Activating Transcription Factor 3 Protects against Restraint Stress-Induced Gastrointestinal Injury in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Mice
2.2. Measurement of Stress-Induced GI Leakage
2.3. qRT-PCR Analysis
2.3.1. RNA Isolation and cDNA Preparation
2.3.2. qRT-PCR
2.4. Flow Cytometry Analysis
2.5. Immunohistochemistry (IHC) Tissue Section Preparation
2.6. Immunofluorescent Staining
2.7. Imaging of Confocal Microscopy
2.8. Statistical Analyses
3. Results
3.1. Restraint Stress-Induced GI Tract Leakage Is Sensitively Revealed through Measurement of Plasma Evans Blue Levels That Leaked from GI after Gavage of Live Mice
3.2. Rescued by PPI Treatments, Restraint Stress-Induced GI Leakage Involves an Ulcer-like Injury
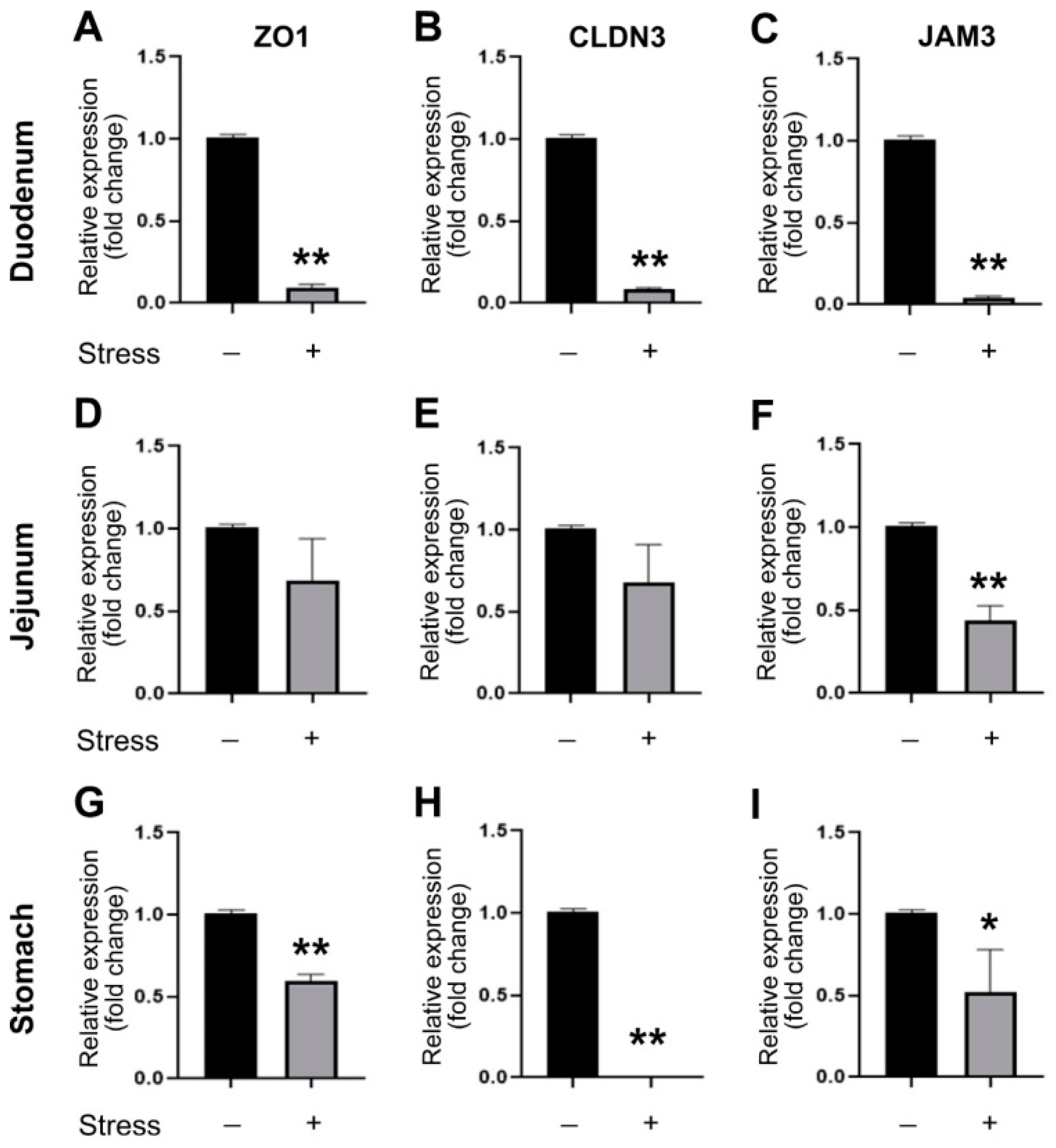
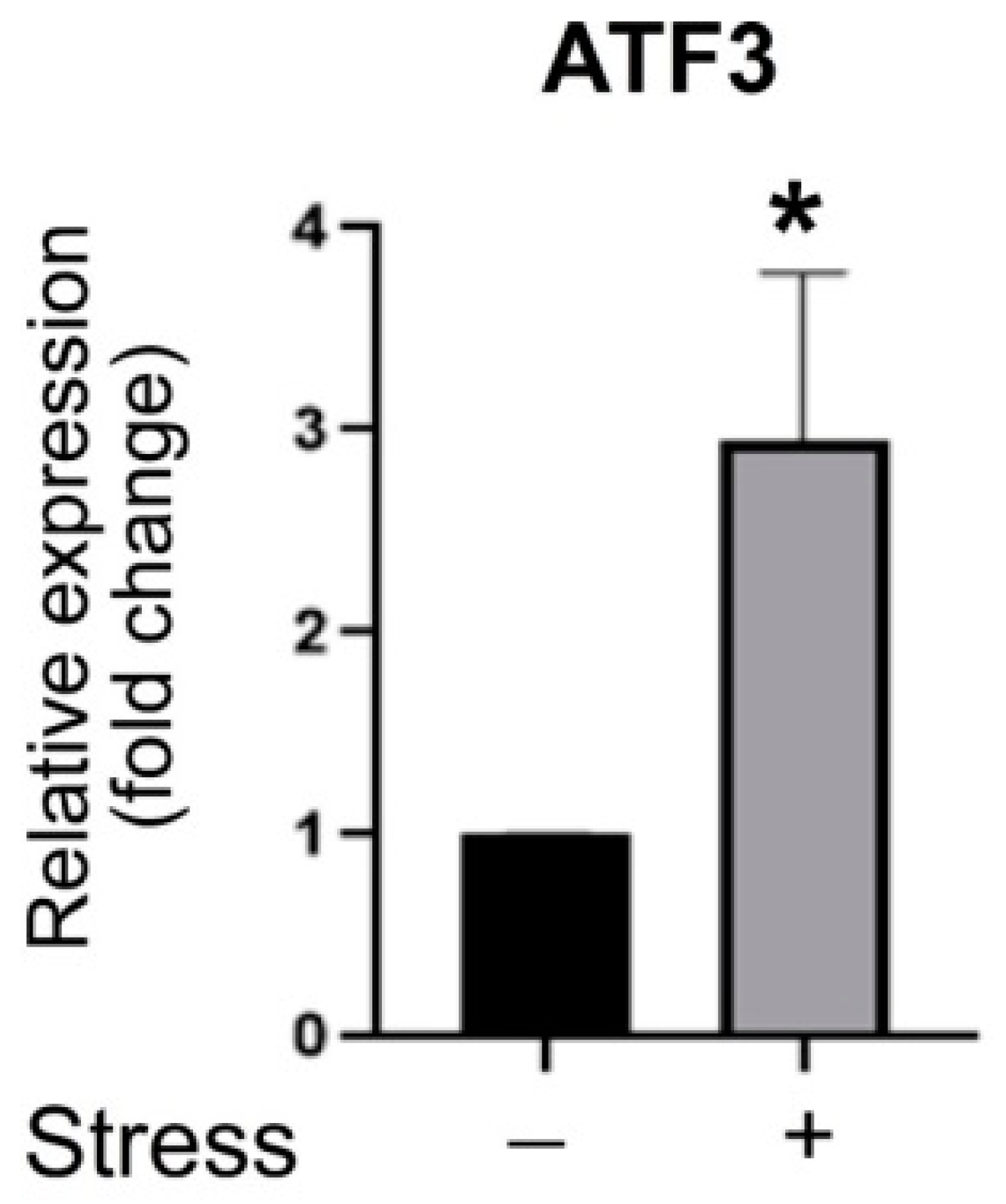
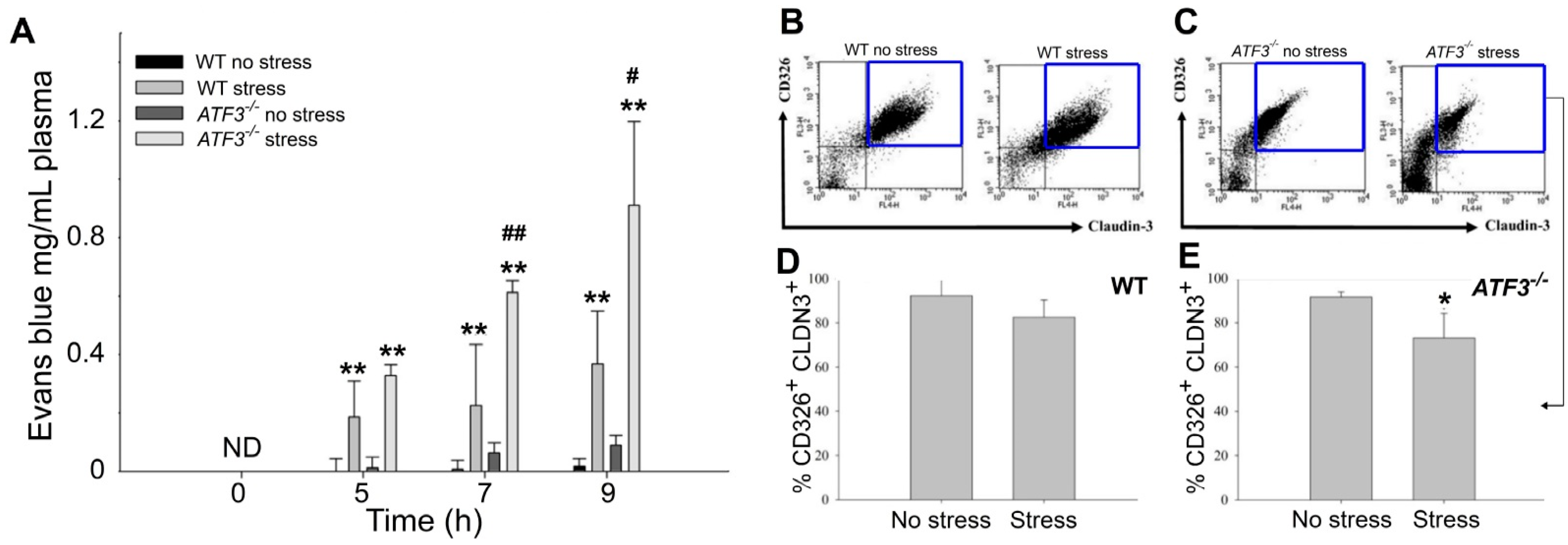
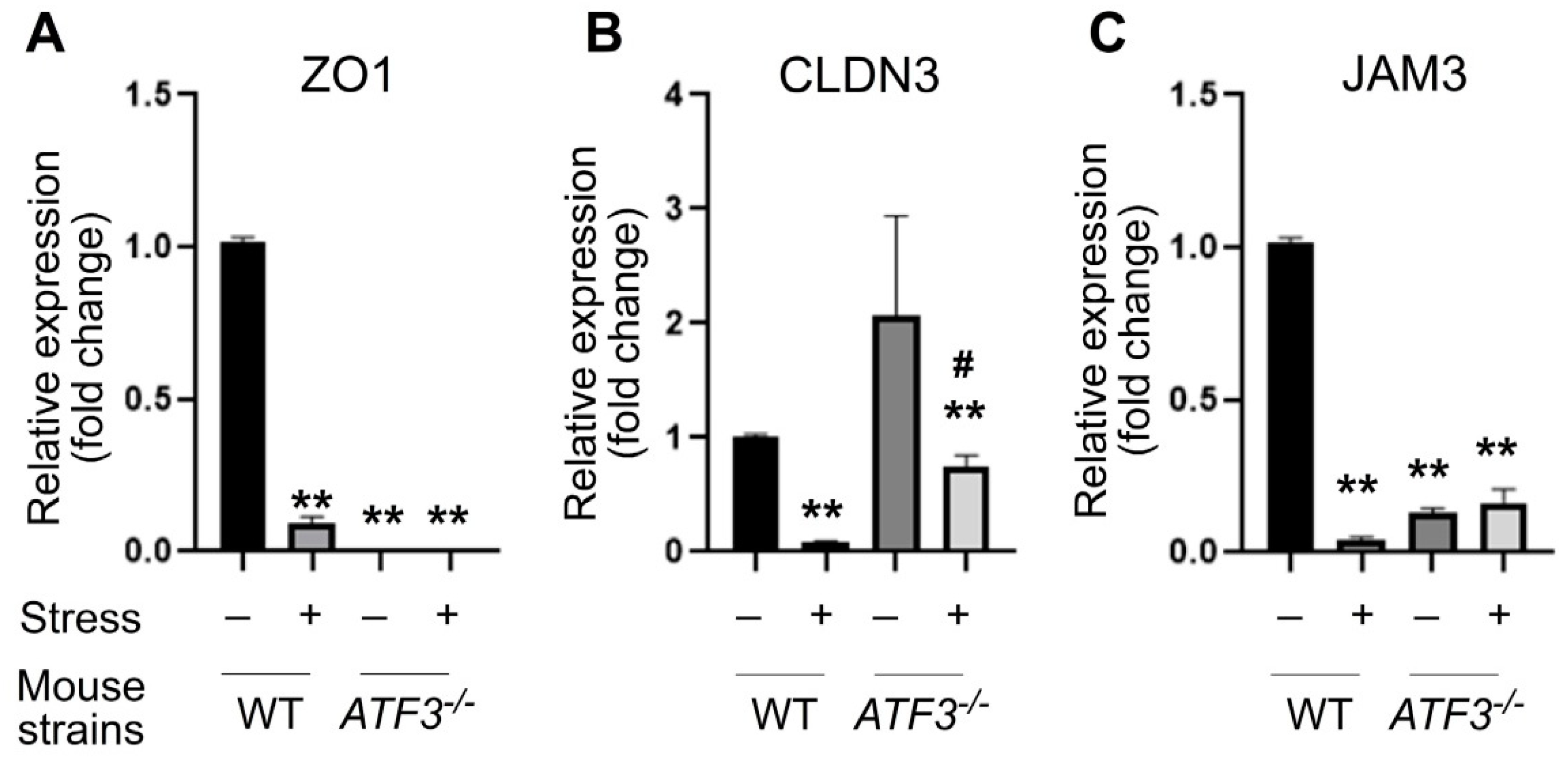
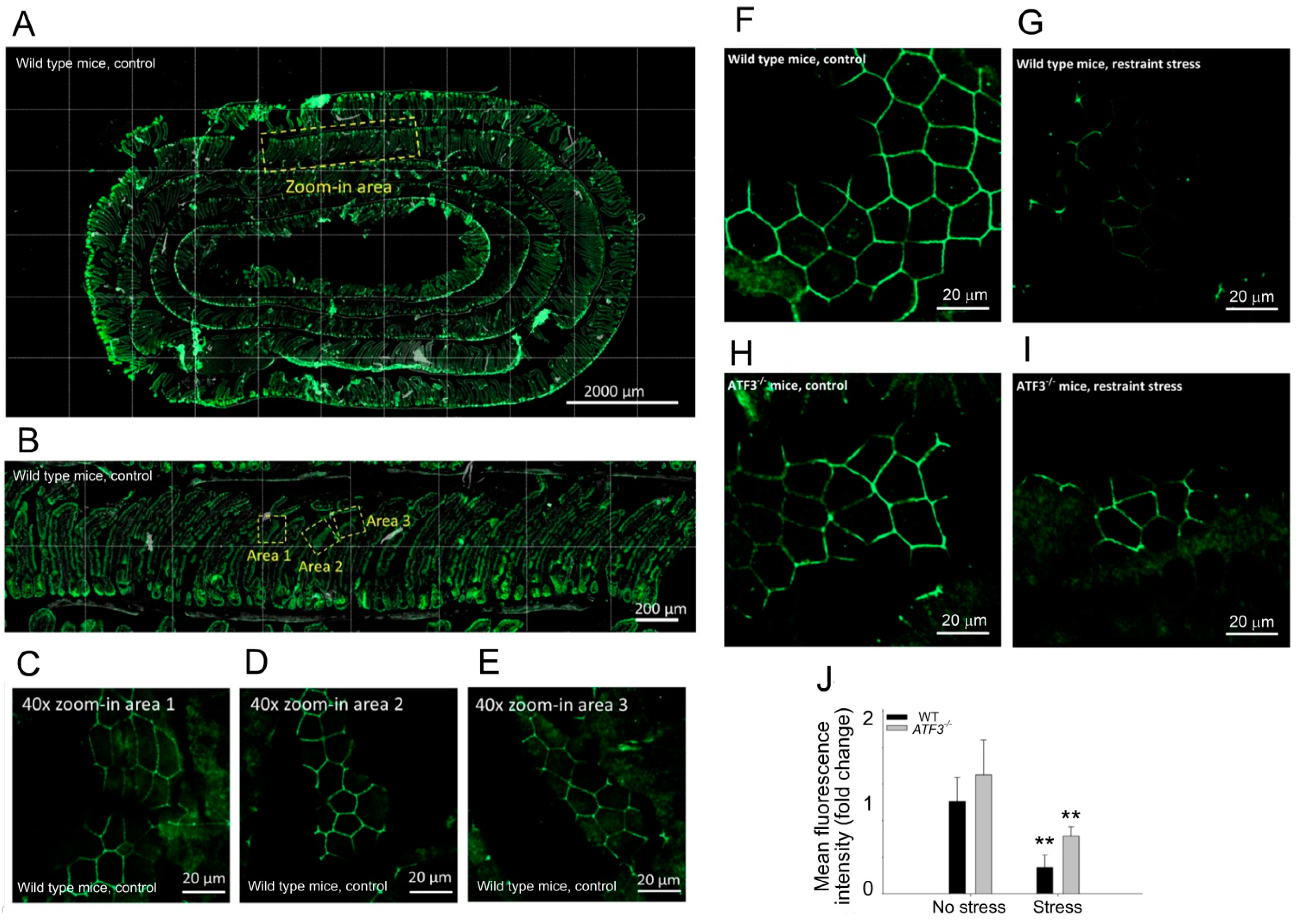
3.3. ATF3 Deficiency Exacerbated Stress-Induced Gut Leakage and Disrupted mRNA of Tight Junction Protein ZO-1, CLDN3, and JAM3 Expression in Mouse Duodenum
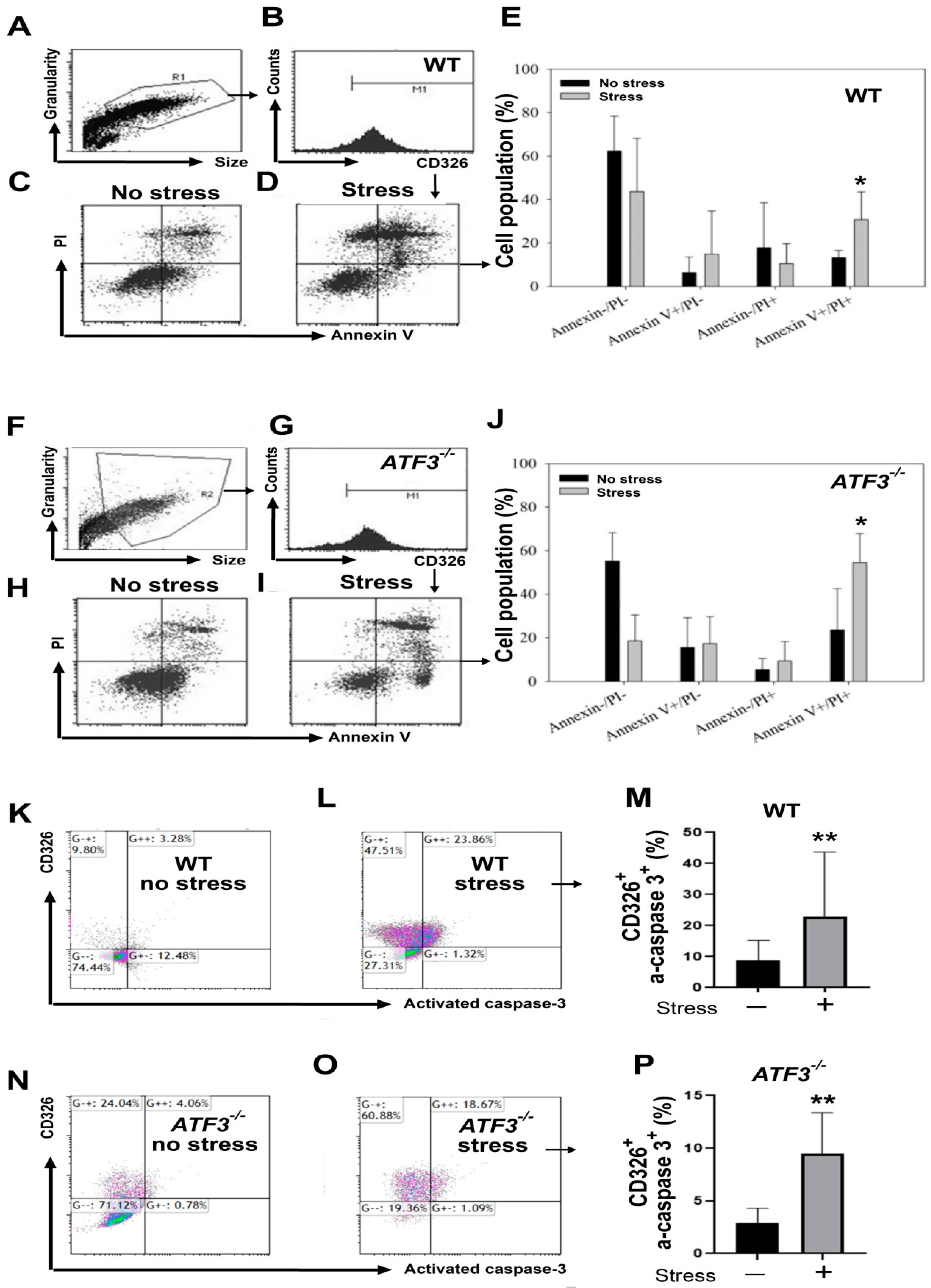
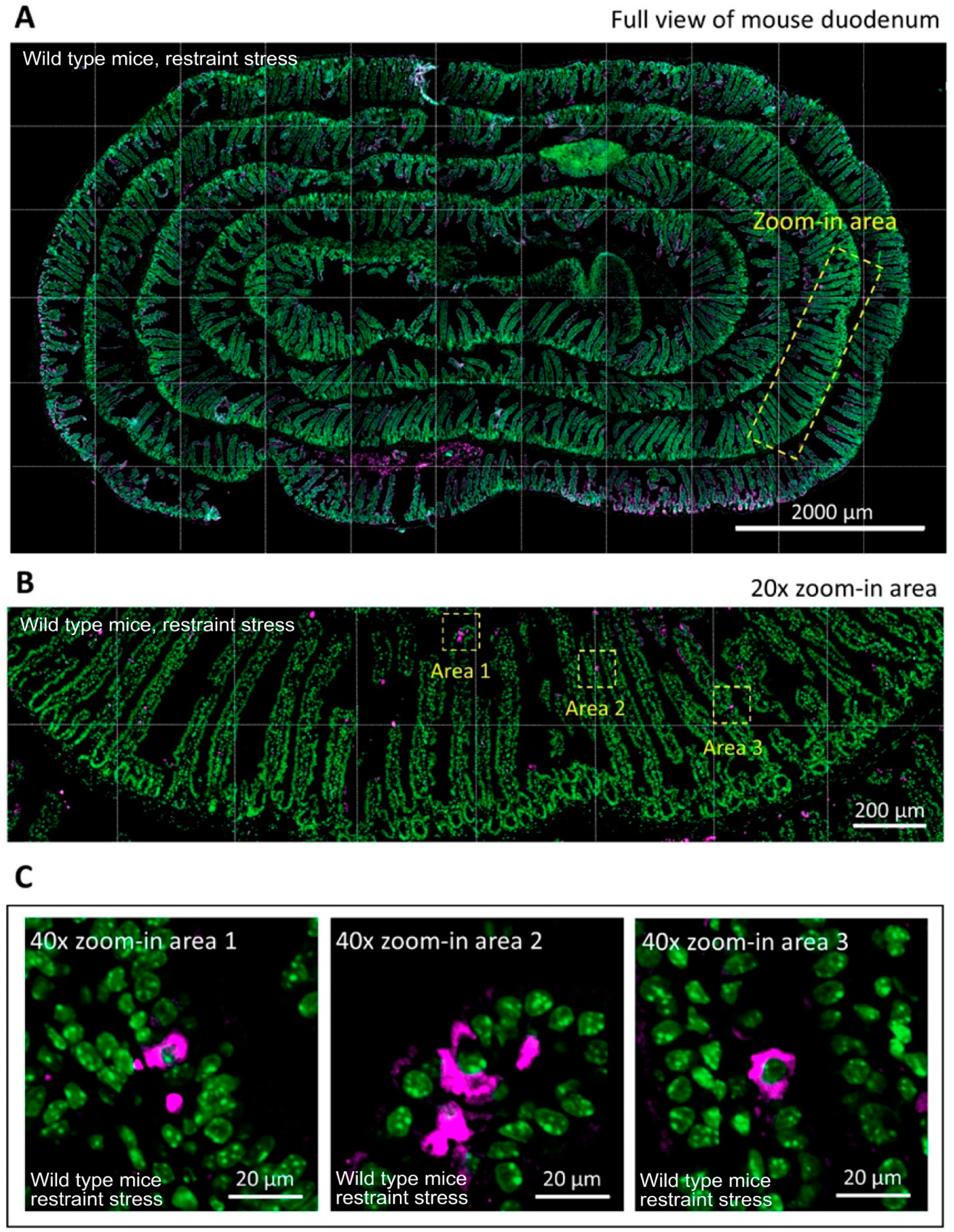
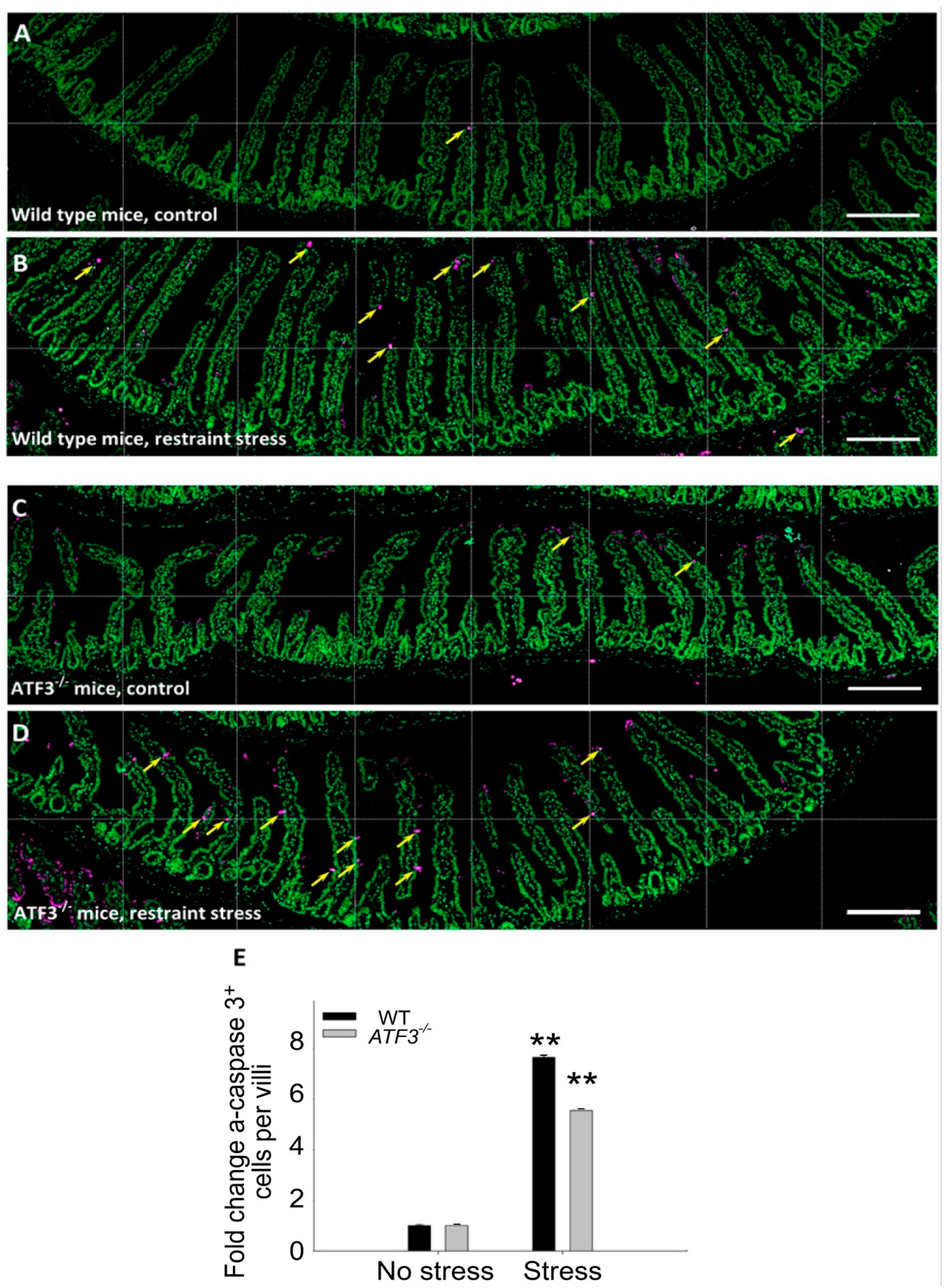
3.4. ATF3 Deficiency Exacerbated Stress-Induced Cell Death of Duodenal Epithelial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sapolsky, R.M. Why Zebras Don’t Get Ulcers, 3rd ed.; W. H. Freeman: New York, NY, USA, 2004. [Google Scholar]
- Oligschlaeger, Y.; Yadati, T.; Houben, T.; Condello Olivan, C.M.; Shiri-Sverdlov, R. Inflammatory Bowel Disease: A Stressed “Gut/Feeling”. Cells 2019, 8, 659. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Gershon, M.D. The bowel and beyond: The enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Karling, P.; Maripuu, M.; Wikgren, M.; Adolfsson, R.; Norrback, K.F. Association between gastrointestinal symptoms and affectivity in patients with bipolar disorder. World J. Gastroenterol. 2016, 22, 8540–8548. [Google Scholar] [CrossRef]
- Severance, E.G.; Prandovszky, E.; Castiglione, J.; Yolken, R.H. Gastroenterology issues in schizophrenia: Why the gut matters. Curr. Psychiatry Rep. 2015, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, H.E.; Bai, Y.M.; Tsai, S.J.; Su, T.P.; Chen, T.J.; Wang, Y.P.; Chen, M.H. Inflammatory bowel disease is associated with higher dementia risk: A nationwide longitudinal study. Gut 2021, 70, 85–91. [Google Scholar] [CrossRef]
- Alkasir, R.; Li, J.; Li, X.; Jin, M.; Zhu, B. Human gut microbiota: The links with dementia development. Protein Cell 2017, 8, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, J.; Klukowski, M. Gastrointestinal symptoms and autism spectrum disorder: Links and risks—A possible new overlap syndrome. Pediatric Health Med. Ther. 2015, 6, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.C.; Cheng, C.F. Master Regulator Activating Transcription Factor 3 (ATF3) in Metabolic Homeostasis and Cancer. Front. Endocrinol. 2020, 11, 556. [Google Scholar] [CrossRef]
