TGF-β Induction of miR-143/145 Is Associated to Exercise Response by Influencing Differentiation and Insulin Signaling Molecules in Human Skeletal Muscle
Abstract
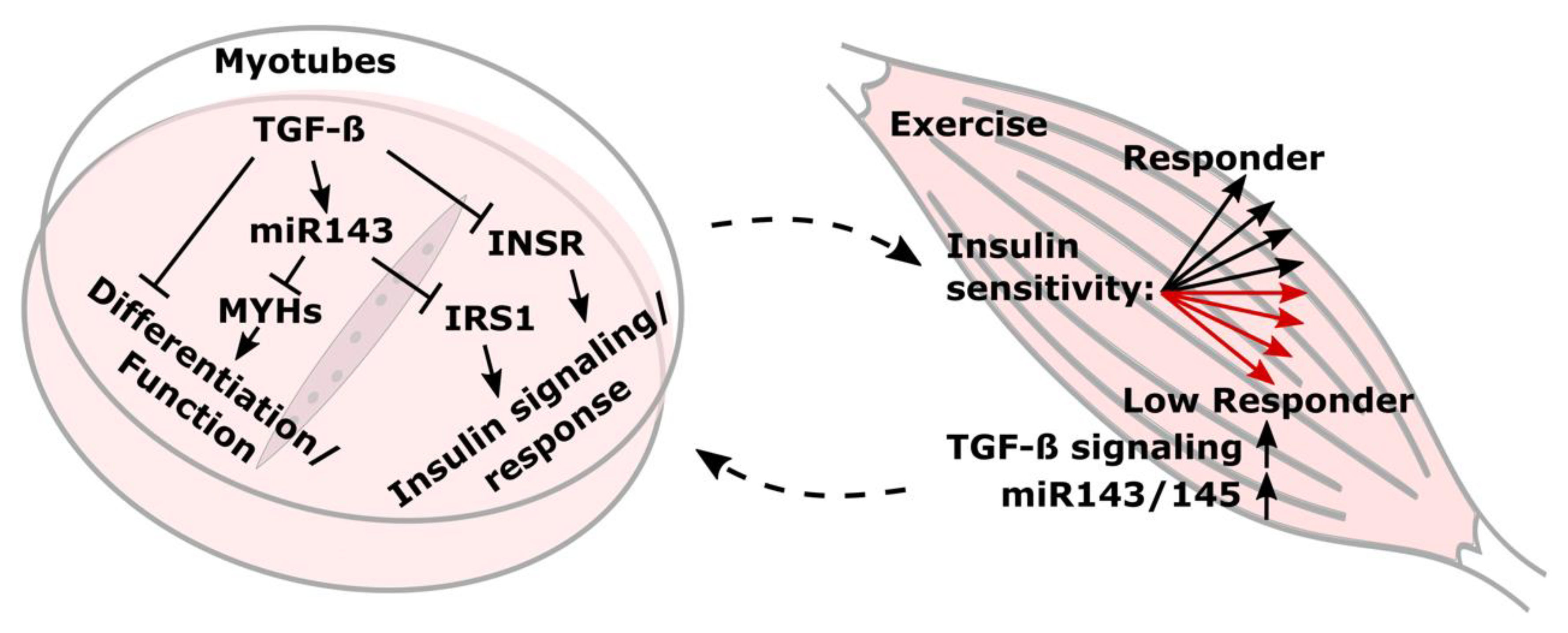
:1. Introduction
2. Materials and Methods
2.1. Primary Cell Culture
2.2. qPCR
2.3. Immunoblot
2.4. Sequencing
2.5. Exercise Intervention Studies
2.6. Statistical Analyses
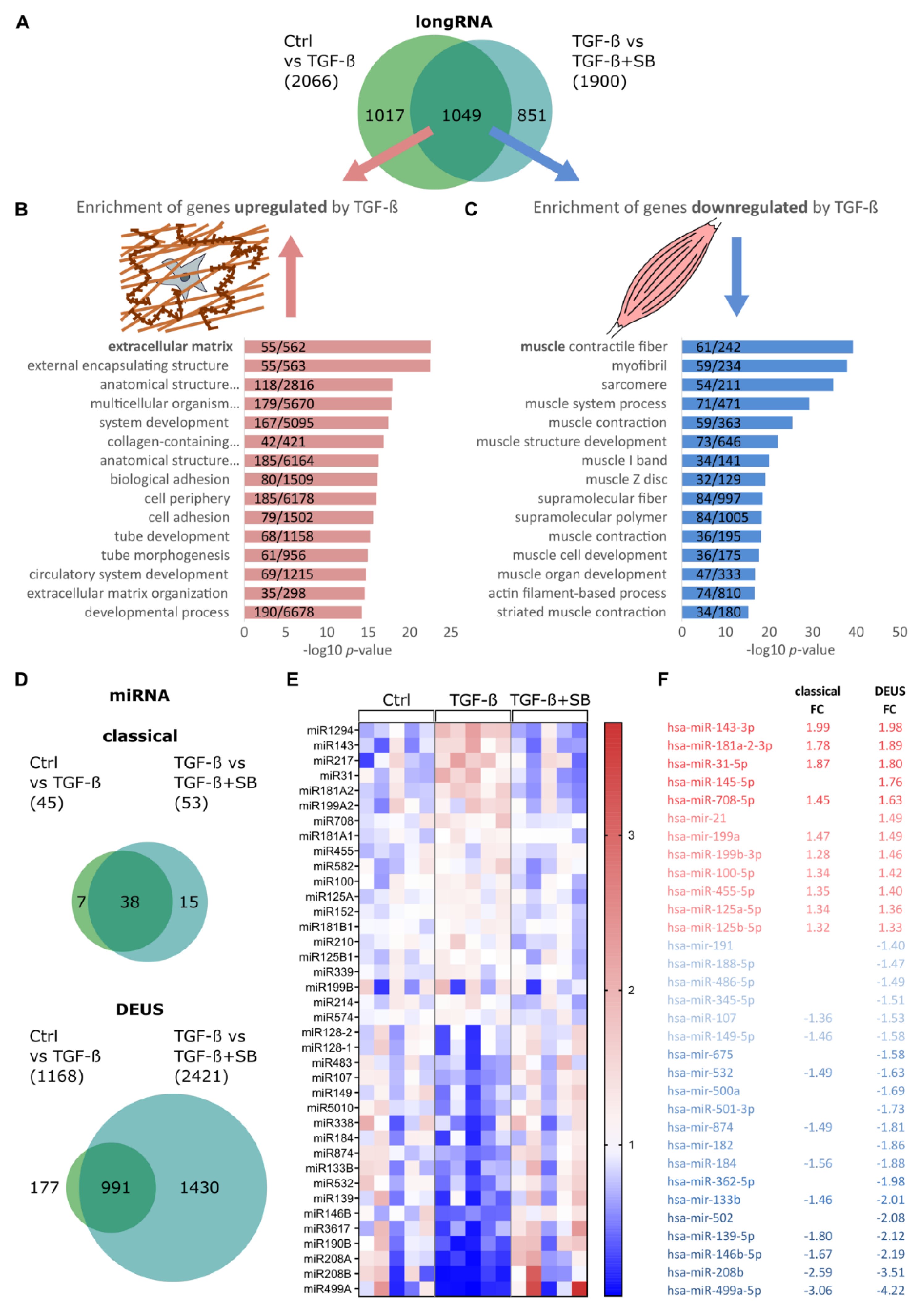
3. Results
3.1. TGF-β1 Modulates Global miRNA Expression Profile in Differentiating Human Skeletal Muscle Cells
3.2. TGF-β1 Regulates miR-143/145 Cluster and miR-181a2 Differentiation-Independantly
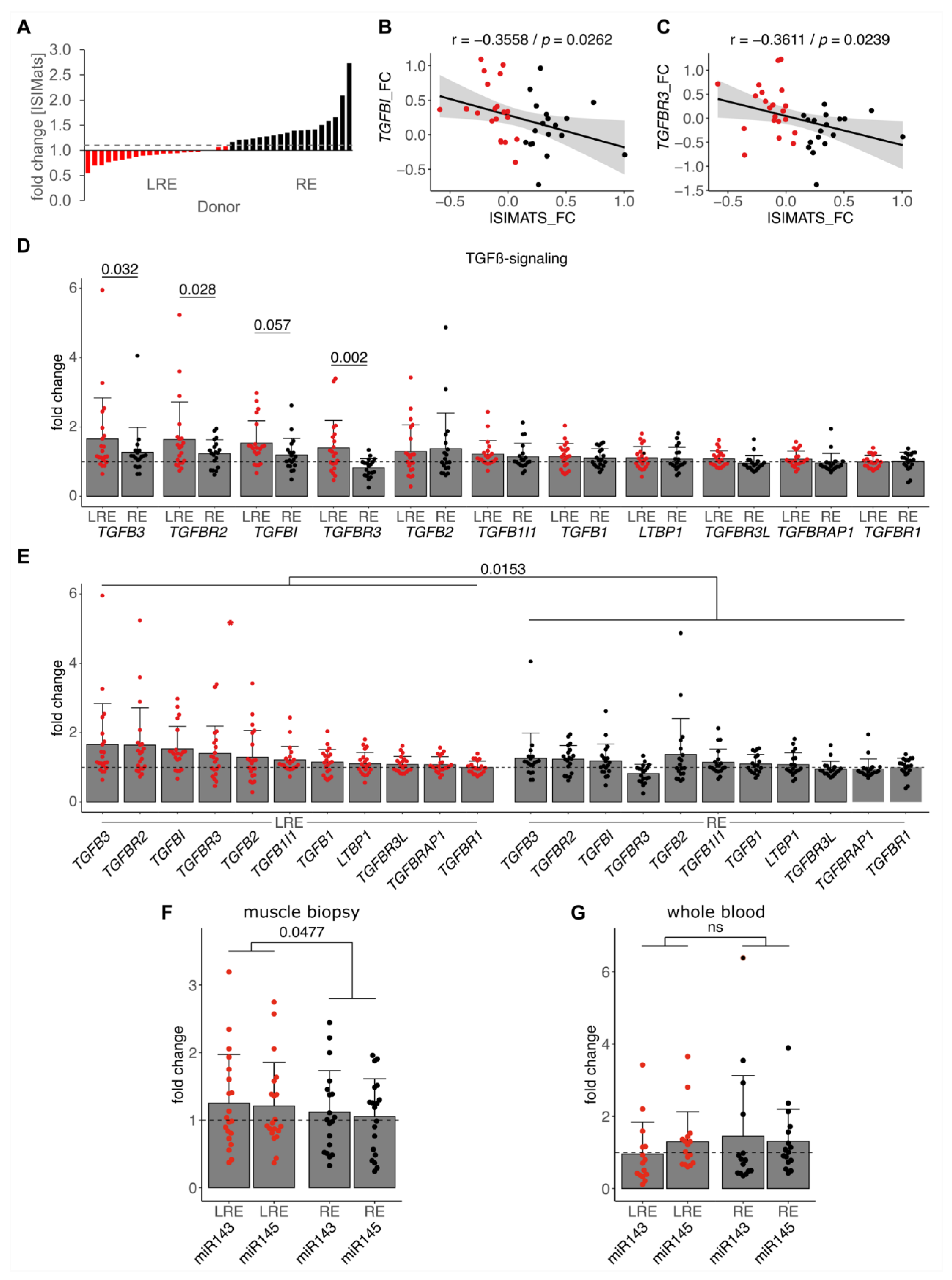
3.3. Low Responders Show Elevated TGF-β Signaling and miR-143/145 Cluster Induction by Training
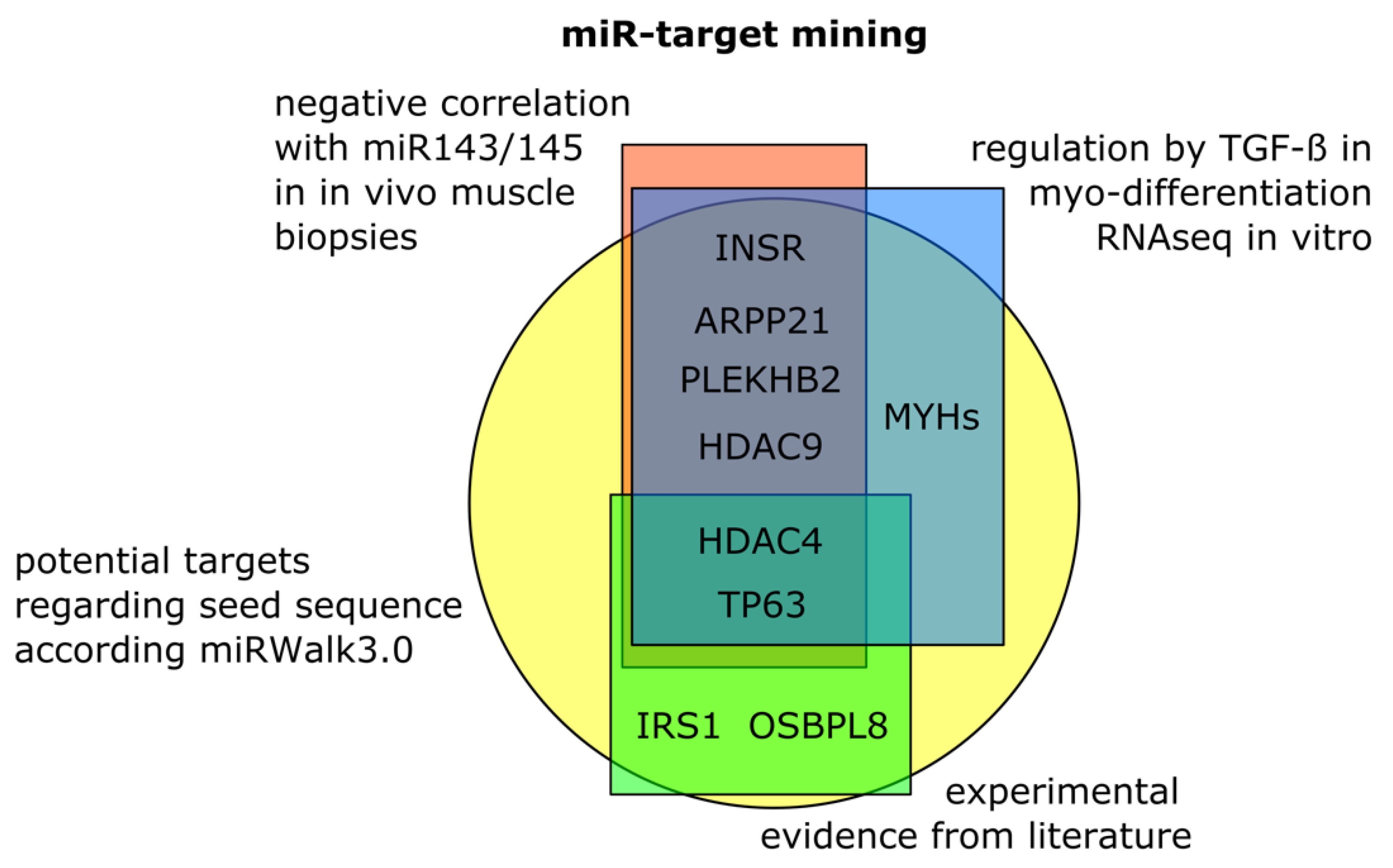
3.4. Target Mining for Potential Skeletal Muscle Specific miR-143/145 Cluster Targets
3.5. TGF-β Negatively Impacts Functional Components and Insulin Signaling in Skeletal Muscle Cells
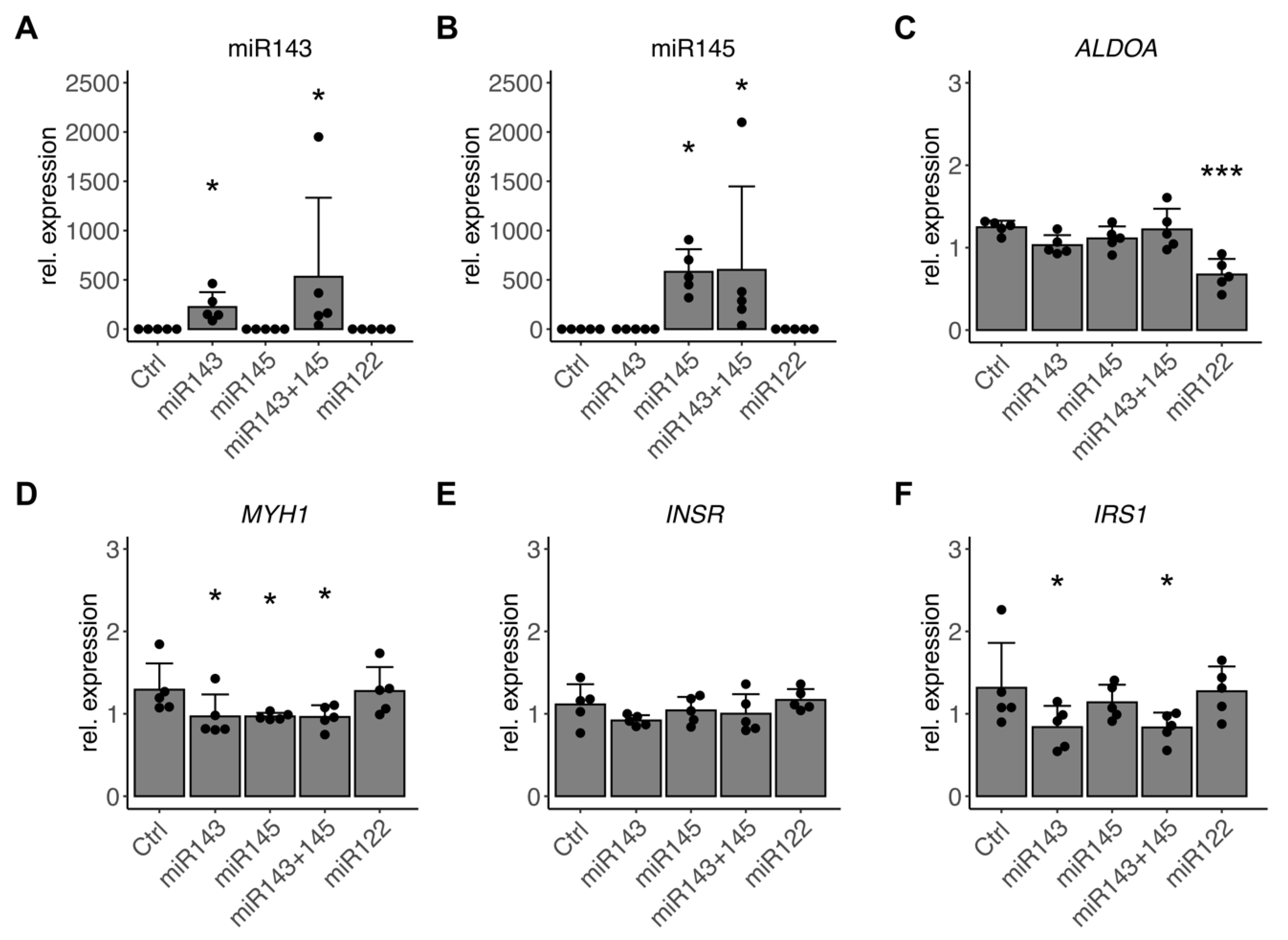
3.6. miR-143/145 Cluster Finetunes TGF-β Effects on Differentiation and Insulin Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Praet, S.F.; van Loon, L.J. Exercise: The brittle cornerstone of type 2 diabetes treatment. Diabetologia 2008, 51, 398–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawley, J.A.; Lessard, S.J. Exercise training-induced improvements in insulin action. Acta Physiol. 2008, 192, 127–135. [Google Scholar] [CrossRef]
- Boule, N.G.; Weisnagel, S.J.; Lakka, T.A.; Tremblay, A.; Bergman, R.N.; Rankinen, T.; Leon, A.S.; Skinner, J.S.; Wilmore, J.H.; Rao, D.C.; et al. Effects of exercise training on glucose homeostasis: The HERITAGE Family Study. Diabetes Care 2005, 28, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Zanuso, S.; Sacchetti, M.; Sundberg, C.J.; Orlando, G.; Benvenuti, P.; Balducci, S. Exercise in type 2 diabetes: Genetic, metabolic and neuromuscular adaptations. A review of the evidence. Br. J. Sports Med. 2017, 51, 1533–1538. [Google Scholar] [CrossRef]
