CrossRef]
- Alsop, D.; Vijayan, M.M. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R711–R719. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.R. Steroid receptor regulated transcription of specific genes and gene networks. Annu. Rev. Genet. 1985, 19, 209–252. [Google Scholar] [CrossRef]
- Hollenberg, S.M.; Weinberger, C.; Ong, E.S.; Cerelli, G.; Oro, A.; Lebo, R.; Thompson, E.B.; Rosenfeld, M.G.; Evans, R. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature 1985, 318, 635–641. [Google Scholar] [CrossRef]
- Miesfeld, R.; Rusconi, S.; Godowski, P.J.; Maler, B.A.; Okret, S.; Wikström, A.-C.; Gustafsson, J.; Yamamoto, K.R. Genetic complementation of a glucocorticoid receptor deficiency by expression of cloned receptor cDNA. Cell 1986, 46, 389–399. [Google Scholar] [CrossRef]
- Jonat, C.; Rahmsdorf, H.J.; Park, K.-K.; Cato, A.; Gebel, S.; Ponta, H.; Herrlich, P. Antitumor promotion and antiinflammation: Down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell 1990, 62, 1189–1204. [Google Scholar] [CrossRef]
- Heck, S.; Kullmann, M.; Gast, A.; Ponta, H.; Rahmsdorf, H.; Herrlich, P.; Cato, A. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J. 1994, 13, 4087–4095. [Google Scholar] [CrossRef]
- Heck, S.; Bender, K.; Kullmann, M.; Göttlicher, M.; Herrlich, P.; Cato, A.C. Ikappa Balpha -independent downregulation of NF-kappa B activity by glucocorticoid receptor. EMBO J. 1997, 16, 4698–4707. [Google Scholar] [CrossRef] [Green Version]
- Yang-Yen, H.-F.; Chambard, J.C.; Sun, Y.-L.; Smeal, T.; Schmidt, T.; Drouin, J.; Karin, M. Transcriptional interference between c-Jun and the glucocorticoid receptor: Mutual inhibition of DNA binding due to direct protein-protein interaction. Cell 1990, 62, 1205–1215. [Google Scholar] [CrossRef]
- Nikolaus, S.; Fölscn, U.; Schreiber, S. Immunopharmacology of 5-aminosalicylic acid and of glucocorticoids in the therapy of inflammatory bowel disease. Hepatogastroenterology 2000, 47, 71–82. [Google Scholar]
- Neeck, G.; Renkawitz, R.; Eggert, M. Molecular aspects of glucocorticoid hormone action in rheumatoid arthritis. Cytokines Cell. Mol. Ther. 2002, 7, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P.; Kino, T. Intracellular Glucocorticoid Signaling: A Formerly Simple System Turns Stochastic. Sci. STKE 2005, 2005, pe48. [Google Scholar] [CrossRef]
- Revollo, J.R.; Cidlowski, J.A. Mechanisms Generating Diversity in Glucocorticoid Receptor Signaling. Ann. N. Y. Acad. Sci. 2009, 1179, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Busillo, J.M.; Cidlowski, J.A. The five Rs of glucocorticoid action during inflammation: Ready, reinforce, repress, resolve, and restore. Trends Endocrinol. Metab. 2013, 24, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Reichardt, S.D.; Amouret, A.; Muzzi, C.; Vettorazzi, S.; Tuckermann, J.P.; Lühder, F.; Reichardt, H.M. The Role of Glucocorticoids in Inflammatory Diseases. Cells 2021, 10, 2921. [Google Scholar] [CrossRef]
- Van Staa, T.P.; Leufkens, H.G.M.; Abenhaim, L.; Begaud, B.; Zhang, B.; Cooper, C. Use of oral corticosteroids in the United Kingdom. QJM Int. J. Med. 2000, 93, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Moghadam-Kia, S.; Werth, V.P. Prevention and treatment of systemic glucocorticoid side effects. Int. J. Dermatol. 2010, 49, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.J.; Adcock, I. Glucocorticoid resistance in inflammatory diseases. Lancet 2009, 373, 1905–1917. [Google Scholar] [CrossRef]
- Barnes, P.J. Glucocorticosteroids: Current and future directions. Br. J. Pharmacol. 2010, 163, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Schaaf, M.J.; Cidlowski, J.A. Molecular mechanisms of glucocorticoid action and resistance. J. Steroid Biochem. Mol. Biol. 2002, 83, 37–48. [Google Scholar] [CrossRef]
- Spies, L.-M.L.; Verhoog, N.J.D.; Louw, A. Acquired Glucocorticoid Resistance Due to Homologous Glucocorticoid Receptor Downregulation: A Modern Look at an Age-Old Problem. Cells 2021, 10, 2529. [Google Scholar] [CrossRef]
- Howell, M.P.; Muglia, L.J. Effects of genetically altered brain glucocorticoid receptor action on behavior and adrenal axis regulation in mice. Front. Neuroendocrinol. 2006, 27, 275–284. [Google Scholar] [CrossRef]
- Zappia, C.D.; Torralba-Agu, V.; Echeverria, E.; Fitzsimons, C.P.; Fernández, N.; Monczor, F. Antihistamines Potentiate Dexamethasone Anti-Inflammatory Effects. Impact on Glucocorticoid Receptor-Mediated Expression of Inflammation-Related Genes. Cells 2021, 10, 3026. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, C.M.; Schulte, H.M.; Chrousos, G.P. Molecular Determinants of Glucocorticoid Receptor Function and Tissue Sensitivity to Glucocorticoids. Endocr. Rev. 1996, 17, 245–261. [Google Scholar] [CrossRef]
- Faught, E.; Vijayan, M.M. Maternal stress and fish reproduction: The role of cortisol revisited. Fish Fish. 2018, 19, 1016–1030. [Google Scholar] [CrossRef]
- Gomez-Sanchez, E.; Gomez-Sanchez, C.E. The Multifaceted Mineralocorticoid Receptor. Compr. Physiol. 2014, 4, 965–994. [Google Scholar] [CrossRef] [Green Version]
- Spach, C.; Streeten, D.H.P. Retardation of Sodium Exchange in Dog Erythrocytes by Physiological Concentrations of Aldosterone, in Vitro. J. Clin. Investig. 1964, 43, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Moura, A.M.; Worcel, M. Direct action of aldosterone on transmembrane 22Na efflux from arterial smooth muscle. Rapid and delayed effects. Hypertension 1984, 6, 425–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buttgereit, F.; Wehling, M.; Burmester, G.R. A new hypothesis of modular glucocorticoid actions: Steroid treatment of rheumatic diseases revisited. Arthritis Rheum. 1998, 41, 761–767. [Google Scholar] [CrossRef]
- Buttgereit, F.; Scheffold, A. Rapid glucocorticoid effects on immune cells. Steroids 2002, 67, 529–534. [Google Scholar] [CrossRef]
- Pereda, M.P.; Lohrer, P.; Kovalovsky, D.; Castro, C.P.; Goldberg, V.; Losa, M.; Chervin, A.; Berner, S.; Molina, H.; Stalla, G.K.; et al. Interleukin-6 is inhibited by glucocorticoids and stimulates ACTH secretion and POMC expression in human corticotroph pituitary adenomas. Exp. Clin. Endocrinol. Diabetes 2000, 108, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Topete, D.; Cidlowski, J. One Hormone, Two Actions: Anti- and Pro-Inflammatory Effects of Glucocorticoids. Neuroimmunomodulation 2015, 22, 20–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnett, M.G.; Muglia, L.M.; Laryea, G.; Muglia, L.J. Genetic Approaches to Hypothalamic-Pituitary-Adrenal Axis Regulation. Neuropsychopharmacology 2015, 41, 245–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertini, R.; Bianchi, M.; Ghezzi, P. Adrenalectomy sensitizes mice to the lethal effects of interleukin 1 and tumor necrosis factor. J. Exp. Med. 1988, 167, 1708–1712. [Google Scholar] [CrossRef]