- Glal, D.; Sudhakar, J.N.; Lu, H.H.; Liu, M.C.; Chiang, H.Y.; Liu, Y.C.; Cheng, C.F.; Shui, J.W. ATF3 Sustains IL-22-Induced STAT3 Phosphorylation to Maintain Mucosal Immunity Through Inhibiting Phosphatases. Front. Immunol. 2018, 9, 2522. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Yang, Q.; Deng, H.; Liu, Y.; Zhou, P.; Xu, H.; Chen, D.; Feng, D.; Zhang, H.; et al. Critical Role of Intestinal Microbiota in ATF3-Mediated Gut Immune Homeostasis. J. Immunol. 2020, 205, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, Q.; Deng, H.; Tang, J.; Hu, J.; Liu, H.; Zhi, M.; Ye, L.; Zou, B.; Liu, Y.; et al. Transcriptional factor ATF3 protects against colitis by regulating follicular helper T cells in Peyer’s patches. Proc. Natl. Acad. Sci. USA 2019, 116, 6286–6291. [Google Scholar] [CrossRef]
- Zhou, J.; Edgar, B.A.; Boutros, M. ATF3 acts as a rheostat to control JNK signalling during intestinal regeneration. Nat. Commun. 2017, 8, 14289. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaca, M.V.; Aguiar, D.C.; Guimaraes, F.S. Animal models of anxiety disorders and stress. Braz. J. Psychiatry 2013, 35 (Suppl. 2), S101–S111. [Google Scholar] [CrossRef] [PubMed]
- Pare, W.P.; Glavin, G.B. Restraint stress in biomedical research: A review. Neurosci. Biobehav. Rev. 1986, 10, 339–370. [Google Scholar] [CrossRef]
- Glavin, G.B.; Pare, W.P.; Sandbak, T.; Bakke, H.K.; Murison, R. Restraint stress in biomedical research: An update. Neurosci. Biobehav. Rev. 1994, 18, 223–249. [Google Scholar] [CrossRef]
- Santha, P.; Veszelka, S.; Hoyk, Z.; Meszaros, M.; Walter, F.R.; Toth, A.E.; Kiss, L.; Kincses, A.; Olah, Z.; Seprenyi, G.; et al. Restraint Stress-Induced Morphological Changes at the Blood-Brain Barrier in Adult Rats. Front. Mol. Neurosci. 2015, 8, 88. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Bai, X.; Xie, Y.; Zhang, T.; Bo, S.; Chen, X. Resveratrol reversed chronic restraint stress-induced impaired cognitive function in rats. Mol. Med. Rep. 2017, 16, 2095–2100. [Google Scholar] [CrossRef]
- Shoji, H.; Miyakawa, T. Differential effects of stress exposure via two types of restraint apparatuses on behavior and plasma corticosterone level in inbred male BALB/cAJcl mice. Neuropsychopharmacol. Rep. 2020, 40, 73–84. [Google Scholar] [CrossRef]
- Dodiya, H.B.; Forsyth, C.B.; Voigt, R.M.; Engen, P.A.; Patel, J.; Shaikh, M.; Green, S.J.; Naqib, A.; Roy, A.; Kordower, J.H.; et al. Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol. Dis. 2020, 135, 104352. [Google Scholar] [CrossRef]
- Gao, X.; Cao, Q.; Cheng, Y.; Zhao, D.; Wang, Z.; Yang, H.; Wu, Q.; You, L.; Wang, Y.; Lin, Y.; et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. USA 2018, 115, E2960–E2969. [Google Scholar] [CrossRef] [PubMed]