- Shahar, J.; Hamdy, O. Medication and exercise interactions: Considering and managing hypoglycemia risk. Diabetes Spectr. 2015, 28, 64–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohm, A.; Weigert, C.; Staiger, H.; Haring, H.U. Exercise and diabetes: Relevance and causes for response variability. Endocrine 2016, 51, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Sparks, L.M. Exercise training response heterogeneity: Physiological and molecular insights. Diabetologia 2017, 60, 2329–2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, R.; Goodpaster, B.H.; Koch, L.G.; Sarzynski, M.A.; Kohrt, W.M.; Johannsen, N.M.; Skinner, J.S.; Castro, A.; Irving, B.A.; Noland, R.C.; et al. Precision exercise medicine: Understanding exercise response variability. Br. J. Sports Med. 2019, 53, 1141–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero, D.; Lundby, C. Refuting the myth of non-response to exercise training: ‘non-responders’ do respond to higher dose of training. J. Physiol. 2017, 595, 3377–3387. [Google Scholar] [CrossRef] [PubMed]
- Bohm, A.; Hoffmann, C.; Irmler, M.; Schneeweiss, P.; Schnauder, G.; Sailer, C.; Schmid, V.; Hudemann, J.; Machann, J.; Schick, F.; et al. TGF-beta Contributes to Impaired Exercise Response by Suppression of Mitochondrial Key Regulators in Skeletal Muscle. Diabetes 2016, 65, 2849–2861. [Google Scholar] [CrossRef] [Green Version]
- Burks, T.N.; Cohn, R.D. Role of TGF-beta signaling in inherited and acquired myopathies. Skelet. Muscle 2011, 1, 19. [Google Scholar] [CrossRef] [Green Version]
- Heinemeier, K.M.; Bjerrum, S.S.; Schjerling, P.; Kjaer, M. Expression of extracellular matrix components and related growth factors in human tendon and muscle after acute exercise. Scand. J. Med. Sci. Sports 2013, 23, e150–e161. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, O.; Sabapathy, S.; Ashton, K.J.; Desbrow, B.; Peake, J.M.; Lazarus, R.; Wessner, B.; Cameron-Smith, D.; Wagner, K.H.; Haseler, L.J.; et al. Time course-dependent changes in the transcriptome of human skeletal muscle during recovery from endurance exercise: From inflammation to adaptive remodeling. J. Appl. Physiol. 2014, 116, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Munoz-Canoves, P. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef] [Green Version]
- Massague, J.; Cheifetz, S.; Endo, T.; Nadal-Ginard, B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc. Natl. Acad. Sci. USA 1986, 83, 8206–8210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiano, J.P.; Springer, D.A.; Rane, S.G. SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1alpha) during exercise. J. Biol. Chem. 2015, 290, 7671–7684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Climent, M.; Quintavalle, M.; Miragoli, M.; Chen, J.; Condorelli, G.; Elia, L. TGFbeta Triggers miR-143/145 Transfer From Smooth Muscle Cells to Endothelial Cells, Thereby Modulating Vessel Stabilization. Circ. Res. 2015, 116, 1753–1764. [Google Scholar] [CrossRef] [Green Version]