- Edwards, C.K., 3rd; Yunger, L.M.; Lorence, R.M.; Dantzer, R.; Kelley, K.W. The pituitary gland is required for protection against lethal effects of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 1991, 88, 2274–2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macphee, I.A.M.; Antoni, F.A.; Mason, D.W. Spontaneous recovery of rats from experimental allergic encephalomyelitis is dependent on regulation of the immune system by endogenous adrenal corticosteroids. J. Exp. Med. 1989, 169, 431–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandra, R.N. Neuro-hormonal host defence in endotoxin shock. Brain Behav. Immun. 1992, 6, 157–169. [Google Scholar] [CrossRef]
- Refojo, D.; Liberman, A.C.; Giacomini, D.; Nagashima, A.C.; Graciarena, M.; Echenique, C.; Pereda, M.P.; Stalla, G.; Holsboer, F.; Arzt, E. Integrating Systemic Information at the Molecular Level: Cross-talk between steroid receptors and cytokine signaling on different target cells. Ann. N. Y. Acad. Sci. 2003, 992, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Ruzek, M.C.; Pearce, B.D.; Miller, A.H.; Biron, C.A. Endogenous glucocorticoids protect against cytokine-mediated lethality during viral infection. J. Immunol. 1999, 162, 3527–3533. [Google Scholar]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid regulation of inflammation and its functional correlates: From HPA axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55–63. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialog. Clin. Neurosci. 2006, 8, 383–395. [Google Scholar]
- De Guia, R.; Rose, A.J.; Herzig, S. Glucocorticoid hormones and energy homeostasis. Horm. Mol. Biol. Clin. Investig. 2014, 19, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Katsu, Y.; Iguchi, T. Subchapter 95D—Cortisol. In Handbook of Hormones; Takei, Y., Ando, H., Tsutsui, K., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 532–533. [Google Scholar] [CrossRef]
- Cummins, E.P.; Taylor, C.T. Hypoxia-responsive transcription factors. Pflügers Arch. 2005, 450, 363–371. [Google Scholar] [CrossRef]
- Bertout, J.A.; Patel, S.A.; Simon, M.C. The impact of O2 availability on human cancer. Nat. Rev. Cancer 2008, 8, 967–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougherty, E.J.; Pollenz, R.S. ARNT: A Key bHLH/PAS Regulatory Protein Across Multiple Pathways. Compr. Toxicol. 2010, 2, 231–252. [Google Scholar] [CrossRef]
- Pelster, B.; Egg, M. Hypoxia-inducible transcription factors in fish: Expression, function and interconnection with the circadian clock. J. Exp. Biol. 2018, 221, jeb163709. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.J.; Heiden, T.C.K.; Heideman, W.; Peterson, R.E. Potential Roles of Arnt2 in Zebrafish Larval Development. Zebrafish 2009, 6, 79–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasch, A.L.; Tanguay, R.L.; Mehta, V.; Heideman, W.; Peterson, R.E. Identification of Zebrafish ARNT1 Homologs: 2,3,7,8-Tetrachlorodibenzo-p-dioxin Toxicity in the Developing Zebrafish Requires ARNT1. Mol. Pharmacol. 2005, 69, 776–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elks, P.M.; Renshaw, S.; Meijer, A.H.; Walmsley, S.; van Eeden, F.J. Exploring the HIFs, buts and maybes of hypoxia signalling in disease: Lessons from zebrafish models. Dis. Model. Mech. 2015, 8, 1349–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lando, D.; Peet, D.J.; Gorman, J.J.; Whelan, D.A.; Whitelaw, M.L.; Bruick, R.K. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002, 16, 1466–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Rooijen, E.; Voest, E.E.; Logister, I.; Korving, J.; Schwerte, T.; Schulte-Merker, S.; Giles, R.H.; van Eeden, F.J. Zebrafish mutants in the von Hippel-Lindau tumor suppressor display a hypoxic response and recapitulate key aspects of Chuvash polycythemia. Blood 2009, 113, 6449–6460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pescador, N.; Cuevas, Y.; Naranjo, S.; Alcaide, M.; Villar, D.; Landázuri, M.O.; del Peso, L. Identification of a functional hypoxia-responsive element that regulates the expression of the egl nine homologue 3 (egln3/phd3) gene. Biochem. J. 2005, 390, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santhakumar, K.; Judson, E.C.; Elks, P.M.; McKee, S.; Elworthy, S.; Van Rooijen, E.; Walmsley, S.S.; Renshaw, S.A.; Cross, S.S.; Van Eeden, F.J. A Zebrafish Model to Study and Therapeutically Manipulate Hypoxia Signaling in Tumorigenesis. Cancer Res. 2012, 72, 4017–4027. [Google Scholar] [CrossRef] [Green Version]
- Basu, M.; Sawhney, R.C.; Kumar, S.; Pal, K.; Prasad, R.; Selvamurthy, W. Glucocorticoids as prophylaxis against acute mountain sickness. Clin. Endocrinol. 2002, 57, 761–767. [Google Scholar] [CrossRef]
- Leonard, M.O.; Godson, C.; Brady, H.R.; Taylor, C. Potentiation of Glucocorticoid Activity in Hypoxia through Induction of the Glucocorticoid Receptor. J. Immunol. 2005, 174, 2250–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodama, T.; Shimizu, N.; Yoshikawa, N.; Makino, Y.; Ouchida, R.; Okamoto, K.; Hisada, T.; Nakamura, H.; Morimoto, C.; Tanaka, H. Role of the Glucocorticoid Receptor for Regulation of Hypoxia-dependent Gene Expression. J. Biol. Chem. 2003, 278, 33384–33391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, A.; Huck, G.; Stiehl, D.; Jelkmann, W.; Hellwig-Bürgel, T. Dexamethasone impairs hypoxia-inducible factor-1 function. Biochem. Biophys. Res. Commun. 2008, 372, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Y.; Wang, C.-Y.; Hsu, M.-F.; Juan, S.-H.; Chang, C.-Y.; Chou, C.-M.; Yang, L.-Y.; Hung, K.-S.; Xu, J.; Lee, Y.-H.; et al. Glucocorticoid Protection of Oligodendrocytes against Excitotoxin Involving Hypoxia-Inducible Factor-1 in a Cell-Type-Specific Manner. J. Neurosci. 2010, 30, 9621–9630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elks, P.; Van Eeden, F.J.; Dixon, G.; Wang, X.; Reyes-Aldasoro, C.C.; Ingham, P.W.; Whyte, M.K.B.; Walmsley, S.; Renshaw, S.A. Activation of hypoxia-inducible factor-1α (Hif-1α) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood 2011, 118, 712–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Rooijen, E.; Santhakumar, K.; Logister, I.; Voest, E.; Schulte-Merker, S.; Giles, R.; van Eeden, F. A Zebrafish Model for VHL and Hypoxia Signaling. Apoptosis 2011, 105, 163–190. [Google Scholar] [CrossRef]
- Schoonheim, P.J.; Chatzopoulou, A.; Schaaf, M.J. The zebrafish as an in vivo model system for glucocorticoid resistance. Steroids 2010, 75, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, M.; Chatzopoulou, A.; Spaink, H. The zebrafish as a model system for glucocorticoid receptor research. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Hammond, F.R.; Lewis, A.; Elks, P.M. If it’s not one thing, HIF’s another: Immunoregulation by hypoxia inducible factors in disease. FEBS J. 2020, 287, 3907–3916. [Google Scholar] [CrossRef] [PubMed]