- Siopi, E.; Chevalier, G.; Katsimpardi, L.; Saha, S.; Bigot, M.; Moigneu, C.; Eberl, G.; Lledo, P.M. Changes in Gut Microbiota by Chronic Stress Impair the Efficacy of Fluoxetine. Cell Rep. 2020, 30, 3682–3690.e3686. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the microbiota-gut-brain axis in chronic restraint stress: Disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 2021, 13, 1869501. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Galipeau, H.J.; Verdu, E.F. The complex task of measuring intestinal permeability in basic and clinical science. Neurogastroenterol. Motil. 2016, 28, 957–965. [Google Scholar] [CrossRef]
- Delvaux, M.; Escourrou, J. Endoscopy in peptic ulcer disease: Diagnosis, prognosis and management. Endoscopy 1992, 24, 41–44. [Google Scholar] [CrossRef]
- Dunlap, J.J.; Patterson, S. Peptic Ulcer Disease. Gastroenterol. Nurs. 2019, 42, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.Y.; Sun, D.S.; Chang, H.H. Silver Nanoparticles Protect Skin from Ultraviolet B-Induced Damage in Mice. Int. J. Mol. Sci 2020, 21, 7082. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Sun, D.S.; Su, M.T.; Lien, T.S.; Chen, Y.H.; Lin, C.Y.; Huang, C.H.; King, C.C.; Li, C.R.; Chen, T.H.; et al. Suppressed humoral immunity is associated with dengue nonstructural protein NS1-elicited anti-death receptor antibody fractions in mice. Sci. Rep. 2020, 10, 6294. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Hu, C.T.; Sun, D.S.; Lien, T.S.; Chang, H.H. Thioacetamide-induced liver damage and thrombocytopenia is associated with induction of antiplatelet autoantibody in mice. Sci. Rep. 2019, 9, 17497. [Google Scholar] [CrossRef]
- Perevedentseva, E.; Krivokharchenko, A.; Karmenyan, A.V.; Chang, H.H.; Cheng, C.L. Raman spectroscopy on live mouse early embryo while it continues to develop into blastocyst in vitro. Sci. Rep. 2019, 9, 6636. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Huang, H.S.; Sun, D.S.; Lee, C.J.; Lien, T.S.; Chang, H.H. TRPM8 and RAAS-mediated hypertension is critical for cold-induced immunosuppression in mice. Oncotarget 2018, 9, 12781–12795. [Google Scholar] [CrossRef][Green Version]
- Huang, C.Y.; Yu, W.S.; Liu, G.C.; Hung, S.C.; Chang, J.H.; Chang, J.C.; Cheng, C.L.; Sun, D.S.; Lin, M.D.; Lin, W.Y.; et al. Opportunistic gill infection is associated with TiO2 nanoparticle-induced mortality in zebrafish. PLoS ONE 2021, 16, e0247859. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.G.; Lu, D.; Kim, M.L.; Kociba, G.G.G.J.; Shukri, T.; Buteau, J.; Wang, X.; Frankel, W.L.; Guttridge, D.; Prentki, M.; et al. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol. Cell Biol. 2004, 24, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.F.; Ku, H.C.; Cheng, J.J.; Chao, S.W.; Li, H.F.; Lai, P.F.; Chang, C.C.; Don, M.J.; Chen, H.H.; Lin, H. Adipocyte browning and resistance to obesity in mice is induced by expression of ATF3. Commun. Biol. 2019, 2, 389. [Google Scholar] [CrossRef] [PubMed]