- Redshaw, N.; Camps, C.; Sharma, V.; Motallebipour, M.; Guzman-Ayala, M.; Oikonomopoulos, S.; Thymiakou, E.; Ragoussis, J.; Episkopou, V. TGF-beta/Smad2/3 signaling directly regulates several miRNAs in mouse ES cells and early embryos. PLoS ONE 2013, 8, e55186. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Komers, R.; Carew, R.; Winbanks, C.E.; Xu, B.; Herman-Edelstein, M.; Koh, P.; Thomas, M.; Jandeleit-Dahm, K.; Gregorevic, P.; et al. Suppression of microRNA-29 expression by TGF-beta1 promotes collagen expression and renal fibrosis. J. Am. Soc. Nephrol. JASN 2012, 23, 252–265. [Google Scholar] [CrossRef] [Green Version]
- Winbanks, C.E.; Wang, B.; Beyer, C.; Koh, P.; White, L.; Kantharidis, P.; Gregorevic, P. TGF-beta regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J. Biol. Chem. 2011, 286, 13805–13814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, B.N.; Hilyard, A.C.; Nguyen, P.H.; Lagna, G.; Hata, A. Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol. Cell 2010, 39, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 2016, 410, 1–13. [Google Scholar] [CrossRef]
- McCarthy, J.J. MicroRNA-206: The skeletal muscle-specific myomiR. Biochim. Biophys. Acta 2008, 1779, 682–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, C.; Hockele, S.; Kappler, L.; Hrabe de Angelis, M.; Haring, H.U.; Weigert, C. The effect of differentiation and TGFbeta on mitochondrial respiration and mitochondrial enzyme abundance in cultured primary human skeletal muscle cells. Sci. Rep. 2018, 8, 737. [Google Scholar] [CrossRef] [Green Version]
- Massart, J.; Sjogren, R.J.O.; Egan, B.; Garde, C.; Lindgren, M.; Gu, W.; Ferreira, D.M.S.; Katayama, M.; Ruas, J.L.; Barres, R.; et al. Endurance exercise training-responsive miR-19b-3p improves skeletal muscle glucose metabolism. Nat. Commun. 2021, 12, 5948. [Google Scholar] [CrossRef]