- Ziv, L.; Muto, A.; Schoonheim, P.J.; Meijsing, S.H.; Strasser, D.; Ingraham, H.A.; Schaaf, M.J.M.; Yamamoto, K.R.; Baier, H. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol. Psychiatry 2012, 18, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.L.; Holmes, G.R.; Bojarczuk, A.N.; Burgon, J.; Loynes, C.A.; Chimen, M.; Sawtell, A.K.; Hamza, B.; Willson, J.; Walmsley, S.R.; et al. A Zebrafish Compound Screen Reveals Modulation of Neutrophil Reverse Migration as an Anti-Inflammatory Mechanism. Sci. Transl. Med. 2014, 6, 225ra29. [Google Scholar] [CrossRef] [Green Version]
- Hruscha, A.; Krawitz, P.; Rechenberg, A.; Heinrich, V.; Hecht, J.; Haass, C.; Schmid, B. Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. Development 2013, 140, 4982–4987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varshney, G.K.; Pei, W.; LaFave, M.C.; Idol, J.; Xu, L.; Gallardo, V.; Carrington, B.; Bishop, K.; Jones, M.; Li, M.; et al. High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res. 2015, 25, 1030–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doudna, J.A.; Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef] [PubMed]
- Nesan, D.; Vijayan, M.M. Maternal Cortisol Mediates Hypothalamus-Pituitary-Interrenal Axis Development in Zebrafish. Sci. Rep. 2016, 6, 22582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsigos, C.; Chrousos, G.P. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, F.; Braunbeck, T. Alterations along the Hypothalamic-Pituitary-Thyroid Axis of the Zebrafish (Danio rerio) after Exposure to Propylthiouracil. J. Thyroid. Res. 2011, 2011, 376243. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Nunez, V. Identification of two proopiomelanocortin genes in zebrafish (Danio rerio). Mol. Brain Res. 2003, 120, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.A.; To, T.T.; Wortmann, S.; Burmester, T.; Winkler, C.; Meyer, S.R.; Neuner, C.; Fassnacht, M.; Allolio, B. The pro-opiomelanocortin gene of the zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2003, 303, 1121–1128. [Google Scholar] [CrossRef]
- To, T.T.; Hahner, S.; Nica, G.; Rohr, K.B.; Hammerschmidt, M.; Winkler, C.; Allolio, B. Pituitary-Interrenal Interaction in Zebrafish Interrenal Organ Development. Mol. Endocrinol. 2007, 21, 472–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagle, M.; Mathur, P.; Guo, S. Corticotropin-Releasing Factor Critical for Zebrafish Camouflage Behavior Is Regulated by Light and Sensitive to Ethanol. J. Neurosci. 2011, 31, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Lu, Y.; Zhai, G.; Huang, J.; Shang, G.; Lou, Q.; Li, D.; Jin, X.; He, J.; Du, Z.; et al. Hyperandrogenism in POMCa-deficient zebrafish enhances somatic growth without increasing adiposity. J. Mol. Cell Biol. 2019, 12, 291–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Nesan, D.; Vijayan, M.M. Role of glucocorticoid in developmental programming: Evidence from zebrafish. Gen. Comp. Endocrinol. 2013, 181, 35–44. [Google Scholar] [CrossRef]
- Spiga, F.; Walker, J.J.; Terry, J.R.; Lightman, S.L. HPA Axis-Rhythms. Compr. Physiol. 2014, 4, 1273–1298. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids. Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Dallman, M.F.; Akana, S.F.; Cascio, C.S.; Darlington, D.N.; Jacobson, L.; Levin, N. Regulation of ACTH Secretion: Variations on a Theme of B. Recent Prog. Horm. Res. 1987, 43, 113–173. [Google Scholar] [CrossRef] [PubMed]
- Drouin, J.; Trifiro, M.A.; Plante, R.K.; Nemer, M.; Eriksson, P.; Wrange, O. Glucocorticoid receptor binding to a specific DNA sequence is required for hormone-dependent repression of pro-opiomelanocortin gene transcription. Mol. Cell. Biol. 1989, 9, 5305–5314. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.; Herman, J. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Nicolaides, N.C.; Galata, Z.; Kino, T.; Chrousos, G.P.; Charmandari, E. The human glucocorticoid receptor: Molecular basis of biologic function. Steroids 2010, 75, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Nicolaides, N.C.; Kyratzi, E.; Lamprokostopoulou, A.; Chrousos, G.P.; Charmandari, E. Stress, the Stress System and the Role of Glucocorticoids. Neuroimmunomodulation 2015, 22, 6–19. [Google Scholar] [CrossRef]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef] [Green Version]
- De Kloet, E.R.; Joels, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]
- Schoneveld, O.J.; Gaemers, I.C.; Lamers, W.H. Mechanisms of glucocorticoid signalling. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 2004, 1680, 114–128. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The Biology of the Glucocorticoid Receptor: New Signaling Mechanism in Health and Disease. J. Allergy Clin. Inmunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.M. The steroid and thyroid hormone receptor superfamily. Science 1988, 240, 889–895. [Google Scholar] [CrossRef]
- Kumar, R.; Thompson, E.B. Gene regulation by the glucocorticoid receptor: Structure:function relationship. J. Steroid Biochem. Mol. Biol. 2005, 94, 383–394. [Google Scholar] [CrossRef]
- Glass, C.K.; Rose, D.W.; Rosenfeld, M.G. Nuclear receptor coactivators. Curr. Opin. Cell Biol. 1997, 9, 222–232. [Google Scholar] [CrossRef]
- Beck, I.; Berghe, W.V.; Vermeulen, L.; Yamamoto, K.R.; Haegeman, G.; De Bosscher, K. Crosstalk in Inflammation: The Interplay of Glucocorticoid Receptor-Based Mechanisms and Kinases and Phosphatases. Endocr. Rev. 2009, 30, 830–882. [Google Scholar] [CrossRef]
- Bledsoe, R.K.; Montana, V.G.; Stanley, T.B.; Delves, C.J.; Apolito, C.J.; McKee, D.D.; Consler, T.G.; Parks, D.J.; Stewart, E.L.; Willson, T.M.; et al. Crystal Structure of the Glucocorticoid Receptor Ligand Binding Domain Reveals a Novel Mode of Receptor Dimerization and Coactivator Recognition. Cell 2002, 110, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Yudt, M.R.; Cidlowski, J.A. Molecular Identification and Characterization of A and B Forms of the Glucocorticoid Receptor. Mol. Endocrinol. 2001, 15, 1093–1103. [Google Scholar] [CrossRef]
- Yudt, M.R.; Cidlowski, J. The Glucocorticoid Receptor: Coding a Diversity of Proteins and Responses through a Single Gene. Mol. Endocrinol. 2002, 16, 1719–1726. [Google Scholar] [CrossRef] [Green Version]
- Oakley, R.H.; Cidlowski, J.A. Cellular Processing of the Glucocorticoid Receptor Gene and Protein: New Mechanisms for Generating Tissue-specific Actions of Glucocorticoids. J. Biol. Chem. 2011, 286, 3177–3184. [Google Scholar] [CrossRef] [Green Version]
- Merkulov, V.M.; Merkulova, T.I. Glucocorticoid receptor isoforms generated by alternative splicing and alternative translation initiation. Russ. J. Genet. Appl. Res. 2012, 2, 205–213. [Google Scholar] [CrossRef]
- Rafacho, A.; Ortsäter, H.; Nadal, A.; Quesada, I. Glucocorticoid treatment and endocrine pancreas function: Implications for glucose homeostasis, insulin resistance and diabetes. J. Endocrinol. 2014, 223, R49–R62. [Google Scholar] [CrossRef]
- Marchi, D. Bidirectional Crosstalk between Hypoxia-Inducible Factor and Glucocorticoid Signalling in Zebrafish Larvae. University of Sheffield. 2020. Available online: https://etheses.whiterose.ac.uk/28224/ (accessed on 2 December 2021).
