Exploring the Binding Pattern of Geraniol with Acetylcholinesterase through In Silico Docking, Molecular Dynamics Simulation, and In Vitro Enzyme Inhibition Kinetics Studies
Abstract
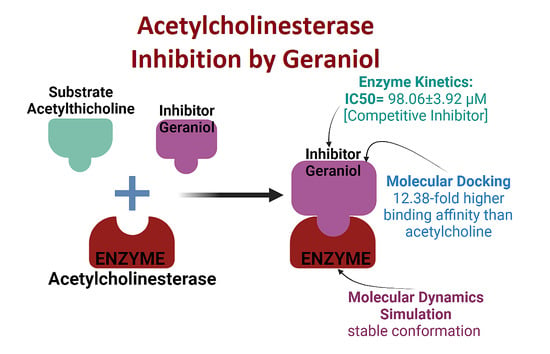
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Hardware and Software Used
2.3. Ligand Preparation
2.4. Protein Target Preparation
2.5. Acetylcholinesterase Inhibition Assay
2.6. Spectrometric Study of the Enzyme Kinetic Assay
2.7. Calculation of Physicochemical, Drug-Likeness, and Pharmacokinetics Properties
2.8. Molecular Docking
2.9. Molecular Dynamics (MD) Simulation
3. Results and Discussion
3.1. Acetylcholinesterase Enzyme Inhibition Activity
3.2. Enzyme Inhibition Kinetics
3.3. Physicochemical Properties, Drug-Likeness, and Pharmacokinetics Prediction of Geraniol
3.4. Molecular Docking Study Confirms AChE Inhibition through Geraniol
3.5. Analysis of Molecular Dynamics Simulation
3.5.1. Root-Mean-Square Deviation (RMSD) Analysis
3.5.2. Root-Mean-Square Fluctuation (RMSF) Analysis
3.5.3. Analysis of Radius of Gyration (Rg) and Different Surface Areas
3.5.4. Total Contacts Formed between Protein and Ligand
3.5.5. Secondary Structure Analysis
3.5.6. Protein–Ligand Interaction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarter, M.; Parikh, V. Choline Transporters, Cholinergic Transmission and Cognition. Nat. Rev. Neurosci. 2005, 6, 48–56. [Google Scholar] [CrossRef]
- Rosenberg, P.B.; Nowrangi, M.A.; Lyketsos, C.G. Neuropsychiatric Symptoms in Alzheimer’s Disease: What Might Be Associated Brain Circuits? Mol. Asp. Med. 2015, 43, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Catasus, C.; Bohnen, N.I.; D’Cruz, N.; Muller, M. Striatal Acetylcholine-Dopamine Imbalance in Parkinson’s Disease: In Vivo Neuroimaging Study with Dual-Tracer PET and Dopaminergic PET-Informed Correlational Tractography. J. Nucl. Med. 2021, 62, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A. Unravelling the Pathogenesis of Myasthenia Gravis. Nat. Rev. Immunol. 2002, 2, 797–804. [Google Scholar] [CrossRef]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical Trials and Late-Stage Drug Development for Alzheimer’s Disease: An Appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef]
- Saxena, M.; Dubey, R. Target Enzyme in Alzheimer’s Disease: Acetylcholinesterase Inhibitors. Curr. Top. Med. Chem. 2019, 19, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The Cholinergic System in the Pathophysiology and Treatment of Alzheimer’s Disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Petrov, K.A.; Kharlamova, A.D.; Lenina, O.A.; Nurtdinov, A.R.; Sitdykova, M.E.; Ilyin, V.I.; Zueva, I.V.; Nikolsky, E.E. Specific Inhibition of Acetylcholinesterase as an Approach to Decrease Muscarinic Side Effects during Myasthenia Gravis Treatment. Sci. Rep. 2018, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.B.; Adiao, K.J. Acetylcholinesterase Inhibitors in Myasthenic Crisis: A Systematic Review of Observational Studies. Neurocrit Care 2021, 35, 528–544. [Google Scholar] [CrossRef]
- Pagano, G.; Rengo, G.; Pasqualetti, G.; Femminella, G.D.; Monzani, F.; Ferrara, N.; Tagliati, M. Cholinesterase Inhibitors for Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 767–773. [Google Scholar] [CrossRef]
- Dou, K.-X.; Tan, M.-S.; Tan, C.-C.; Cao, X.-P.; Hou, X.-H.; Guo, Q.-H.; Tan, L.; Mok, V.; Yu, J.-T. Comparative Safety and Effectiveness of Cholinesterase Inhibitors and Memantine for Alzheimer’s Disease: A Network Meta-Analysis of 41 Randomized Controlled Trials. Alzheimer’s Res. Ther. 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.-K.; Chao, S.-P.; Hu, C.-J. Clinical Trials of New Drugs for Alzheimer Disease. J. Biomed. Sci. 2020, 27, 18. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Alvi, S.S.; Iqbal, D.; Khan, M.S. Insights into Pharmacological Mechanisms of Polydatin in Targeting Risk Factors-Mediated Atherosclerosis. Life Sci. 2020, 254, 117756. [Google Scholar] [CrossRef]