- Suzuki, H.I. MicroRNA Control of TGF-beta Signaling. Int. J. Mol. Sci. 2018, 19, 1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, T.; Hu, C.; Yang, H.; Cao, L.; An, J. Transforming growth factor-beta-induced miR143 expression in regulation of non-small cell lung cancer cell viability and invasion capacity in vitro and in vivo. Int. J. Oncol. 2014, 45, 1977–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Fan, J.; Chen, N. A Novel Regulator of Type II Diabetes: MicroRNA-143. Trends Endocrinol. Metab. 2018, 29, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.D.; Kruger, M.; Willmes, D.M.; Redemann, N.; Wunderlich, F.T.; Bronneke, H.S.; Merkwirth, C.; Kashkar, H.; Olkkonen, V.M.; Bottger, T.; et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat. Cell Biol. 2011, 13, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Blumensatt, M.; Wronkowitz, N.; Wiza, C.; Cramer, A.; Mueller, H.; Rabelink, M.J.; Hoeben, R.C.; Eckel, J.; Sell, H.; Ouwens, D.M. Adipocyte-derived factors impair insulin signaling in differentiated human vascular smooth muscle cells via the upregulation of miR-143. Biochim. Biophys. Acta 2014, 1842, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, S.; Albinsson, S. Regulation of IRS-1, insulin signaling and glucose uptake by miR-143/145 in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2020, 529, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Gottmann, P.; Ouni, M.; Saussenthaler, S.; Roos, J.; Stirm, L.; Jahnert, M.; Kamitz, A.; Hallahan, N.; Jonas, W.; Fritsche, A.; et al. A computational biology approach of a genome-wide screen connected miRNAs to obesity and type 2 diabetes. Mol. Metab. 2018, 11, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Jeske, T.; Huypens, P.; Stirm, L.; Hockele, S.; Wurmser, C.M.; Bohm, A.; Weigert, C.; Staiger, H.; Klein, C.; Beckers, J.; et al. DEUS: An R package for accurate small RNA profiling based on differential expression of unique sequences. Bioinformatics 2019, 35, 4834–4836. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, C.; Schneeweiss, P.; Randrianarisoa, E.; Schnauder, G.; Kappler, L.; Machann, J.; Schick, F.; Fritsche, A.; Heni, M.; Birkenfeld, A.; et al. Response of Mitochondrial Respiration in Adipose Tissue and Muscle to 8 Weeks of Endurance Exercise in Obese Subjects. J. Clin. Endocrinol. Metab. 2020, 105, e4023–e4037. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- RCoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Inman, G.J.; Nicolas, F.J.; Callahan, J.F.; Harling, J.D.; Gaster, L.M.; Reith, A.D.; Laping, N.J.; Hill, C.S. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002, 62, 65–74. [Google Scholar] [CrossRef]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef]