- Encío, I.J.; Detera-Wadleigh, S.D. The genomic structure of the human glucocorticoid receptor. J. Biol. Chem. 1991, 266, 7182–7188. [Google Scholar] [CrossRef]
- Yudt, M.R.; Jewell, C.M.; Bienstock, R.; Cidlowski, J.A. Molecular Origins for the Dominant Negative Function of Human Glucocorticoid Receptor Beta. Mol. Cell. Biol. 2003, 23, 4319–4330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oakley, R.H.; Sar, M.; Cidlowski, J.A. The Human Glucocorticoid Receptor β Isoform: Expression, biochemical properties, and putative function. J. Biol. Chem. 1996, 271, 9550–9559. [Google Scholar] [CrossRef] [Green Version]
- Bamberger, C.M.; Bamberger, A.M.; de Castro, M.; Chrousos, G.P. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J. Clin. Investig. 1995, 95, 2435–2441. [Google Scholar] [CrossRef] [Green Version]
- Oakley, R.H.; Jewell, C.M.; Yudt, M.R.; Bofetiado, D.M.; Cidlowski, J. The Dominant Negative Activity of the Human Glucocorticoid Receptor β Isoform. Specificity and mechanisms of action. J. Biol. Chem. 1999, 274, 27857–27866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charmandari, E.; Chrousos, G.P.; Ichijo, T.; Bhattacharyya, N.; Vottero, A.; Souvatzoglou, E.; Kino, T. The Human Glucocorticoid Receptor (hGR) β Isoform Suppresses the Transcriptional Activity of hGRα by Interfering with Formation of Active Coactivator Complexes. Mol. Endocrinol. 2005, 19, 52–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauk, P.J.; Goleva, E.; Strickland, I.; Vottero, A.; Chrousos, G.P.; Kisich, K.O.; Leung, D.Y.M. Increased Glucocorticoid ReceptorβExpression Converts Mouse Hybridoma Cells to a Corticosteroid-Insensitive Phenotype. Am. J. Respir. Cell Mol. Biol. 2002, 27, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Strickland, I.; Kisich, K.; Hauk, P.J.; Vottero, A.; Chrousos, G.P.; Klemm, D.J.; Leung, D.Y. High Constitutive Glucocorticoid Receptor β in Human Neutrophils Enables Them to Reduce Their Spontaneous Rate of Cell Death in Response to Corticosteroids. J. Exp. Med. 2001, 193, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef] [Green Version]
- Lewis-Tuffin, L.J.; Jewell, C.M.; Bienstock, R.; Collins, J.B.; Cidlowski, J. Human Glucocorticoid Receptor β Binds RU-486 and Is TranscriptionallyActive. Mol. Cell. Biol. 2007, 27, 2266–2282. [Google Scholar] [CrossRef] [Green Version]
- Kino, T.; Manoli, I.; Kelkar, S.; Wang, Y.; Su, Y.A.; Chrousos, G.P. Glucocorticoid receptor (GR) β has intrinsic, GRα-independent transcriptional activity. Biochem. Biophys. Res. Commun. 2009, 381, 671–675. [Google Scholar] [CrossRef] [Green Version]
- Honda, M.; Orii, F.; Ayabe, T.; Imai, S.; Ashida, T.; Obara, T.; Kohgo, Y. Expression of glucocorticoid receptor β in lymphocytes of patients with glucocorticoid-resistant ulcerative colitis. Gastroenterology 2000, 118, 859–866. [Google Scholar] [CrossRef]
- Shahidi, H.; Vottero, A.; Stratakis, C.A.; Taymans, S.E.; Karl, M.; Longui, C.A.; Chrousos, G.P.; Daughaday, W.H.; Gregory, S.A.; Plate, J.M. Imbalanced Expression of the Glucocorticoid Receptor Isoforms in Cultured Lymphocytes from a Patient with Systemic Glucocorticoid Resistance and Chronic Lymphocytic Leukemia. Biochem. Biophys. Res. Commun. 1999, 254, 559–565. [Google Scholar] [CrossRef]
- Bergeron, C.; Fukakusa, M.; Olivenstein, R.; Lemiere, C.; Shannon, J.; Ernst, P.; Martin, J.G.; Hamid, Q. Increased glucocorticoid receptor–β expression, but not decreased histone deacetylase 2, in severe asthma. J. Allergy Clin. Immunol. 2006, 117, 703–705. [Google Scholar] [CrossRef]
- Hamid, Q.A.; Wenzel, S.E.; Hauk, P.J.; Tsicopoulos, A.; Wallaert, B.; Lafitte, J.-J.; Chrousos, G.P.; Szefler, S.J.; Leung, D.Y.M. Increased Glucocorticoid Receptor β in Airway Cells of Glucocorticoid-insensitive Asthma. Am. J. Respir. Crit. Care Med. 1999, 159, 1600–1604. [Google Scholar] [CrossRef]
- Goleva, E.; Li, L.-B.; Eves, P.T.; Strand, M.; Martin, R.J.; Leung, D.Y.M. Increased Glucocorticoid Receptor β Alters Steroid Response in Glucocorticoid-insensitive Asthma. Am. J. Respir. Crit. Care Med. 2006, 173, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Leung, D.Y.; Hamid, Q.; Vottero, A.; Szefler, S.J.; Surs, W.; Minshall, E.; Chrousos, G.P.; Klemm, D.J. Association of Glucocorticoid Insensitivity with Increased Expression of Glucocorticoid Receptor β. J. Exp. Med. 1997, 186, 1567–1574. [Google Scholar] [CrossRef]
- Chatzopoulou, A.; Roy, U.; Meijer, A.H.; Alia, A.; Spaink, H.; Schaaf, M.J.M. Transcriptional and Metabolic Effects of Glucocorticoid Receptor α and β Signaling in Zebrafish. Endocrinology 2015, 156, 1757–1769. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Ramírez, P.; Tliba, O. Glucocorticoid Receptor β (GRβ): Beyond Its Dominant-Negative Function. Int. J. Mol. Sci. 2021, 22, 3649. [Google Scholar] [CrossRef]
- Holownia, A.; Mroz, R.M.; Kolodziejczyk, A.; Chyczewska, E.; Braszko, J.J. Increased FKBP51 in induced sputum cells of chronic obstructive pulmonary disease patients after therapy. Eur. J. Med. Res. 2009, 14 (Suppl. 4), 108–111. [Google Scholar] [CrossRef] [Green Version]
- Storer, C.L.; Dickey, C.A.; Galigniana, M.D.; Rein, T.; Cox, M.B. FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol. Metab. 2011, 22, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, P.G.; Boushey, H.A.; Dolganov, G.M.; Barker, C.S.; Yang, J.; Donnelly, S.; Ellwanger, A.; Sidhu, S.S.; Dao-Pick, T.P.; Pantoja, C.; et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc. Natl. Acad. Sci. USA 2007, 104, 15858–15863. [Google Scholar] [CrossRef] [Green Version]
- Binder, E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009, 34, S186–S195. [Google Scholar] [CrossRef]
- Liberman, A.C.; Budziñski, M.L.; Sokn, C.; Gobbini, R.P.; Steininger, A.; Arzt, E. Regulatory and Mechanistic Actions of Glucocorticoids on T and Inflammatory Cells. Front. Endocrinol. 2018, 9, 235. [Google Scholar] [CrossRef]
- Vandevyver, S.; Dejager, L.; Tuckermann, J.; Libert, C. New Insights into the Anti-inflammatory Mechanisms of Glucocorticoids: An Emerging Role for Glucocorticoid-Receptor-Mediated Transactivation. Endocrinology 2013, 154, 993–1007. [Google Scholar] [CrossRef] [Green Version]
- Presman, D.M.; Hager, G.L. More than meets the dimer: What is the quaternary structure of the glucocorticoid receptor? Transcription 2016, 8, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Reichardt, H.M.; Kaestner, K.H.; Tuckermann, J.; Kretz, O.; Wessely, O.; Bock, R.; Gass, P.; Schmid, W.; Herrlich, P.; Angel, P.; et al. DNA Binding of the Glucocorticoid Receptor Is Not Essential for Survival. Cell 1998, 93, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Prager, E.M.; Johnson, L.R. Stress at the Synapse: Signal Transduction Mechanisms of Adrenal Steroids at Neuronal Membranes. Sci. Signal. 2009, 2, re5. [Google Scholar] [CrossRef]
- Groeneweg, F.L.; Karst, H.; de Kloet, R.; Joels, M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 2011, 209, 153–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlenhaut, H.; Barish, G.D.; Yu, R.T.; Downes, M.; Karunasiri, M.; Liddle, C.; Schwalie, P.C.; Hübner, N.; Evans, R.M. Insights into Negative Regulation by the Glucocorticoid Receptor from Genome-wide Profiling of Inflammatory Cistromes. Mol. Cell 2012, 49, 158–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guenther, M.G.; Barak, O.; Lazar, M.A. The SMRT and N-CoR Corepressors Are Activating Cofactors for Histone Deacetylase 3. Mol. Cell. Biol. 2001, 21, 6091–6101. [Google Scholar] [CrossRef] [Green Version]