- Zimprich, A.; Garrett, L.; Deussing, J.M.; Wotjak, C.T.; Fuchs, H.; Gailus-Durner, V.; de Angelis, M.H.; Wurst, W.; Holter, S.M. A robust and reliable non-invasive test for stress responsivity in mice. Front. Behav. Neurosci. 2014, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Zhou, Y.; Hu, Z.; Lou, J.; Song, W.; Li, J.; Liang, X.; Chen, C.; Wang, S.; Yang, B.; et al. 24-hour-restraint stress induces long-term depressive-like phenotypes in mice. Sci. Rep. 2016, 6, 32935. [Google Scholar] [CrossRef]
- Lien, T.S.; Sun, D.S.; Hung, S.C.; Wu, W.S.; Chang, H.H. Dengue Virus Envelope Protein Domain III Induces Nlrp3 Inflammasome-Dependent NETosis-Mediated Inflammation in Mice. Front. Immunol. 2021, 12, 618577. [Google Scholar] [CrossRef]
- Lien, T.S.; Sun, D.S.; Wu, C.Y.; Chang, H.H. Exposure to Dengue Envelope Protein Domain III Induces Nlrp3 Inflammasome-Dependent Endothelial Dysfunction and Hemorrhage in Mice. Front. Immunol 2021, 12, 617251. [Google Scholar] [CrossRef]
- Lien, T.S.; Chan, H.; Sun, D.S.; Wu, J.C.; Lin, Y.Y.; Lin, G.L.; Chang, H.H. Exposure of Platelets to Dengue Virus and Envelope Protein Domain III Induces Nlrp3 Inflammasome-Dependent Platelet Cell Death and Thrombocytopenia in Mice. Front. Immunol. 2021, 12, 616394. [Google Scholar] [CrossRef] [PubMed]
- Wilcz-Villega, E.M.; McClean, S.; O’Sullivan, M.A. Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: Implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am. J. Gastroenterol. 2013, 108, 1140–1151. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, H.; Lei, L.; Tong, X.; Yang, L.; Yang, Y.; Li, S.; Zhou, Y.; Luo, L.; Huang, J.; et al. Tight junctions and their regulation by non-coding RNAs. Int. J. Biol. Sci. 2021, 17, 712–727. [Google Scholar] [CrossRef]
- Zeisel, M.B.; Dhawan, P.; Baumert, T.F. Tight junction proteins in gastrointestinal and liver disease. Gut 2019, 68, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Luissint, A.C.; Nusrat, A.; Parkos, C.A. JAM-related proteins in mucosal homeostasis and inflammation. Semin. Immunopathol. 2014, 36, 211–226. [Google Scholar] [CrossRef]
- Ouchi, T.; Morimura, S.; Dow, L.E.; Miyoshi, H.; Udey, M.C. EpCAM (CD326) Regulates Intestinal Epithelial Integrity and Stem Cells via Rho-Associated Kinase. Cells 2021, 10, 256. [Google Scholar] [CrossRef]
- Balfe, A.; Lennon, G.; Lavelle, A.; Docherty, N.G.; Coffey, J.C.; Sheahan, K.; Winter, D.C.; O’Connell, P.R. Isolation and gene expression profiling of intestinal epithelial cells: Crypt isolation by calcium chelation from in vivo samples. Clin. Exp. Gastroenterol. 2018, 11, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.F.; Lin, G.L.; Chu, S.C.; Chen, C.C.; Liou, Y.S.; Chang, H.H.; Sun, D.S. AQP0 is a novel surface marker for deciphering abnormal erythropoiesis. Stem. Cell Res. Ther. 2021, 12, 274. [Google Scholar] [CrossRef]
