Differential Dose- and Tissue-Dependent Effects of foxo on Aging, Metabolic and Proteostatic Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Drosophila Stocks and Maintenance
2.2. Flies Sorting, Tissue Microdissections, and Hemolymph Isolation
2.3. Gustatory Assay, Body Mass Measurement, and Progeny
2.4. Locomotion and Longevity
2.5. Stress Sensitivity Assays
2.5.1. Heat Sensitivity Assay
2.5.2. Cold Sensitivity Assay
2.5.3. Nutrients’ Sensitivity Assays
2.6. Total RNA Extraction and Quantitative Real-Time (Q-RT-PCR) Analyses
2.7. Isolation of Total Protein, Immunoblotting Analyses and Detection of Carbonyl Groups
2.8. Measurement of Reactive Oxygen Species (ROS), 26S Proteasome CT-L/C-L and Cathepsins Activity, as well as of Tissues’ Sugar Content
2.9. CSLM and Immunofluorescence Staining
2.10. Antibodies
2.11. Statistical Analyses
3. Results
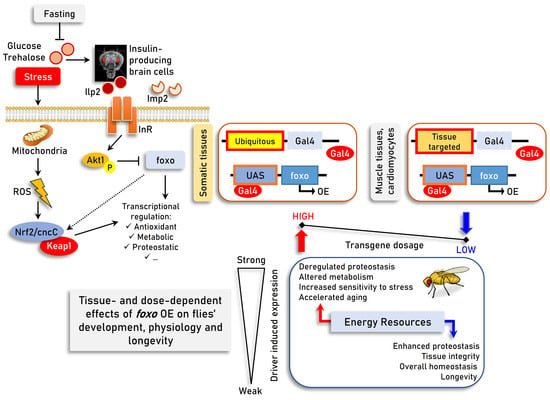
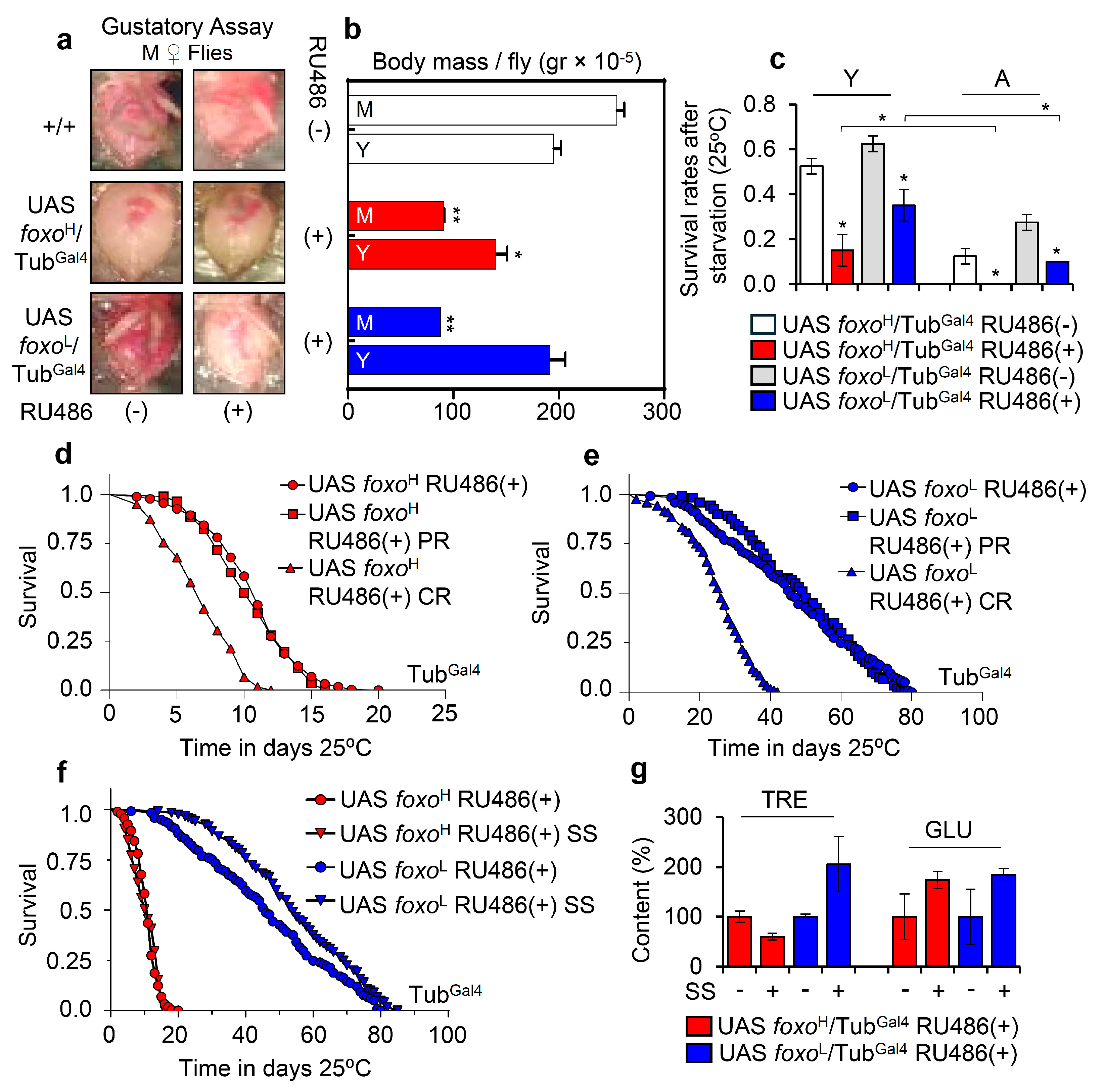
3.1. Prolonged Ubiquitous foxo OE Accelerates Aging Phenotypes and Impairs Thermal Stress Responses Dose-Dependently
3.2. Sustained foxo OE Perturbs Cellular Energetics and Reduces Tolerance to Nutritional Deprivation
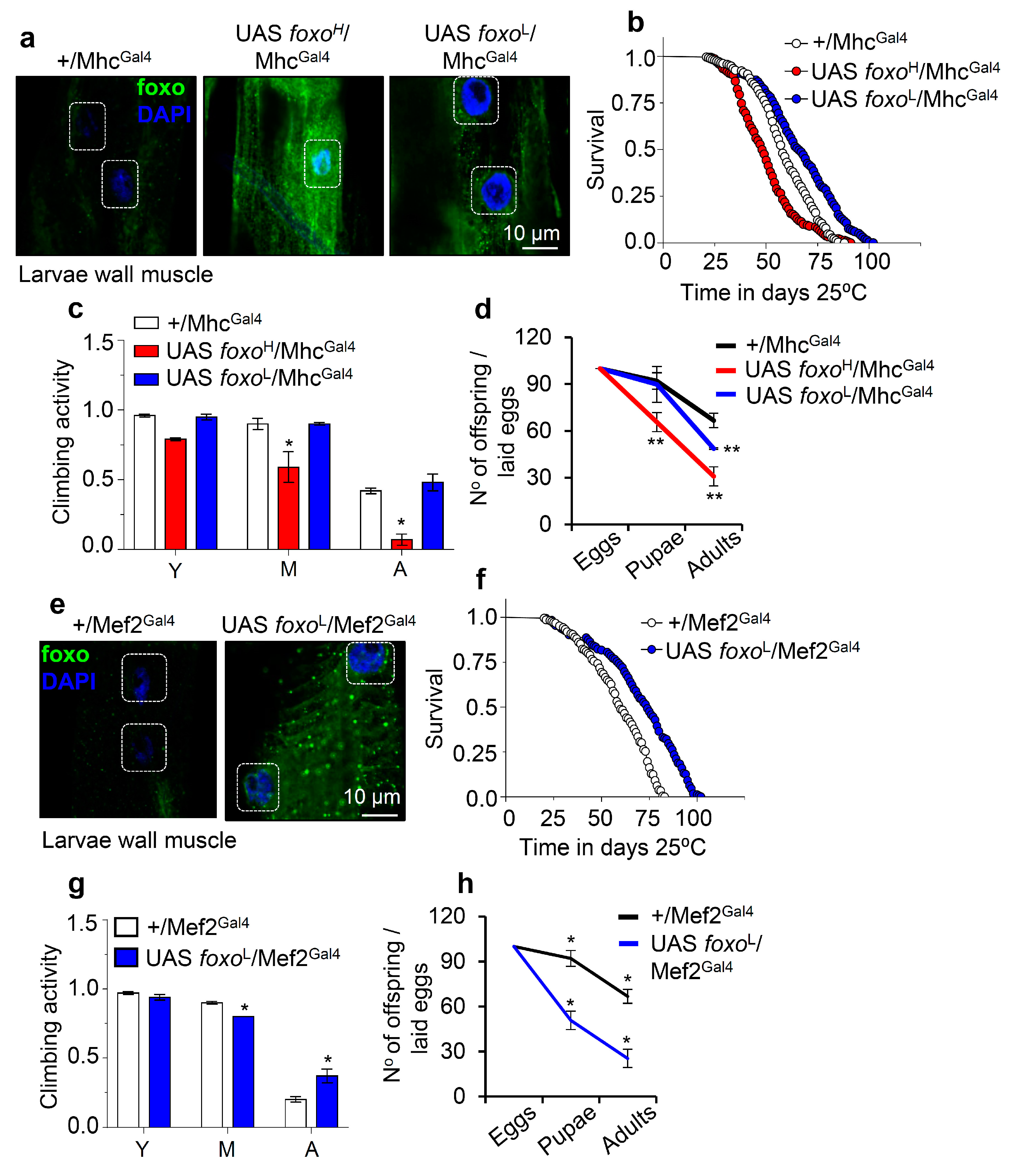
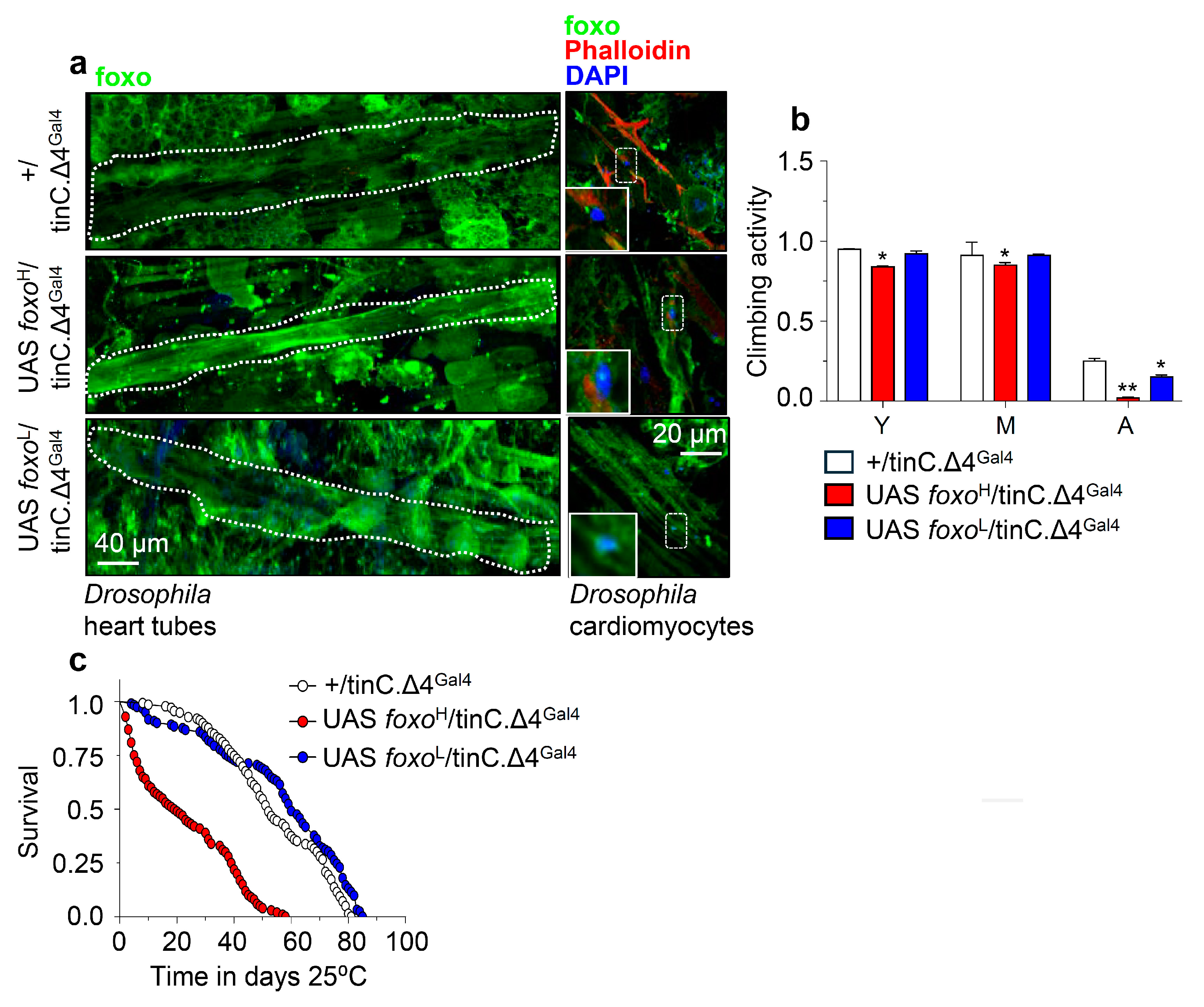
3.3. While High Levels of Muscle- or Cardiomyocytes-Targeted foxo OE Are Toxic, Moderate OE Increases Healthspan and Delays Age-Related Phenotypes
3.4. Inducible foxo OE Activates Proteostatic Pathways