- Surjit, M.; Ganti, K.P.; Mukherji, A.; Ye, T.; Hua, G.; Metzger, D.; Li, M.; Chambon, P. Widespread Negative Response Elements Mediate Direct Repression by Agonist- Liganded Glucocorticoid Receptor. Cell 2011, 145, 224–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramamoorthy, S.; Cidlowski, J.A. Exploring the Molecular Mechanisms of Glucocorticoid Receptor Action from Sensitivity to Resistance. Endocr. Dev. 2013, 24, 41–56. [Google Scholar] [CrossRef] [Green Version]
- Escoter-Torres, L.; Caratti, G.; Mechtidou, A.; Tuckermann, J.; Uhlenhaut, N.H.; Vettorazzi, S. Fighting the Fire: Mechanisms of Inflammatory Gene Regulation by the Glucocorticoid Receptor. Front. Immunol. 2019, 10, 1859. [Google Scholar] [CrossRef]
- Oh, K.-S.; Patel, H.; Gottschalk, R.A.; Lee, W.S.; Baek, S.; Fraser, I.; Hager, G.L.; Sung, M.-H. Anti-Inflammatory Chromatinscape Suggests Alternative Mechanisms of Glucocorticoid Receptor Action. Immunity 2017, 47, 298–309.e5. [Google Scholar] [CrossRef]
- Ratman, D.; Berghe, W.V.; Dejager, L.; Libert, C.; Tavernier, J.; Beck, I.; De Bosscher, K. How glucocorticoid receptors modulate the activity of other transcription factors: A scope beyond tethering. Mol. Cell. Endocrinol. 2013, 380, 41–54. [Google Scholar] [CrossRef]
- Baudrand, R.; Pojoga, L.; Romero, J.R. Chapter 10—Aldosterone’s Mechanism of Action: Genomic and Nongenomic Signaling. In Textbook of Nephro-Endocrinology, 2nd ed.; Singh, A.K., Williams, G.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 173–188. [Google Scholar] [CrossRef]
- Reul, J.M.H.M.; de Kloet, R. Two Receptor Systems for Corticosterone in Rat Brain: Microdistribution and Differential Occupation. Endocrinology 1985, 117, 2505–2511. [Google Scholar] [CrossRef]
- Reul, J.M.; Collins, A.; Saliba, R.S.; Mifsud, K.; Carter, S.D.; Gutierrez-Mecinas, M.; Qian, X.; Linthorst, A.C. Glucocorticoids, epigenetic control and stress resilience. Neurobiol. Stress 2014, 1, 44–59. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, C.; Gekle, M. Non-classical actions of the mineralocorticoid receptor: Misuse of EGF receptors? Mol. Cell. Endocrinol. 2007, 277, 6–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sireeni, J.; Bakker, N.; Jaikumar, G.; Obdam, D.; Slabbekoorn, H.; Tudorache, C.; Schaaf, M. Profound effects of glucocorticoid resistance on anxiety-related behavior in zebrafish adults but not in larvae. Gen. Comp. Endocrinol. 2020, 292, 113461. [Google Scholar] [CrossRef]
- Müller, M.B.; Holsboer, F.; Keck, M.E. Genetic modification of corticosteroid receptor signalling: Novel insights into pathophysiology and treatment strategies of human affective disorders. Neuropeptides 2002, 36, 117–131. [Google Scholar] [CrossRef]
- Van Eekelen, J.; Bohn, M.; De Kloet, E. Postnatal ontogeny of mineralocorticoid and glucocorticoid receptor gene expression in regions of the rat tel- and diencephalon. Dev. Brain Res. 1991, 61, 33–43. [Google Scholar] [CrossRef]
- Hartmann, J.; Bajaj, T.; Klengel, C.; Chatzinakos, C.; Ebert, T.; Dedic, N.; McCullough, K.M.; Lardenoije, R.; Joëls, M.; Meijer, O.C.; et al. Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5. Cell Rep. 2021, 35, 109185. [Google Scholar] [CrossRef]
- De Kloet, E.; Meijer, O.; de Nicola, A.; de Rijk, R.; Joëls, M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front. Neuroendocrinol. 2018, 49, 124–145. [Google Scholar] [CrossRef]
- Lee, H.B.; Schwab, T.L.; Sigafoos, A.N.; Gauerke, J.L.; Ii, R.G.K.; Serres, M.R.; Jacobs, D.C.; Cotter, R.P.; Das, B.; Petersen, M.O.; et al. Novel zebrafish behavioral assay to identify modifiers of the rapid, nongenomic stress response. Genes Brain Behav. 2018, 18, e12549. [Google Scholar] [CrossRef]
- Marchi, D.; Santhakumar, K.; Markham, E.; Li, N.; Storbeck, K.-H.; Krone, N.; Cunliffe, V.T.; van Eeden, F.J.M. Bidirectional crosstalk between Hypoxia-Inducible Factor and glucocorticoid signalling in zebrafish larvae. PLoS Genet. 2020, 16, e1008757. [Google Scholar] [CrossRef]
- Ince, L.M.; Weber, J.; Scheiermann, C. Control of Leukocyte Trafficking by Stress-Associated Hormones. Front. Immunol. 2019, 9, 3143. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Schmidt, S.; Rainer, J.; Ploner, C.; Presul, E.; Riml, S.; Kofler, R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: Molecular mechanisms and clinical relevance. Cell Death Differ. 2004, 11, S45–S55. [Google Scholar] [CrossRef] [Green Version]
- Planey, S.L.; Litwack, G. Glucocorticoid-Induced Apoptosis in Lymphocytes. Biochem. Biophys. Res. Commun. 2000, 279, 307–312. [Google Scholar] [CrossRef]
- Ashwell, J.D.; Lu, F.W.M.; Vacchio, M.S. Glucocorticoids in T Cell Development and Function. Annu. Rev. Immunol. 2000, 18, 309–345. [Google Scholar] [CrossRef]
- Herold, M.J.; McPherson, K.G.; Reichardt, H.M. Glucocorticoids in T cell apoptosis and function. Cell. Mol. Life Sci. 2005, 63, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McColl, A.; Bournazos, S.; Franz, S.; Perretti, M.; Morgan, B.P.; Haslett, C.; Dransfield, I. Glucocorticoids Induce Protein S-Dependent Phagocytosis of Apoptotic Neutrophils by Human Macrophages. J. Immunol. 2009, 183, 2167–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.H.; Spencer, R.L.; Stein, M.; McEwen, B.S. Adrenal steroid receptor binding in spleen and thymus after stress or dexamethasone. Am. J. Physiol. Metab. 1990, 259, E405. [Google Scholar] [CrossRef] [PubMed]
- Barish, G.D.; Downes, M.; Alaynick, W.A.; Yu, R.T.; Ocampo, C.B.; Bookout, A.L.; Mangelsdorf, D.J.; Evans, R.M. A Nuclear Receptor Atlas: Macrophage Activation. Mol. Endocrinol. 2005, 19, 2466–2477. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.-Y.; Müller, N.; Herold, M.J.; Brandt, J.V.D.; Reichardt, H.M. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology 2007, 122, 47–53. [Google Scholar] [CrossRef]
- Harizi, H.; Mormede, P.; Corcuff, J. Inter-strain differences in glucocorticoid and mineralocorticoid effects on macrophage and lymphocyte functions in mice. J. Neuroimmunol. 2008, 204, 38–42. [Google Scholar] [CrossRef]
- Bene, N.C.; Alcaide, P.; Wortis, H.H.; Jaffe, I.Z. Mineralocorticoid receptors in immune cells: Emerging role in cardiovascular disease. Steroids 2014, 91, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Durango, N.; Vecchiola, A.; Gonzalez-Gomez, L.M.; Simon, F.; Riedel, C.; Fardella, C.E.; Kalergis, A.M. Modulation of Immunity and Inflammation by the Mineralocorticoid Receptor and Aldosterone. BioMed Res. Int. 2015, 2015, 652738. [Google Scholar] [CrossRef] [Green Version]
- Van Der Heijden, C.D.C.C.; Deinum, J.; Joosten, L.A.B.; Netea, M.G.; Riksen, N.P. The mineralocorticoid receptor as a modulator of innate immunity and atherosclerosis. Cardiovasc. Res. 2018, 114, 944–953. [Google Scholar] [CrossRef] [Green Version]
- Vandevyver, S.; Dejager, L.; Libert, C. On the Trail of the Glucocorticoid Receptor: Into the Nucleus and Back. Traffic 2011, 13, 364–374. [Google Scholar] [CrossRef]
- Ronchetti, S.; Migliorati, G.; Riccardi, C. GILZ as a Mediator of the Anti-Inflammatory Effects of Glucocorticoids. Front. Endocrinol. 2015, 6, 170. [Google Scholar] [CrossRef] [Green Version]