- Mandal, J.P.; Shiue, C.N.; Chen, Y.C.; Lee, M.C.; Yang, H.H.; Chang, H.H.; Hu, C.T.; Liao, P.C.; Hui, L.C.; You, R.I.; et al. PKCdelta mediates mitochondrial ROS generation and oxidation of HSP60 to relieve RKIP inhibition on MAPK pathway for HCC progression. Free Radic. Biol. Med. 2021, 163, 69–87. [Google Scholar] [CrossRef]
- Chen, T.L.; Chiang, Y.W.; Lin, G.L.; Chang, H.H.; Lien, T.S.; Sheh, M.H.; Sun, D.S. Different effects of granulocyte colony-stimulating factor and erythropoietin on erythropoiesis. Stem. Cell. Res. Ther. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Moolenbeek, C.; Ruitenberg, E.J. The “Swiss roll”: A simple technique for histological studies of the rodent intestine. Lab. Anim. 1981, 15, 57–59. [Google Scholar] [CrossRef]
- Bialkowska, A.B.; Ghaleb, A.M.; Nandan, M.O.; Yang, V.W. Improved Swiss-rolling Technique for Intestinal Tissue Preparation for Immunohistochemical and Immunofluorescent Analyses. J. Vis. Exp. 2016, 2016, 54161. [Google Scholar] [CrossRef]
- Naud, S. Writing a Rationale Justifying Animal Numbers. In Statistical Justification of Animal Numbers Used in Research and Teaching; COM Biostatistics Unit, The University of Vermont: Burlington, VT, USA, 2020; Available online: https://www.uvm.edu/sites/default/files/Research-Protections-Office/Statistical_Justification_of_Animal_Numbers_Used_in_Research_and_Training.pdf (accessed on 2 November 2021).
- Festing, M.F.W.; Overend, P.; Das, R.G.; Borja, C.M.; Berdoy, M. Reducing the Use of Animals in Research through Better Experimental Design. In The Design of Animal Experiments; Royal Society of Medicine Press Limited: London, UK, 2002. [Google Scholar]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Mateer, S.W.; Cardona, J.; Marks, E.; Goggin, B.J.; Hua, S.; Keely, S. Ex Vivo Intestinal Sacs to Assess Mucosal Permeability in Models of Gastrointestinal Disease. J. Vis. Exp. 2016, 2016, e53250. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Sugiyama, M.; Mukai, T. Adhesion Properties of Lactic Acid Bacteria on Intestinal Mucin. Microorganisms 2016, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Milosavljevic, T.; Kostic-Milosavljevic, M.; Jovanovic, I.; Krstic, M. Complications of peptic ulcer disease. Dig. Dis 2011, 29, 491–493. [Google Scholar] [CrossRef]
- Kamada, T.; Satoh, K.; Itoh, T.; Ito, M.; Iwamoto, J.; Okimoto, T.; Kanno, T.; Sugimoto, M.; Chiba, T.; Nomura, S.; et al. Evidence-based clinical practice guidelines for peptic ulcer disease 2020. J. Gastroenterol. 2021, 56, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Reshetnyak, V.I.; Burmistrov, A.I.; Maev, I.V. Helicobacter pylori: Commensal, symbiont or pathogen? World J. Gastroenterol. 2021, 27, 545–560. [Google Scholar] [CrossRef]