3.5. Proteasome Activation in foxo OE Transgenic Flies Is Nrf2/cncC-Mediated
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability statement:
Acknowledgments
Conflicts of Interest
References
- da Costa, J.P.; Vitorino, R.; Silva, G.M.; Vogel, C.; Duarte, A.C.; Rocha-Santos, T. A synopsis on aging—Theories, mechanisms and future prospects. Ageing Res. Rev. 2016, 29, 90–112. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Maria, A.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Richard, I. Aging: A Common Driver of Chronic Diseases and a Target for Novel Interventions. Cell 2016, 159, 709–713. [Google Scholar] [CrossRef]
- Martínez Corrales, G.; Alic, N. Evolutionary Conservation of Transcription Factors Affecting Longevity. Trends Genet. 2020, 36, 373–382. [Google Scholar] [CrossRef]
- Friedman, D.; Johnson, T. A Mutation in the Age-1 Gene in Caenorhabditis elegans Lengthens Life and Reduces Hermaphrodite Fertility. Genetics 1988, 118, 75–86. [Google Scholar] [CrossRef]
- Johnson, T.E. 25years after age-1: Genes, interventions and the revolution in aging research. Exp. Gerontol. 2013, 48, 640–643. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Proteostasis and aging. Nat. Med. 2015, 21, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Klaips, C.L.; Jayaraj, G.G.; Hartl, F.U. Pathways of Cellular Proteostasis in Aging and Disease. J. Cell Biol. 2018, 217, 51–63. [Google Scholar] [CrossRef]