- Scheinman, R.I.; Gualberto, A.; Jewell, C.M.; Cidlowski, J.A.; Baldwin, A.S. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol. Cell. Biol. 1995, 15, 943–953. [Google Scholar] [CrossRef] [Green Version]
- Escoter-Torres, L.; Greulich, F.; Quagliarini, F.; Wierer, M.; Uhlenhaut, N.H. Anti-inflammatory functions of the glucocorticoid receptor require DNA binding. Nucleic Acids Res. 2020, 48, 8393–8407. [Google Scholar] [CrossRef]
- Stellato, C. Post-transcriptional and Nongenomic Effects of Glucocorticoids. Proc. Am. Thorac. Soc. 2004, 1, 255–263. [Google Scholar] [CrossRef]
- De Bosscher, K.; Van Craenenbroeck, K.; Meijer, O.C.; Haegeman, G. Selective transrepression versus transactivation mechanisms by glucocorticoid receptor modulators in stress and immune systems. Eur. J. Pharmacol. 2008, 583, 290–302. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, K.; Haegeman, G.; Elewaut, D. Targeting inflammation using selective glucocorticoid receptor modulators. Curr. Opin. Pharmacol. 2010, 10, 497–504. [Google Scholar] [CrossRef]
- Davies, T.H.; Ning, Y.-M.; Sánchez, E.R. Differential Control of Glucocorticoid Receptor Hormone-Binding Function by Tetratricopeptide Repeat (TPR) Proteins and the Immunosuppressive Ligand FK506. Biochemistry 2005, 44, 2030–2038. [Google Scholar] [CrossRef] [Green Version]
- Liberman, A.C.; Druker, J.; Perone, M.J.; Arzt, E. Glucocorticoids in the regulation of transcription factors that control cytokine synthesis. Cytokine Growth Factor Rev. 2007, 18, 45–56. [Google Scholar] [CrossRef]
- Pascual, G.; Glass, C.K. Nuclear receptors versus inflammation: Mechanisms of transrepression. Trends Endocrinol. Metab. 2006, 17, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Limbourg, F.P.; Liao, J.K. Nontranscriptional actions of the glucocorticoid receptor. J. Mol. Med. 2003, 81, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Liberman, A.C.; Refojo, D.; Druker, J.; Toscano, M.; Rein, T.; Holsboer, F.; Arzt, E. The activated glucocorticoid receptor inhibits the transcription factor T-bet by direct protein-protein interaction. FASEB J. 2007, 21, 1177–1188. [Google Scholar] [CrossRef]
- Vayssière, B.M.; Dupont, S.; Choquart, A.; Petit, F.; Garcia, T.; Marchandeau, C.; Gronemeyer, H.; Resche-Rigon, M. Synthetic Glucocorticoids That Dissociate Transactivation and AP-1 Transrepression Exhibit Antiinflammatory Activity in Vivo. Mol. Endocrinol. 1997, 11, 1245–1255. [Google Scholar] [CrossRef] [Green Version]
- Belvisi, M.G.; Wicks, S.L.; Battram, C.H.; Bottoms, S.E.W.; Redford, J.E.; Woodman, P.; Brown, T.J.; Webber, S.E.; Foster, M.L. Therapeutic Benefit of a Dissociated Glucocorticoid and the Relevance of In Vitro Separation of Transrepression from Transactivation Activity. J. Immunol. 2001, 166, 1975–1982. [Google Scholar] [CrossRef] [Green Version]
- Schacke, H.; Schottelius, A.; Docke, W.-D.; Strehlke, P.; Jaroch, S.; Schmees, N.; Rehwinkel, H.; Hennekes, H.; Asadullah, K. Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc. Natl. Acad. Sci. USA 2003, 101, 227–232. [Google Scholar] [CrossRef] [Green Version]
- De Bosscher, K.; Beck, I.M.; Dejager, L.; Bougarne, N.; Gaigneaux, A.; Chateauvieux, S.; Ratman, D.; Bracke, M.; Tavernier, J.; Berghe, W.V.; et al. Selective modulation of the glucocorticoid receptor can distinguish between transrepression of NF-κB and AP-1. Cell. Mol. Life Sci. 2013, 71, 143–163. [Google Scholar] [CrossRef] [Green Version]
- De Bosscher, K.; Berghe, W.V.; Beck, I.; Van Molle, W.; Hennuyer, N.; Hapgood, J.; Libert, C.; Staels, B.; Louw, A.; Haegeman, G. A fully dissociated compound of plant origin for inflammatory gene repression. Proc. Natl. Acad. Sci. USA 2005, 102, 15827–15832. [Google Scholar] [CrossRef] [Green Version]
- Duque, E.D.A.; Munhoz, C.D. The Pro-inflammatory Effects of Glucocorticoids in the Brain. Front. Endocrinol. 2016, 7, 78. [Google Scholar] [CrossRef] [Green Version]
- Langlais, D.; Couture, C.; Balsalobre, A.; Drouin, J. Regulatory Network Analyses Reveal Genome-Wide Potentiation of LIF Signaling by Glucocorticoids and Define an Innate Cell Defense Response. PLoS Genet. 2008, 4, e1000224. [Google Scholar] [CrossRef]
- Langlais, D.; Couture, C.; Balsalobre, A.; Drouin, J. The Stat3/GR Interaction Code: Predictive Value of Direct/Indirect DNA Recruitment for Transcription Outcome. Mol. Cell 2012, 47, 38–49. [Google Scholar] [CrossRef] [Green Version]
- Dittrich, A.; Khouri, C.; Sackett, S.D.; Ehlting, C.; Böhmer, O.; Albrecht, U.; Bode, J.G.; Trautwein, C.; Schaper, F. Glucocorticoids increase interleukin-6-dependent gene induction by interfering with the expression of the suppressor of cytokine signaling 3 feedback inhibitor. Hepatology 2011, 55, 256–266. [Google Scholar] [CrossRef]
- Xie, Y.; Tolmeijer, S.; Oskam, J.M.; Tonkens, T.; Meijer, A.H.; Schaaf, M.J.M. Glucocorticoids inhibit macrophage differentiation towards a pro-inflammatory phenotype upon wounding without affecting their migration. Dis. Model. Mech. 2019, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bos, R.V.D.; Cromwijk, S.; Tschigg, K.; Althuizen, J.; Zethof, J.; Whelan, R.; Flik, G.; Schaaf, M. Early Life Glucocorticoid Exposure Modulates Immune Function in Zebrafish (Danio rerio) Larvae. Front. Immunol. 2020, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Sabo, P.J.; Thurman, R.E.; Sung, M.-H.; Biddie, S.; Johnson, T.A.; Hager, G.L.; Stamatoyannopoulos, J.A. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat. Genet. 2011, 43, 264–268. [Google Scholar] [CrossRef]
- John, S.; Sabo, P.J.; Johnson, T.A.; Sung, M.-H.; Biddie, S.C.; Lightman, S.L.; Voss, T.C.; Davis, S.R.; Meltzer, P.S.; Stamatoyannopoulos, J.A.; et al. Interaction of the Glucocorticoid Receptor with the Chromatin Landscape. Mol. Cell 2008, 29, 611–624. [Google Scholar] [CrossRef]
- Ito, K.; Barnes, P.J.; Adcock, I.M. Glucocorticoid Receptor Recruitment of Histone Deacetylase 2 Inhibits Interleukin-1β-Induced Histone H4 Acetylation on Lysines 8 and 12. Mol. Cell. Biol. 2000, 20, 6891–6903. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. Hypoxia. Cross talk between oxygen sensing and the cell cycle machinery. Am. J. Physiol. Physiol. 2011, 301, C550–C552. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001, 13, 167–171. [Google Scholar] [CrossRef]
- Webb, J.D.; Coleman, M.L.; Pugh, C.W. Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell. Mol. Life Sci. 2009, 66, 3539. [Google Scholar] [CrossRef]
- Pollenz, R.S.; Sattler, C.A.; Poland, A. The aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein show distinct subcellular localizations in Hepa 1c1c7 cells by immunofluorescence microscopy. Mol. Pharmacol. 1994, 45, 428–438. [Google Scholar] [PubMed]
- Eguchi, H.; Ikuta, T.; Tachibana, T.; Yoneda, Y.; Kawajiri, K. A Nuclear Localization Signal of Human Aryl Hydrocarbon Receptor Nuclear Translocator/Hypoxia-inducible Factor 1β Is a Novel Bipartite Type Recognized by the Two Components of Nuclear Pore-targeting Complex. J. Biol. Chem. 1997, 272, 17640–17647. [Google Scholar] [CrossRef] [Green Version]
- Iwai, K.; Yamanaka, K.; Kamura, T.; Minato, N.; Conaway, R.C.; Conaway, J.; Klausner, R.D.; Pause, A. Identification of the von Hippel-Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc. Natl. Acad. Sci. USA 1999, 96, 12436–12441. [Google Scholar] [CrossRef] [Green Version]