- Akhter, F.; Alvi, S.S.; Ahmad, P.; Iqbal, D.; Alshehri, B.M.; Khan, M.S. Therapeutic Efficacy of Boerhaavia Diffusa (Linn.) Root Methanolic Extract in Attenuating Streptozotocin-Induced Diabetes, Diabetes-Linked Hyperlipidemia and Oxidative-Stress in Rats. Biomed. Res. Ther. 2019, 6, 3293–3306. [Google Scholar] [CrossRef] [Green Version]
- Alvi, S.; Ahmad, P.; Ishrat, M.; Iqbal, D.; Khan, S. Secondary Metabolites from Rosemary (Rosmarinus officinalis L.): Structure, Biochemistry and Therapeutic Implications Against Neurodegenerative Diseases. In Natural Bio-active Compounds; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–24. ISBN 9789811372049. [Google Scholar]
- Alvi, S.S.; Iqbal, D.; Ahmad, S.; Khan, M.S. Molecular Rationale Delineating the Role of Lycopene as a Potent HMG-CoA Reductase Inhibitor: In Vitro and in Silico Study. Nat. Prod. Res. 2016, 30, 2111–2114. [Google Scholar] [CrossRef]
- Iqbal, D.; Khan, M.S.; Khan, A.; Khan, M.S.; Ahmad, S.; Srivastava, A.K.; Bagga, P. In Vitro Screening for β-Hydroxy-β-Methylglutaryl-CoA Reductase Inhibitory and Antioxidant Activity of Sequentially Extracted Fractions of Ficus palmata Forsk. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, D.; Khan, M.S.; Khan, M.S.; Ahmad, S.; Srivastava, A.K. An in Vitro and Molecular Informatics Study to Evaluate the Antioxidative and β-Hydroxy-β-Methylglutaryl-CoA Reductase Inhibitory Property of Ficus virens Ait. Phytother. Res. 2014, 28, 899–908. [Google Scholar] [CrossRef]
- Iqbal, D.; Khan, M.S.; Khan, A.; Ahmad, S. Extenuating the Role of Ficus virens Ait and Its Novel Bioactive Compound on Antioxidant Defense System and Oxidative Damage in Cigarette Smoke Exposed Rats. Biomed. Res. Ther. 2016, 3, 723–732. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, D.; Khan, A.; Ansari, I.A.; Khan, M.S. Investigating the Role of Novel Bioactive Compound from Ficus Virens Ait on Cigarette Smoke Induced Oxidative Stress and Hyperlipidemia in Rats. Iran. J. Pharm. Res. 2017, 16, 1089–1103. [Google Scholar]
- Khatoon, A.; Khan, F.; Ahmad, N.; Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Al-Qahtani, M.H.; Abuzenadah, A.M.; Tabrez, S.; Ahmed, A.B.F.; et al. Silver Nanoparticles from Leaf Extract of Mentha Piperita: Eco-Friendly Synthesis and Effect on Acetylcholinesterase Activity. Life Sci. 2018, 209, 430–434. [Google Scholar] [CrossRef]
- Nabi, R.; Alvi, S.S.; Shah, A.; Chaturvedi, C.P.; Iqbal, D.; Ahmad, S.; Khan, M.S. Modulatory Role of HMG-CoA Reductase Inhibitors and Ezetimibe on LDL-AGEs-Induced ROS Generation and RAGE-Associated Signalling in HEK-293 Cells. Life Sci. 2019, 235, 116823. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Mode of Inhibition of Acetylcholinesterase by Monoterpenoids and Implications for Pest Control. Ind. Crop. Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hejazian, L.B.; Amani, R.; Siahchehreh Badeli, N. Geraniol Attenuates Oxidative Stress, Bioaccumulation, Serological and Histopathological Changes during Aluminum Chloride-Hepatopancreatic Toxicity in Male Wistar Rats. Environ. Sci. Pollut. Res. 2020, 27, 20076–20089. [Google Scholar] [CrossRef]
- Pavan, B.; Dalpiaz, A.; Marani, L.; Beggiato, S.; Ferraro, L.; Canistro, D.; Paolini, M.; Vivarelli, F.; Valerii, M.C.; Comparone, A.; et al. Geraniol Pharmacokinetics, Bioavailability and Its Multiple Effects on the Liver Antioxidant and Xenobiotic-Metabolizing Enzymes. Front. Pharmacol. 2018, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.N.; Muralidhara, M. Analysis of the Antioxidant Activity of Geraniol Employing Various In-Vitro Models: Relevance to Neurodegeneration in Diabetic Neuropathy. Asian J. Pharm. Clin. Res. 2017, 10, 101–105. [Google Scholar] [CrossRef]