- Levenstein, S.; Rosenstock, S.; Jacobsen, R.K.; Jorgensen, T. Psychological stress increases risk for peptic ulcer, regardless of Helicobacter pylori infection or use of nonsteroidal anti-inflammatory drugs. Clin. Gastroenterol. Hepatol. 2015, 13, 498–506.e491. [Google Scholar] [CrossRef]
- Lee, Y.B.; Yu, J.; Choi, H.H.; Jeon, B.S.; Kim, H.K.; Kim, S.W.; Kim, S.S.; Park, Y.G.; Chae, H.S. The association between peptic ulcer diseases and mental health problems: A population-based study: A STROBE compliant article. Medicine 2017, 96, e7828. [Google Scholar] [CrossRef] [PubMed]
- Melinder, C.; Udumyan, R.; Hiyoshi, A.; Brummer, R.J.; Montgomery, S. Decreased stress resilience in young men significantly increases the risk of subsequent peptic ulcer disease—A prospective study of 233 093 men in Sweden. Aliment. Pharmacol. Ther. 2015, 41, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Fossmark, R.; Martinsen, T.C.; Waldum, H.L. Adverse Effects of Proton Pump Inhibitors-Evidence and Plausibility. Int. J. Mol. Sci. 2019, 20, 5203. [Google Scholar] [CrossRef]
- Bjorkman, D.J. Psychological Stress and Peptic Ulcer. N. Engl. J. Med. J. Watch. 2014, 2014, 1. [Google Scholar]
- Lange, S.; Delbro, D.S.; Jennische, E. Evans blue permeation of intestinal mucosa in the rat. Scand. J. Gastroenterol. 1994, 29, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wick, M.J.; Harral, J.W.; Loomis, Z.L.; Dempsey, E.C. An Optimized Evans Blue Protocol to Assess Vascular Leak in the Mouse. J. Vis. Exp. 2018, 139, e57037. [Google Scholar] [CrossRef]
- Tolstanova, G.; Deng, X.; French, S.W.; Lungo, W.; Paunovic, B.; Khomenko, T.; Ahluwalia, A.; Kaplan, T.; Dacosta-Iyer, M.; Tarnawski, A.; et al. Early endothelial damage and increased colonic vascular permeability in the development of experimental ulcerative colitis in rats and mice. Lab. Investig. 2012, 92, 9–21. [Google Scholar] [CrossRef]
- Evans, H.M.; Schulemann, W. The Action of Vital Stains Belonging to the Benzidine Group. Science 1914, 39, 443–454. [Google Scholar] [CrossRef]
- Yao, L.; Xue, X.; Yu, P.; Ni, Y.; Chen, F. Evans Blue Dye: A Revisit of Its Applications in Biomedicine. Contrast Media Mol. Imaging 2018, 2018, 7628037. [Google Scholar] [CrossRef]
- Sun, D.S.; Chang, Y.C.; Lien, T.S.; King, C.C.; Shih, Y.L.; Huang, H.S.; Wang, T.Y.; Li, C.R.; Lee, C.C.; Hsu, P.N.; et al. Endothelial Cell Sensitization by Death Receptor Fractions of an Anti-Dengue Nonstructural Protein 1 Antibody Induced Plasma Leakage, Coagulopathy, and Mortality in Mice. J. Immunol. 2015, 195, 2743–2753. [Google Scholar] [CrossRef]