- Long, X.; Miano, J.M. Transforming growth factor-beta1 (TGF-beta1) utilizes distinct pathways for the transcriptional activation of microRNA 143/145 in human coronary artery smooth muscle cells. J. Biol. Chem. 2011, 286, 30119–30129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riches, K.; Alshanwani, A.R.; Warburton, P.; O’Regan, D.J.; Ball, S.G.; Wood, I.C.; Turner, N.A.; Porter, K.E. Elevated expression levels of miR-143/5 in saphenous vein smooth muscle cells from patients with Type 2 diabetes drive persistent changes in phenotype and function. J. Mol. Cell. Cardiol. 2014, 74, 240–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Chen, C.; Liu, Q.; Liu, B.; Song, C.; Zhu, S.; Wu, C.; Liu, S.; Yu, H.; Yao, D.; et al. The role of the miR-31/FIH1 pathway in TGF-beta-induced liver fibrosis. Clin. Sci. 2015, 129, 305–317. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Tsuyada, A.; Ren, X.; Wu, X.; Stubblefield, K.; Rankin-Gee, E.K.; Wang, S.E. Transforming growth factor-beta regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene 2011, 30, 1470–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Li, S.; Zhang, W.; Tong, H.; Li, S.; Yan, Y. MiR-139 promotes differentiation of bovine skeletal muscle-derived satellite cells by regulating DHFR gene expression. J. Cell. Physiol. 2018, 234, 632–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, N.; Ge, Y.; Chen, J. MicroRNA-146b promotes myogenic differentiation and modulates multiple gene targets in muscle cells. PLoS ONE 2014, 9, e100657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liang, X.; Zhou, D.; Lai, L.; Xiao, L.; Liu, L.; Fu, T.; Kong, Y.; Zhou, Q.; Vega, R.B.; et al. Coupling of mitochondrial function and skeletal muscle fiber type by a miR-499/Fnip1/AMPK circuit. EMBO Mol. Med. 2016, 8, 1212–1228. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, J.J.; Esser, K.A.; Peterson, C.A.; Dupont-Versteegden, E.E. Evidence of MyomiR network regulation of beta-myosin heavy chain gene expression during skeletal muscle atrophy. Physiol. Genom. 