- Shay, J.E.; Simon, M.C. Hypoxia-inducible factors: Crosstalk between inflammation and metabolism. Semin. Cell Dev. Biol. 2012, 23, 389–394. [Google Scholar] [CrossRef]
- Siddiq, A.; Aminova, L.R.; Ratan, R.R. Hypoxia Inducible Factor Prolyl 4-Hydroxylase Enzymes: Center Stage in the Battle Against Hypoxia, Metabolic Compromise and Oxidative Stress. Neurochem. Res. 2007, 32, 931–946. [Google Scholar] [CrossRef]
- Pan, Y.; Mansfield, K.D.; Bertozzi, C.C.; Rudenko, V.; Chan, D.A.; Giaccia, A.J.; Simon, M.C. Multiple Factors Affecting Cellular Redox Status and Energy Metabolism Modulate Hypoxia-Inducible Factor Prolyl Hydroxylase Activity In Vivo and In Vitro. Mol. Cell. Biol. 2007, 27, 912–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, N.; Singleton, R.S.; Sekirnik, R.; Trudgian, D.C.; Ambrose, L.J.; Miranda, M.X.; Tian, Y.; Kessler, B.M.; Schofield, C.J.; Ratcliffe, P.J. The FIH hydroxylase is a cellular peroxide sensor that modulates HIF transcriptional activity. EMBO Rep. 2012, 13, 251–257. [Google Scholar] [CrossRef]
- Rhee, S.G.; Woo, H.A.; Kil, I.S.; Bae, S.H. Peroxiredoxin Functions as a Peroxidase and a Regulator and Sensor of Local Peroxides. J. Biol. Chem. 2012, 287, 4403–4410. [Google Scholar] [CrossRef] [Green Version]
- Palazon, A.; Goldrath, A.W.; Nizet, V.; Johnson, R.S. HIF Transcription Factors, Inflammation, and Immunity. Immunity 2014, 41, 518–528. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. Oxygen Sensing, Homeostasis, and Disease. N. Engl. J. Med. 2011, 365, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Safronova, O.; Morita, I. Transcriptome Remodeling in Hypoxic Inflammation. J. Dent. Res. 2010, 89, 430–444. [Google Scholar] [CrossRef]
- Bosco, M.C.; Puppo, M.; Blengio, F.; Fraone, T.; Cappello, P.; Giovarelli, M.; Varesio, L. Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration. Immunobiology 2008, 213, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C.; Muthana, M.; Lewis, C.E. Hypoxia Regulates Macrophage Functions in Inflammation. J. Immunol. 2005, 175, 6257–6263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, C.; Murdoch, C. Macrophage Responses to Hypoxia: Implications for Tumor Progression and Anti-Cancer Therapies. Am. J. Pathol. 2005, 167, 627–635. [Google Scholar] [CrossRef]
- Safronova, O.; Pluemsampant, S.; Nakahama, K.-I.; Morita, I. Regulation of chemokine gene expression by hypoxia via cooperative activation of NF-κB and histone deacetylase. Int. J. Biochem. Cell Biol. 2009, 41, 2270–2280. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yin, M.-J.; Gaynor, R.B. IκB Kinase α (IKKα) Regulation of IKKβ Kinase Activity. Mol. Cell. Biol. 2000, 20, 3655–3666. [Google Scholar] [CrossRef]
- Cummins, E.P.; Berra, E.; Comerford, K.M.; Ginouves, A.; Fitzgerald, K.T.; Seeballuck, F.; Godson, C.; Nielsen, J.E.; Moynagh, P.; Pouyssegur, J.; et al. Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. Proc. Natl. Acad. Sci. USA 2006, 103, 18154–18159. [Google Scholar] [CrossRef] [Green Version]
- Cummins, E.; Comerford, K.M.; Scholz, C.; Bruning, U.; Taylor, C. Hypoxic Regulation of NF-κB Signaling. Methods Enzymol. 2007, 435, 479–492. [Google Scholar] [CrossRef]
- van Uden, P.; Kenneth, N.S.; Rocha, S. Regulation of hypoxia-inducible factor-1α by NF-κB. Biochem. J. 2008, 412, 477–484. [Google Scholar] [CrossRef] [Green Version]
- van Uden, P.; Kenneth, N.S.; Webster, R.; Müller, H.A.; Mudie, S.; Rocha, S. Evolutionary Conserved Regulation of HIF-1β by NF-κB. PLoS Genet. 2011, 7, e1001285. [Google Scholar] [CrossRef] [Green Version]
- Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A.S.; Nizet, V.; Johnson, R.S.; Haddad, G.G.; Karin, M. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 2008, 453, 807–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ignazio, L.; Bandarra, D.; Rocha, S. NF-κB and HIF crosstalk in immune responses. FEBS J. 2015, 283, 413–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schild, Y.; Mohamed, A.; Wootton, E.J.; Lewis, A.; Elks, P.M. Hif-1alpha stabilisation is protective against infection in zebrafish comorbid models. FEBS J. 2020, 287, 3925–3943. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.E.; Chou, P.-C.; Coutts, D.J.C.; Kumar, V.; To, M.; Akashi, K.; Pinhu, L.; Griffiths, M.; Adcock, I.; Barnes, P.J.; et al. Hypoxia-inducible Factor 1α Induces Corticosteroid-insensitive Inflammation via Reduction of Histone Deacetylase-2 Transcription. J. Biol. Chem. 2009, 284, 36047–36054. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhao, J.-J.; Lv, Y.-Y.; Ding, P.-S.; Liu, R.-Y. Hypoxia down-regulates glucocorticoid receptor alpha and attenuates the anti-inflammatory actions of dexamethasone in human alveolar epithelial A549 cells. Life Sci. 2009, 85, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Vanderhaeghen, T.; Timmermans, S.; Watts, D.; Paakinaho, V.; Eggermont, M.; Vandewalle, J.; Wallaeys, C.; Van Wyngene, L.; Van Looveren, K.; Nuyttens, L.; et al. Reprogramming of glucocorticoid receptor function by hypoxia. EMBO Rep. 2021, e53083. [Google Scholar] [CrossRef] [PubMed]
- Elsby, L.M.; Donn, R.; Alourfi, Z.; Green, L.M.; Beaulieu, E.; Ray, D.W. Hypoxia and glucocorticoid signaling converge to regulate macrophage migration inhibitory factor gene expression. Arthritis Rheum. 2009, 60, 2220–2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ma, Y.-Y.; Song, X.-L.; Cai, H.-Y.; Chen, J.-C.; Song, L.-N.; Yang, R.; Lu, J. Upregulations of Glucocorticoid-Induced Leucine Zipper by Hypoxia and Glucocorticoid Inhibit Proinflammatory Cytokines under Hypoxic Conditions in Macrophages. J. Immunol. 2011, 188, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Ayroldi, E.; Riccardi, C. Glucocorticoid-induced leucine zipper (GILZ): A new important mediator of glucocorticoid action. FASEB J. 2009, 23, 3649–3658. [Google Scholar] [CrossRef] [Green Version]
- Cannarile, L.; Delfino, D.V.; Adorisio, S.; Riccardi, C.; Ayroldi, E. Implicating the Role of GILZ in Glucocorticoid Modulation of T-Cell Activation. Front. Immunol. 2019, 10, 1823. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Choi, E.Y. Macrophages and Inflammation. J. Rheum. Dis. 2018, 25, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Xue, L.; Greisler, H.P. Angiogenic effect of fibroblast growth factor-1 and vascular endothelial growth factor and their synergism in a novel in vitro quantitative fibrin-based 3-dimensional angiogenesis system. Surgery 2002, 132, 259–267. [Google Scholar] [CrossRef]
- Ke, X.; Chen, C.; Song, Y.; Cai, Q.; Li, J.; Tang, Y.; Han, X.; Qu, W.; Chen, A.; Wang, H.; et al. Hypoxia modifies the polarization of macrophages and their inflammatory microenvironment, and inhibits malignant behavior in cancer cells. Oncol. Lett. 2019, 18, 5871–5878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Goodwin, J.E. The Effect of Glucocorticoids on Angiogenesis in the Treatment of Solid Tumors. J. Cell. Signal. 2020, 1, 42–49. [Google Scholar] [CrossRef]
- Bernhardt, W.M.; Câmpean, V.; Kany, S.; Jürgensen, J.-S.; Weidemann, A.; Warnecke, C.; Arend, M.; Klaus, S.; Günzler, V.; Amann, K.; et al. Preconditional Activation of Hypoxia-Inducible Factors Ameliorates Ischemic Acute Renal Failure. J. Am. Soc. Nephrol. 2006, 17, 1970–1978. [Google Scholar] [CrossRef]