- Tsakiri, E.N.; Trougakos, I.P. The amazing ubiquitin-proteasome system: Structural components and implication inaging. Int. Rev. Cell Mol. Biol. 2015, 314, 171–237. [Google Scholar]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Webb, A.E.; Brunet, A. FOXO transcription factors: Key regulators of cellular quality control. Trends Biochem. Sci. 2014, 39, 159–169. [Google Scholar] [CrossRef]
- Martins, R.; Lithgow, G.J.; Link, W. Long live FOXO: Unraveling the role of FOXO proteins in aging and longevity. Aging Cell 2016, 15, 196–207. [Google Scholar] [CrossRef]
- Eijkelenboom, A.; Burgering, B.M.T. FOXOs: Signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 2013, 15, 83–97. [Google Scholar] [CrossRef]
- Sen, P.; Shah, P.P.; Nativio, R.; Berger, S.L. Epigenetic Mechanisms of Longevity and Aging. Cell 2016, 166, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z. The FoxO–Autophagy Axis in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 658–671. [Google Scholar] [CrossRef]
- Demontis, F.; Perrimon, N. FOXO/4E-BP Signaling in Drosophila Muscles Regulates Organism-wide Proteostasis during Aging. Cell 2010, 143, 813–825. [Google Scholar] [CrossRef]
- Hwangbo, D.S.; Gersham, B.; Tu, M.; Palmer, M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 2004, 429, 562–567. [Google Scholar] [CrossRef]
- Giannakou, M.E.; Goss, M.; Juenger, M.A.; Ju, M.A.; Hafen, E.; Leevers, S.J.; Partridge, L. Long-Lived Drosophila with Over- Expressed DFOXO in Adult Fat Body. Science 2004, 305, 361. [Google Scholar] [CrossRef] [PubMed]