- Farokhcheh, M.; Hejazian, L.; Akbarnejad, Z.; Pourabdolhossein, F.; Hosseini, S.M.; Mehraei, T.M.; Soltanpour, N. Geraniol Improved Memory Impairment and Neurotoxicity Induced by Zinc Oxide Nanoparticles in Male Wistar Rats through Its Antioxidant Effect. Life Sci. 2021, 282, 119823. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Fu, P.; Jun, X.; Cheng, P. Pharmacological Properties of Geraniol—A Review. Planta Med. 2019, 85, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rekha, K.R.; Selvakumar, G.P.; Sethupathy, S.; Santha, K.; Sivakamasundari, R.I. Geraniol Ameliorates the Motor Behavior and Neurotrophic Factors Inadequacy in MPTP-Induced Mice Model of Parkinson’s Disease. J. Mol. Neurosci. 2013, 51, 851–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.N. Muralidhara Mitigation of acrylamide-induced behavioral deficits, oxidative impairments and neurotoxicity by oral supplements of geraniol (a monoterpene) in a rat model. Chem. Interactions 2014, 223, 27–37. [Google Scholar] [CrossRef]
- Oboh, G.; Olasehinde, T.A.; Ademosun, A.O. Essential Oil from Lemon Peels Inhibit Key Enzymes Linked to Neurodegenerative Conditions and Pro-Oxidant Induced Lipid Peroxidation. J. Oleo Sci. 2014, 63, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhananjayan, K.; Sumathy, A.; Palanisamy, S. Molecular Docking Studies and In-Vitro Acetylcholinesterase Inhibition by Terpenoids and Flavonoids. Asian J. Res. Chem. 2013, 6, 1011–1017. [Google Scholar]
- Perry, N.S.L.; Houghton, P.J.; Theobald, A.; Jenner, P.; Perry, E.K. In-Vitro Inhibition of Human Erythrocyte Acetylcholinesterase by Salvia Lavandulaefolia Essential Oil and Constituent Terpenes. J. Pharm. Pharmacol. 2000, 52, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Zarred, K.; Laarif, A.; Hamouda, A.B.; Chaieb, I.; Jemaa, J.M.-B. Anticholinesterase Potential of Monoterpenoids on the Whitefly Bemisia Tabaci and Their Kinetic Studies. J. Agric. Sci. Technol. 2017, 19, 643–652. [Google Scholar]
- Fatima, K.; Wani, Z.A.; Meena, A.; Luqman, S. Geraniol Exerts Its Antiproliferative Action by Modulating Molecular Targets in Lung and Skin Carcinoma Cells. Phytother. Res. 2021, 35, 3861–3874. [Google Scholar] [CrossRef]
- Jabir, N.R.; Shakil, S.; Tabrez, S.; Khan, M.S.; Rehman, M.T.; Ahmed, B.A. In Silico Screening of Glycogen Synthase Kinase-3β Targeted Ligands against Acetylcholinesterase and Its Probable Relevance to Alzheimer’s Disease. J. Biomol. Struct. Dyn. 2021, 39, 5083–5092. [Google Scholar] [CrossRef]
- Rehman, M.T.; AlAjmi, M.F.; Hussain, A.; Rather, G.M.; Khan, M.A. High-Throughput Virtual Screening, Molecular Dynamics Simulation, and Enzyme Kinetics Identified ZINC84525623 as a Potential Inhibitor of NDM-1. Int. J. Mol. Sci. 2019, 20, E819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamsi, A.; Mohammad, T.; Khan, M.S.; Shahwan, M.; Husain, F.M.; Rehman, M.T.; Hassan, M.I.; Ahmad, F.; Islam, A. Unraveling Binding Mechanism of Alzheimer’s Drug Rivastigmine Tartrate with Human Transferrin: Molecular Docking and Multi-Spectroscopic Approach towards Neurodegenerative Diseases. Biomolecules 2019, 9, 495. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, D.; Rehman, M.T.; Bin Dukhyil, A.; Rizvi, S.M.D.; AlAjmi, M.F.; Alshehri, B.M.; Banawas, S.; Khan, M.S.; Alturaiki, W.; Alsaweed, M. High-Throughput Screening and Molecular Dynamics Simulation of Natural Product-like Compounds against Alzheimer’s Disease through Multitarget Approach. Pharmaceuticals 2021, 14, 937. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- BIOVIA Discovery Studio—BIOVIA—Dassault Systèmes®. Available online: https://www.3ds.com/products-services/biovia/products/molecular-modeling-simulation/biovia-discovery-studio/ (accessed on 29 August 2021).