- Wan, C.; Gilbert, S.R.; Wang, Y.; Cao, X.; Shen, X.; Ramaswamy, G.; Jacobsen, K.A.; Alaql, Z.S.; Eberhardt, A.W.; Gerstenfeld, L.C.; et al. Activation of the hypoxia-inducible factor-1 pathway accelerates bone regeneration. Proc. Natl. Acad. Sci. USA 2008, 105, 686–691. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J. Clin. Investig. 2013, 123, 3664–3671. [Google Scholar] [CrossRef] [Green Version]
- Vettori, A.; Greenald, D.; Wilson, G.K.; Peron, M.; Facchinello, N.; Markham, E.; Sinnakaruppan, M.; Matthews, L.C.; McKeating, J.A.; Argenton, F.; et al. Glucocorticoids promote Von Hippel Lindau degradation and Hif-1α stabilization. Proc. Natl. Acad. Sci. USA 2017, 114, 9948–9953. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.D.; Brearey, S.P.; Imray, C.H.E. High hopes at high altitudes: Pharmacotherapy for acute mountain sickness and high-altitude cerebral and pulmonary oedema. Expert Opin. Pharmacother. 2007, 9, 119–127. [Google Scholar] [CrossRef]
- Dardzinski, B.J.; Smith, S.L.; Towfighi, J.; Williams, G.D.; Vannucci, R.C.; Smith, M.B. Increased Plasma Beta-Hydroxybutyrate, Preserved Cerebral Energy Metabolism, and Amelioration of Brain Damage During Neonatal Hypoxia Ischemia with Dexamethasone Pretreatment. Pediatr. Res. 2000, 48, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Limbourg, F.P.; Huang, Z.; Plumier, J.-C.; Simoncini, T.; Fujioka, M.; Tuckermann, J.; Schutz, G.; Moskowitz, M.A.; Liao, J.K. Rapid nontranscriptional activation of endothelial nitric oxide synthase mediates increased cerebral blood flow and stroke protection by corticosteroids. J. Clin. Investig. 2002, 110, 1729–1738. [Google Scholar] [CrossRef]
- Glanemann, M.; Strenziok, R.; Kuntze, R.; Münchow, S.; Dikopoulos, N.; Lippek, F.; Langrehr, J.M.; Dietel, M.; Neuhaus, P.; Nussler, A.K. Ischemic preconditioning and methylprednisolone both equally reduce hepatic ischemia/reperfusion injury. Surgery 2004, 135, 203–214. [Google Scholar] [CrossRef]
- Tokudome, S.; Sano, M.; Shinmura, K.; Matsuhashi, T.; Morizane, S.; Moriyama, H.; Tamaki, K.; Hayashida, K.; Nakanishi, H.; Yoshikawa, N.; et al. Glucocorticoid protects rodent hearts from ischemia / reperfusion injury by activating lipocalin-type prostaglandin D synthase—Derived PGD 2 biosynthesis. J. Clin. Investig. 2009, 119, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qiang, Q.; Jiang, Y.; Hu, L.; Ding, X.; Lu, Y.; Hu, G. Effects of hypoxia inducible factor-1α on apoptotic inhibition and glucocorticoid receptor downregulation by dexamethasone in AtT-20 cells. BMC Endocr. Disord. 2015, 15, 24. [Google Scholar] [CrossRef] [Green Version]
- Gaber, T.; Schellmann, S.; Erekul, K.B.; Fangradt, M.; Tykwinska, K.; Hahne, M.; Maschmeyer, P.; Wagegg, M.; Stahn, C.; Kolar, P.; et al. Macrophage Migration Inhibitory Factor Counterregulates Dexamethasone-Mediated Suppression of Hypoxia-Inducible Factor-1α Function and Differentially Influences Human CD4+ T Cell Proliferation under Hypoxia. J. Immunol. 2010, 186, 764–774. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.S.; Lam, I.I.; Clay, H.; Duong, D.N.; Deo, R.C.; Coughlin, S.R. A Rapid Method for Directed Gene Knockout for Screening in G0 Zebrafish. Dev. Cell 2018, 46, 112–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, A.; Lindsay, H.; Felker, A.; Hess, C.; Anders, C.; Chiavacci, E.; Zaugg, J.; Weber, L.M.; Catena, R.; Jinek, M.; et al. Maximizing mutagenesis with solubilized CRISPR-Cas9 ribonucleoprotein complexes. Development 2016, 143, 2025–2037. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, L.; Verhoog, N.; Louw, A. Novel role for receptor dimerization in post-translational processing and turnover of the GRα. Sci. Rep. 2018, 8, 14266. [Google Scholar] [CrossRef] [Green Version]
- Pace, T.W.; Hu, F.; Miller, A.H. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav. Immun. 2007, 21, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.; Yu, R.M.K.; Wu, R.S.S.; Kong, R.Y.C. Overexpression and Knockdown of Hypoxia-Inducible Factor 1 Disrupt the Expression of Steroidogenic Enzyme Genes and Early Embryonic Development in Zebrafish. Gene Regul. Syst. Biol. 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Egg, M.; Köblitz, L.; Hirayama, J.; Schwerte, T.; Folterbauer, C.; Kurz, A.; Fiechtner, B.; Möst, M.; Salvenmoser, W.; Sassone-Corsi, P.; et al. Linking Oxygen to Time: The Bidirectional Interaction Between the Hypoxic Signaling Pathway and the Circadian Clock. Chronobiol. Int. 2013, 30, 510–529. [Google Scholar] [CrossRef] [PubMed]
- Egg, M.; Paulitsch, M.; Ennemoser, Y.; Wüstenhagen, A.; Schwerte, T.; Sandbichler, A.M.; Fiechtner, B.; Köblitz, L.; Prem, C.; Pelster, B. Chronodisruption increases cardiovascular risk in zebrafish via reduced clearance of senescent erythrocytes. Chronobiol. Int. 2014, 31, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Pelster, B.; Egg, M. Multiplicity of Hypoxia-Inducible Transcription Factors and Their Connection to the Circadian Clock in the Zebrafish. Physiol. Biochem. Zool. 2015, 88, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Peek, C.B.; Levine, D.; Cedernaes, J.; Taguchi, A.; Kobayashi, Y.; Tsai, S.J.; Bonar, N.A.; McNulty, M.R.; Ramsey, K.M.; Bass, J. Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab. 2016, 25, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, N.; Berry, A.; Sauer, C.; Baxter, M.; Donaldson, I.J.; Forbes, K.; Donn, R.; Matthews, L.; Ray, D. Hypoxia regulates GR function through multiple mechanisms involving microRNAs 103 and 107. Mol. Cell. Endocrinol. 2020, 518, 111007. [Google Scholar] [CrossRef]
- Watts, D.; Stein, J.; Meneses, A.; Bechmann, N.; Neuwirth, A.; Kaden, D.; Krüger, A.; Sinha, A.; Alexaki, V.I.; Perez-Rivas, L.G.; et al. HIF1α is a direct regulator of steroidogenesis in the adrenal gland. Cell. Mol. Life Sci. 2021, 78, 3577–3590. [Google Scholar] [CrossRef] [PubMed]
- Rybnikova, E.; Glushchenko, T.; Churilova, A.; Pivina, S.; Samoilov, M. Expression of glucocorticoid and mineralocorticoid receptors in hippocampus of rats exposed to various modes of hypobaric hypoxia: Putative role in hypoxic preconditioning. Brain Res. 2011, 1381, 66–77. [Google Scholar] [CrossRef]
- Robinson, P.C.; Morand, E. Divergent effects of acute versus chronic glucocorticoids in COVID-19. Lancet Rheumatol. 2021, 3, e168–e170. [Google Scholar] [CrossRef]
- van Paassen, J.; Vos, J.S.; Hoekstra, E.M.; Neumann, K.M.I.; Boot, P.C.; Arbous, S.M. Corticosteroid use in COVID-19 patients: A systematic review and meta-analysis on clinical outcomes. Crit. Care 2020, 24, 696. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchi, D.; van Eeden, F.J.M. Homeostatic Regulation of Glucocorticoid Receptor Activity by Hypoxia-Inducible Factor 1: From Physiology to Clinic. Cells 2021, 10, 3441. https://doi.org/10.3390/cells10123441
Marchi D, van Eeden FJM. Homeostatic Regulation of Glucocorticoid Receptor Activity by Hypoxia-Inducible Factor 1: From Physiology to Clinic. Cells. 2021; 10(12):3441. https://doi.org/10.3390/cells10123441
Chicago/Turabian StyleMarchi, Davide, and Fredericus J. M. van Eeden. 2021. "Homeostatic Regulation of Glucocorticoid Receptor Activity by Hypoxia-Inducible Factor 1: From Physiology to Clinic" Cells 10, no. 12: 3441. https://doi.org/10.3390/cells10123441
APA StyleMarchi, D., & van Eeden, F. J. M. (2021). Homeostatic Regulation of Glucocorticoid Receptor Activity by Hypoxia-Inducible Factor 1: From Physiology to Clinic. Cells, 10(12), 3441. https://doi.org/10.3390/cells10123441