2009, 39, 219–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Essential amino acids increase microRNA-499, -208b, and -23a and downregulate myostatin and myocyte enhancer factor 2C mRNA expression in human skeletal muscle. J. Nutr. 2009, 139, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Griffiths-Jones, S.; Ashurst, J.L.; Bradley, A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004, 14, 1902–1910. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Shyr, Y.; Cai, J.; Liu, Q. Interplay between miRNAs and host genes and their role in cancer. Brief. Funct. Genom. 2018, 18, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacante, F.; Denby, L.; Sluimer, J.C.; Baker, A.H. The function of miR-143, miR-145 and the MiR-143 host gene in cardiovascular development and disease. Vascul. Pharmacol. 2019, 112, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Yasui, Y.; Iwasaki, J.; Kumazaki, M.; Yamada, N.; Naito, S.; Akao, Y. Replacement treatment with microRNA-143 and -145 induces synergistic inhibition of the growth of human bladder cancer cells by regulating PI3K/Akt and MAPK signaling pathways. Cancer Lett. 2013, 328, 353–361. [Google Scholar] [CrossRef]
- Copps, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.C.; Cheng, Z.; Copps, K.D.; White, M.F. Insulin receptor substrates Irs1 and Irs2 coordinate skeletal muscle growth and metabolism via the Akt and AMPK pathways. Mol. Cell. Biol. 2011, 31, 430–441. [Google Scholar] [CrossRef] [Green Version]
- Coletta, R.; Roberts, N.A.; Randles, M.J.; Morabito, A.; Woolf, A.S. Exogenous transforming growth factor-beta1 enhances smooth muscle differentiation in embryonic mouse jejunal explants. J. Tissue Eng. Regen. Med. 2018, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valcourt, U.; Kowanetz, M.; Niimi, H.; Heldin, C.H.; Moustakas, A. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol. Biol. Cell 2005, 16, 1987–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avalle, L.; Incarnato, D.; Savino, A.; Gai, M.; Marino, F.; Pensa, S.; Barbieri, I.; Stadler, M.B.; Provero, P.; Oliviero, S.; et al. MicroRNAs-143 and -145 induce epithelial to mesenchymal transition and modulate the expression of junction proteins. Cell Death Differ. 2017, 24, 1750–1760. [Google Scholar] [CrossRef] [Green Version]