- Harel, M.; Schalk, I.; Ehret-Sabatier, L.; Bouet, F.; Goeldner, M.; Hirth, C.; Axelsen, P.H.; Silman, I.; Sussman, J.L. Quaternary Ligand Binding to Aromatic Residues in the Active-Site Gorge of Acetylcholinesterase. Proc. Natl. Acad. Sci. USA 1993, 90, 9031–9035. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The Determination of Enzyme Dissociation Constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Dixon, M. The Determination of Enzyme Inhibitor Constants. Biochem. J. 1953, 55, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cherukupalli, S.; Jing, L.; Liu, X.; Zhan, P. Fsp3: A New Parameter for Drug-Likeness. Drug Discov. Today 2020, 25, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Sussman, J.L.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Toker, L.; Silman, I. Atomic Structure of Acetylcholinesterase from Torpedo Californica: A Prototypic Acetylcholine-Binding Protein. Science 1991, 253, 872–879. [Google Scholar] [CrossRef]
- Barak, D.; Kronman, C.; Ordentlich, A.; Ariel, N.; Bromberg, A.; Marcus, D.; Lazar, A.; Velan, B.; Shafferman, A. Acetylcholinesterase Peripheral Anionic Site Degeneracy Conferred by Amino Acid Arrays Sharing a Common Core. J. Biol. Chem. 1994, 269, 6296–6305. [Google Scholar] [CrossRef]
- Rabbani, N.; Tabrez, S.; Islam, B.U.; Rehman, M.T.; Alsenaidy, A.M.; AlAjmi, M.F.; Khan, R.A.; Alsenaidy, M.A.; Khan, M.S. Characterization of Colchicine Binding with Normal and Glycated Albumin: In Vitro and Molecular Docking Analysis. J. Biomol. Struct. Dyn. 2018, 36, 3453–3462. [Google Scholar] [CrossRef] [PubMed]
- AlAjmi, M.F.; Rehman, M.T.; Hussain, A.; Rather, G.M. Pharmacoinformatics Approach for the Identification of Polo-like Kinase-1 Inhibitors from Natural Sources as Anti-Cancer Agents. Int. J. Biol. Macromol. 2018, 116, 173–181. [Google Scholar] [CrossRef]
- Brańka, A.C. Nosé-Hoover Chain Method for Nonequilibrium Molecular Dynamics Simulation. Phys. Rev. E 2000, 61, 4769–4773. [Google Scholar] [CrossRef]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant Pressure Molecular Dynamics Algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase Inhibitors as Alzheimer’s Therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Watkins, P.B.; Zimmerman, H.J.; Knapp, M.J.; Gracon, S.I.; Lewis, K.W. Hepatotoxic Effects of Tacrine Administration in Patients with Alzheimer’s Disease. JAMA 1994, 271, 992–998. [Google Scholar] [CrossRef]
- Prasad, S.N. Muralidhara Neuroprotective effect of geraniol and curcumin in an acrylamide model of neurotoxicity in Drosophila melanogaster: Relevance to neuropathy. J. Insect Physiol. 2014, 60, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Shaw, F.H.; Bentley, G.A. The Pharmacology of Some New Anti-Cholinesterases. Aust. J. Exp. Biol. Med Sci. 1953, 31, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Marquis, J.K. Pharmacological Significance of Acetylcholinesterase Inhibition by Tetrahydroaminoacridine. Biochem. Pharmacol. 1990, 40, 1071–1076. [Google Scholar] [CrossRef]