- Sun, D.S.; Lee, P.C.; Kau, J.H.; Shih, Y.L.; Huang, H.H.; Li, C.R.; Lee, C.C.; Wu, Y.P.; Chen, K.C.; Chang, H.H. Acquired coagulant factor VIII deficiency induced by Bacillus anthracis lethal toxin in mice. Virulence 2015, 6, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.S.; Chang, Y.W.; Kau, J.H.; Huang, H.H.; Ho, P.H.; Tzeng, Y.J.; Chang, H.H. Soluble P-selectin rescues mice from anthrax lethal toxin-induced mortality through PSGL-1 pathway-mediated correction of hemostasis. Virulence 2017, 8, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Diao, L.; Xu, J.M.; Liu, X.C.; Jin, J. A protective effect of melatonin on intestinal permeability is induced by diclofenac via regulation of mitochondrial function in mice. Acta Pharmacol. Sin. 2011, 32, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Helgadottir, H.; Bjornsson, E.S. Problems Associated with Deprescribing of Proton Pump Inhibitors. Int. J. Mol. Sci. 2019, 20, 5469. [Google Scholar] [CrossRef]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric mucosal defense and cytoprotection: Bench to bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.S.; Chang, H.H. Emerging role of the itaconate-mediated rescue of cellular metabolic stress. Tzu Chi Med. J. 2021, 33, 1–5. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Xie, R.; Wang, B.; Jiang, K.; Cao, H. Stress Triggers Flare of Inflammatory Bowel Disease in Children and Adults. Front. Pediatrics 2019, 7, 432. [Google Scholar] [CrossRef]
- Rengarajan, S.; Knoop, K.A.; Rengarajan, A.; Chai, J.N.; Grajales-Reyes, J.G.; Samineni, V.K.; Russler-Germain, E.V.; Ranganathan, P.; Fasano, A.; Sayuk, G.S.; et al. A Potential Role for Stress-Induced Microbial Alterations in IgA-Associated Irritable Bowel Syndrome with Diarrhea. Cell Rep. Med. 2020, 1, 100124. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef]
- Sheng, N.; Ma, Z.; Zhou, Y.; Xu, J.; Gao, Y.; Fu, X.Y. Cholesterol 25-hydroxylase protects against experimental colitis in mice by modulating epithelial gut barrier function. Sci. Rep. 2020, 10, 14246. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Chen, C.C.; Luther, J.; Kao, J.Y. Intestinal dysbiosis in inflammatory bowel disease. Gut Microbes 2011, 2, 211–216. [Google Scholar] [CrossRef]
- Israeli, E.; Hershcovici, T.; Berenshtein, E.; Zannineli, G.; Wengrower, D.; Weiss, O.; Chevion, M.; Goldin, E. The effect of restraint stress on the normal colon and on intestinal inflammation in a model of experimental colitis. Dig. Dis. Sci. 2008, 53, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Duan, Z.; Guan, Y.; Wu, H.; Hu, K.; Gao, X.; Yuan, F.; Jiang, Z.; Fan, Y.; He, B.; et al. Increased expression of tight junction protein occludin is associated with the protective effect of mosapride against aspirin-induced gastric injury. Exp. Ther. Med. 2018, 15, 1626–1632. [Google Scholar] [CrossRef]
- Slifer, Z.M.; Blikslager, A.T. The Integral Role of Tight Junction Proteins in the Repair of Injured Intestinal Epithelium. Int. J. Mol. Sci. 2020, 21, 972. [Google Scholar] [CrossRef]