- Davis-Dusenbery, B.N.; Chan, M.C.; Reno, K.E.; Weisman, A.S.; Layne, M.D.; Lagna, G.; Hata, A. down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J. Biol. Chem. 2011, 286, 28097–28110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, E.N.; Sternberg, E.; Hu, J.S.; Spizz, G.; Wilcox, C. Regulation of myogenic differentiation by type beta transforming growth factor. J. Cell Biol. 1986, 103, 1799–1805. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.Y.; Gonzalez-Martin, A.; Miletic, A.V.; Lai, M.; Knight, S.; Sabouri-Ghomi, M.; Head, S.R.; Macauley, M.S.; Rickert, R.C.; Xiao, C. Transfection of microRNA Mimics Should Be Used with Caution. Front. Genet. 2015, 6, 340. [Google Scholar] [CrossRef] [Green Version]
- Creugny, A.; Fender, A.; Pfeffer, S. Regulation of primary microRNA processing. FEBS Lett. 2018, 592, 1980–1996. [Google Scholar] [CrossRef]
- Sokilde, R.; Newie, I.; Persson, H.; Borg, A.; Rovira, C. Passenger strand loading in overexpression experiments using microRNA mimics. RNA Biol. 2015, 12, 787–791. [Google Scholar] [CrossRef] [Green Version]
- Thomson, D.W.; Bracken, C.P.; Szubert, J.M.; Goodall, G.J. On measuring miRNAs after transient transfection of mimics or antisense inhibitors. PLoS ONE 2013, 8, e55214. [Google Scholar] [CrossRef] [Green Version]
- Sapp, R.M.; Shill, D.D.; Roth, S.M.; Hagberg, J.M. Circulating microRNAs in acute and chronic exercise: More than mere biomarkers. J. Appl. Physiol. 2017, 122, 702–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Sex | 25 females/15 males | |
| Age | 36.84 ± 12.23 | Years |
| WHR | 0.90 ± 0.05 | cm/cm |
| BMI | 31.68 ± 4.39 | kg/m2 |
| Fasting glucose | 5.29 ± 0.45 | mmol/L |
| Fasting insulin | 93.52 ± 40.25 | pmol/L |
| VO2peak | 25.42 ± 4.82 | ml/min∗kg |
| HbA1c | 35.03 ± 3.40 | mmol/mol Hb |
| TGF-ß | p vs. Ctrl | TGF-ß + SB | p vs. TGF-ß | ||
|---|---|---|---|---|---|
| Differentiation | MYH1 | 0.12 | 1.32 × 10−5 | 0.99 | 1.86 × 10−5 |
| MYH2 | 0.03 | 4.73 × 10−6 | 1.22 | 4.11 × 10−7 | |
| MYH4 | 0.04 | 6.01 × 10−8 | 0.96 | 1.40 × 10−7 | |
| MYH7 | 0.03 | 8.40 × 10−8 | 0.78 | 5.15 × 10−6 | |
| Targets | ARPP21 | 0.35 | 1.80 × 10−9 | 0.72 | 3.18 × 10−6 |
| HDAC4 | 0.41 | 8.07 × 10−6 | 0.84 | 5.44 × 10−4 | |
| HDAC9 | 0.21 | 3.03 × 10−6 | 1.12 | 1.81 × 10−6 | |
| INSR | 0.44 | 5.88 × 10−8 | 0.76 | 5.90 × 10−5 | |
| IRS1 | 0.42 | 1.06 × 10−7 | 0.98 | 2.21 × 10−7 | |
| OSBPL8 | 1.46 | 4.22 × 10−5 | 0.94 | 8.71 × 10−6 | |
| PLEKHB2 | 0.87 | n.s. | 1.02 | n.s. | |
| TP63 | 0.15 | 6.69 × 10−6 | 0.59 | 7.00 × 10−3 | |
| miR143 | p vs. Ctrl | miR145 | p vs. Ctrl | miR143 + 145 | p vs. Ctrl | ||