- Pietsch, M.; Christian, L.; Inhester, T.; Petzold, S.; Gütschow, M. Kinetics of Inhibition of Acetylcholinesterase in the Presence of Acetonitrile. FEBS J. 2009, 276, 2292–2307. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.A.; Leonard, K. Interaction of Tetrahydroaminoacridine with Acetylcholinesterase and Butyrylcholinesterase. Mol. Pharmacol. 1992, 41, 412–418. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hitchcock, S.A.; Pennington, L.D. Structure-Brain Exposure Relationships. J. Med. Chem. 2006, 49, 7559–7583. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Alvarez, A.; Pérez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase Accelerates Assembly of Amyloid-Beta-Peptides into Alzheimer’s Fibrils: Possible Role of the Peripheral Site of the Enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef] [Green Version]
- Inestrosa, N.C.; Dinamarca, M.C.; Alvarez, A. Amyloid-Cholinesterase Interactions. Implications for Alzheimer’s Disease. FEBS J. 2008, 275, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Colletier, J.-P.; Fournier, D.; Greenblatt, H.M.; Stojan, J.; Sussman, J.L.; Zaccai, G.; Silman, I.; Weik, M. Structural Insights into Substrate Traffic and Inhibition in Acetylcholinesterase. EMBO J. 2006, 25, 2746–2756. [Google Scholar] [CrossRef] [PubMed]








| ADME Study of Molecule | Geraniol | Tacrine | |
|---|---|---|---|
| Canonical SMILES | OC/C=C(/CCC=C(C)C)\C | Nc1c2CCCCc2nc2c1cccc2 | |
| Formula | C10H18O | C13H14N2 | |
| Physico-chemical properties | MW | 154.25 | 198.26 |
| #Heavy atoms | 11 | 15 | |
| #Aromatic heavy atoms | 0 | 10 | |
| Fraction Csp3 | 0.6 | 0.31 | |
| #Rotatable bonds | 4 | 0 | |
| #H-bond acceptors | 1 | 1 | |
| #H-bond donors | 1 | 1 | |
| Molar Refractivity | 50.4 | 63.58 | |
| TPSA | 20.23 | 38.91 | |
| Lipophilicity | iLOGP | 2.52 | 2.09 |
| XLOGP3 | 3.56 | 2.71 | |
| WLOGP | 2.67 | 2.7 | |
| MLOGP | 2.59 | 2.33 | |
| Silicos-IT Log P | 2.35 | 3.12 | |
| Consensus Log P | 2.74 | 2.59 | |
| Water Solubility | ESOL Class | Soluble | Soluble |
| Ali Class | Soluble | Soluble | |
| Silicos-IT class | Soluble | Moderately soluble | |
| Pharmacokinetics | GI absorption | High | High |
| BBB permeant | Yes | Yes | |
| Pgp substrate | No | Yes | |
| CYP1A2 inhibitor | No | Yes | |
| CYP2C19 inhibitor | No | No | |
| CYP2C9 inhibitor | No | No | |
| CYP2D6 inhibitor | No | No | |
| CYP3A4 inhibitor | No | Yes | |
| log Kp (cm/s) | −4.71 | −5.59 | |
| Drug-likeness | Lipinski #violations | 0 | 0 |
| Ghose #violations | 1 | 0 | |
| Veber #violations | 0 | 0 | |
| Egan #violations | 0 | 0 | |
| Muegge #violations | 2 | 1 | |
| Bioavailability Score | 0.55 | 0.55 | |
| Medicinal Chemistry | PAINS #alerts | 0 | 0 |
| Brenk #alerts | 1 | 0 | |
| Leadlikeness #violations | 2 | 1 | |
| Synthetic Accessibility | 2.58 | 2.08 | |
| Donor–Acceptor Pair | Distance (Å) | Category of Interaction | Type of Interaction | Docking Energy, kcal mol−1 | Binding Affinity, M−1 |
|---|---|---|---|---|---|