- Caron, T.J.; Scott, K.E.; Fox, J.G.; Hagen, S.J. Tight junction disruption: Helicobacter pylori and dysregulation of the gastric mucosal barrier. World J. Gastroenterol. 2015, 21, 11411–11427. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Meng, D.; Zou, F.F.; Fan, L.; Zhang, P.; Yu, Y.; Fang, J. Activating transcription factor 3 regulates survivability and migration of vascular smooth muscle cells. IUBMB Life 2011, 63, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, J.; Zhang, C.; Nobori, K.; Hashimoto, Y.; Adachi, M.T.; Noda, A.; Sunamori, M.; Kitajima, S. Transcriptional repressor activating transcription factor 3 protects human umbilical vein endothelial cells from tumor necrosis factor-alpha-induced apoptosis through down-regulation of p53 transcription. J. Biol. Chem. 2002, 277, 39025–39034. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.; Raivich, G.; Anderson, P.N. Activating transcription factor 3 and the nervous system. Front. Mol. Neurosci. 2012, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Nobori, K.; Ito, H.; Tamamori-Adachi, M.; Adachi, S.; Ono, Y.; Kawauchi, J.; Kitajima, S.; Marumo, F.; Isobe, M. ATF3 inhibits doxorubicin-induced apoptosis in cardiac myocytes: A novel cardioprotective role of ATF3. J. Mol. Cell. Cardiol. 2002, 34, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Sugiura, H.; Mitobe, M.; Tsuchiya, K.; Shirota, S.; Nishimura, S.; Shiohira, S.; Ito, H.; Nobori, K.; Gullans, S.R.; et al. ATF3 protects against renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2008, 19, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Liu, X.M.; Wang, Y.; Chen, Z.Y. Activating transcription factor 3 (ATF3) regulates cell growth, apoptosis, invasion and collagen synthesis in keloid fibroblast through transforming growth factor beta (TGF-beta)/SMAD signaling pathway. Bioengineered 2021, 12, 117–126. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, D.-J.; Pethaperumal, S.; Siwakoti, B.; Chien, H.-J.; Cheng, C.-F.; Hung, S.-C.; Lien, T.-S.; Sun, D.-S.; Chang, H.-H. Activating Transcription Factor 3 Protects against Restraint Stress-Induced Gastrointestinal Injury in Mice. Cells 2021, 10, 3530. https://doi.org/10.3390/cells10123530
Chuang D-J, Pethaperumal S, Siwakoti B, Chien H-J, Cheng C-F, Hung S-C, Lien T-S, Sun D-S, Chang H-H. Activating Transcription Factor 3 Protects against Restraint Stress-Induced Gastrointestinal Injury in Mice. Cells. 2021; 10(12):3530. https://doi.org/10.3390/cells10123530
Chicago/Turabian StyleChuang, Dun-Jie, Subhashree Pethaperumal, Bijaya Siwakoti, Hung-Jen Chien, Ching-Feng Cheng, Shih-Che Hung, Te-Sheng Lien, Der-Shan Sun, and Hsin-Hou Chang. 2021. "Activating Transcription Factor 3 Protects against Restraint Stress-Induced Gastrointestinal Injury in Mice" Cells 10, no. 12: 3530. https://doi.org/10.3390/cells10123530
APA StyleChuang, D.-J., Pethaperumal, S., Siwakoti, B., Chien, H.-J., Cheng, C.-F., Hung, S.-C., Lien, T.-S., Sun, D.-S., & Chang, H.-H. (2021). Activating Transcription Factor 3 Protects against Restraint Stress-Induced Gastrointestinal Injury in Mice. Cells, 10(12), 3530. https://doi.org/10.3390/cells10123530