|---|---|---|---|---|---|---|---|
| Differentiation | MYH1 | 0.79 | 0.0326 | 0.77 | 0.0325 | 0.79 | 0.0293 |
| MYH2 | 0.88 | n.s. | 0.90 | n.s. | 0.96 | n.s. | |
| MYH4 | 0.77 | 0.0021 | 0.70 | 0.0002 | 0.78 | 0.0033 | |
| MYH7 | 0.93 | n.s. | 1.02 | n.s. | 0.91 | n.s. | |
| Targets | ARPP21 | 0.86 | n.s. | 0.88 | n.s. | 0.84 | 0.0479 |
| HDAC4 | 0.87 | n.s. | 1.11 | n.s. | 0.96 | n.s. | |
| HDAC9 | 0.93 | n.s. | 0.94 | n.s. | 0.98 | n.s. | |
| INSR | 0.87 | n.s. | 1.00 | n.s. | 0.97 | n.s. | |
| IRS1 | 0.74 | 0.0302 | 0.99 | n.s. | 0.70 | 0.0287 | |
| OSBPL8 | 1.02 | n.s. | 1.01 | n.s. | 1.06 | n.s. | |
| PLEKHB2 | 1.02 | n.s. | 1.04 | n.s. | 1.00 | n.s. | |
| TP63 | 0.76 | 0.0121 | 0.91 | n.s. | 0.91 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dreher, S.I.; Höckele, S.; Huypens, P.; Irmler, M.; Hoffmann, C.; Jeske, T.; Hastreiter, M.; Moller, A.; Birkenfeld, A.L.; Häring, H.-U.; et al. TGF-β Induction of miR-143/145 Is Associated to Exercise Response by Influencing Differentiation and Insulin Signaling Molecules in Human Skeletal Muscle. Cells 2021, 10, 3443. https://doi.org/10.3390/cells10123443
Dreher SI, Höckele S, Huypens P, Irmler M, Hoffmann C, Jeske T, Hastreiter M, Moller A, Birkenfeld AL, Häring H-U, et al. TGF-β Induction of miR-143/145 Is Associated to Exercise Response by Influencing Differentiation and Insulin Signaling Molecules in Human Skeletal Muscle. Cells. 2021; 10(12):3443. https://doi.org/10.3390/cells10123443
Chicago/Turabian StyleDreher, Simon I., Selina Höckele, Peter Huypens, Martin Irmler, Christoph Hoffmann, Tim Jeske, Maximilian Hastreiter, Anja Moller, Andreas L. Birkenfeld, Hans-Ulrich Häring, and et al. 2021. "TGF-β Induction of miR-143/145 Is Associated to Exercise Response by Influencing Differentiation and Insulin Signaling Molecules in Human Skeletal Muscle" Cells 10, no. 12: 3443. https://doi.org/10.3390/cells10123443
APA StyleDreher, S. I., Höckele, S., Huypens, P., Irmler, M., Hoffmann, C., Jeske, T., Hastreiter, M., Moller, A., Birkenfeld, A. L., Häring, H.-U., Peter, A., Beckers, J., Hrabě de Angelis, M., & Weigert, C. (2021). TGF-β Induction of miR-143/145 Is Associated to Exercise Response by Influencing Differentiation and Insulin Signaling Molecules in Human Skeletal Muscle. Cells, 10(12), 3443. https://doi.org/10.3390/cells10123443