| Acetylcholine at catalytic active site (CAS) * | |||||
| LIG:N–GLU199:OE1 | 4.09 | Electrostatic | Attractive Charge | −4.1 | 1.02 × 103 |
| HIS440:CD2–LIG:O | 3.77 | Hydrogen Bond | Carbon Hydrogen Bond | ||
| LIG:C–GLU199:OE1 | 3.62 | Hydrogen Bond | Carbon Hydrogen Bond | ||
| LIG:C–GLU199:OE1 | 3.67 | Hydrogen Bond | Carbon Hydrogen Bond | ||
| LIG:C–SER200:OG | 3.65 | Hydrogen Bond | Carbon Hydrogen Bond | ||
| LIG:N–TRP84 | 4.88 | Electrostatic | Pi-Cation | ||
| LIG:N–TRP84 | 4.24 | Electrostatic | Pi-Cation | ||
| LIG:C–TRP84 | 3.8 | Hydrophobic | Pi-Sigma | ||
| LIG:C–TRP84 | 3.81 | Hydrophobic | Pi-Sigma | ||
| LIG:C–PHE330 | 3.58 | Hydrophobic | Pi-Sigma | ||
| Geraniol at catalytic active site (CAS) | |||||
| HIS440:CD2–LIG:O | 3.54 | Hydrogen Bond | Carbon Hydrogen Bond | −5.6 | 1.28 × 104 |
| LIG:C–HIS440:O | 3.44 | Hydrogen Bond | Carbon Hydrogen Bond | ||
| LIG:C–PHE330 | 3.93 | Hydrophobic | Pi-Sigma | ||
| TRP84–LIG:C | 4.05 | Hydrophobic | Pi-Alkyl | ||
| TRP84–LIG:C | 4.66 | Hydrophobic | Pi-Alkyl | ||
| PHE330–LIG:C | 5.23 | Hydrophobic | Pi-Alkyl | ||
| PHE330–LIG:C | 5.39 | Hydrophobic | Pi-Alkyl | ||
| PHE331–LIG:C | 4.77 | Hydrophobic | Pi-Alkyl | ||
| PHE331–LIG:C | 4.65 | Hydrophobic | Pi-Alkyl | ||
| HIS440–LIG:C | 5.12 | Hydrophobic | Pi-Alkyl | ||
| Geraniol at peripheral anionic site (PAS) | |||||
| TYR70–LIG:C | 4.1 | Hydrophobic | Pi-Alkyl | −6.8 | 9.72 × 104 |
| TYR121–LIG:C | 4.01 | Hydrophobic | Pi-Alkyl | ||
| TYR121–LIG:C | 4.63 | Hydrophobic | Pi-Alkyl | ||
| TRP279–LIG:C | 4.99 | Hydrophobic | Pi-Alkyl | ||
| TRP279–LIG:C | 5.03 | Hydrophobic | Pi-Alkyl | ||
| TRP279–LIG:C | 4.52 | Hydrophobic | Pi-Alkyl | ||
| TRP279–LIG:C | 4.56 | Hydrophobic | Pi-Alkyl | ||
| PHE290–LIG:C | 5.05 | Hydrophobic | Pi-Alkyl | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, D.; Khan, M.S.; Waiz, M.; Rehman, M.T.; Alaidarous, M.; Jamal, A.; Alothaim, A.S.; AlAjmi, M.F.; Alshehri, B.M.; Banawas, S.; et al. Exploring the Binding Pattern of Geraniol with Acetylcholinesterase through In Silico Docking, Molecular Dynamics Simulation, and In Vitro Enzyme Inhibition Kinetics Studies. Cells 2021, 10, 3533. https://doi.org/10.3390/cells10123533
Iqbal D, Khan MS, Waiz M, Rehman MT, Alaidarous M, Jamal A, Alothaim AS, AlAjmi MF, Alshehri BM, Banawas S, et al. Exploring the Binding Pattern of Geraniol with Acetylcholinesterase through In Silico Docking, Molecular Dynamics Simulation, and In Vitro Enzyme Inhibition Kinetics Studies. Cells. 2021; 10(12):3533. https://doi.org/10.3390/cells10123533
Chicago/Turabian StyleIqbal, Danish, M. Salman Khan, Mohd Waiz, Md Tabish Rehman, Mohammed Alaidarous, Azfar Jamal, Abdulaziz S. Alothaim, Mohamed F AlAjmi, Bader Mohammed Alshehri, Saeed Banawas, and et al. 2021. "Exploring the Binding Pattern of Geraniol with Acetylcholinesterase through In Silico Docking, Molecular Dynamics Simulation, and In Vitro Enzyme Inhibition Kinetics Studies" Cells 10, no. 12: 3533. https://doi.org/10.3390/cells10123533