- Poirier, L.; Shane, A.; Zheng, J.; Seroude, L. Characterization of the Drosophila Gene-Switch System in Aging Studies: A Cautionary Tale. Aging Cell 2008, 7, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.J.; Sharma, A.; Mair, W.B. Metabolic Communication and Healthy Aging: Where Should We Focus Our Energy? Dev. Cell 2020, 54, 196–211. [Google Scholar] [CrossRef]
- Ugur, B.; Chen, K.; Bellen, H.J. Drosophila Tools and Assays for the Study of Human Diseases. Dis. Model. Mech. 2016, 9, 235–244. [Google Scholar] [CrossRef]
- Hales, K.G.; Korey, C.A.; Larracuente, A.M.; Roberts, D.M. Genetics on the Fly: A Primer on the Drosophila Model System. Genetics 2015, 201, 815–842. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Margaritis, L.H. Immunolocalization of the Temporally “early” Secreted Major Structural Chorion Proteins, Dvs38 and Dvs36, in the Eggshell Layers and Regions of Drosophila Virilis. J. Struct. Biol. 1998, 123, 111–123. [Google Scholar] [CrossRef]
- Tsakiri, E.N.; Sykiotis, G.P.; Papassideri, I.S.; Gorgoulis, V.G.; Bohmann, D.; Trougakos, I.P. Differential Regulation of Proteasome Functionality in Reproductive vs. Somatic Tissues of Drosophila during Aging or Oxidative Stress. FASEB J. 2013, 27, 2407–2420. [Google Scholar] [CrossRef]
- Tsakiri, E.; Gumeni, S.; Iliaki, K.; Benaki, D.; Sykiotis, G.P.; Gorgoulis, V.G.; Scorrano, L.; Trougakos, I.P.; Iliaki, K.K.; Tsakiri, E.N.; et al. Hyperactivation of Nrf2 Increases Stress Tolerance at the Cost of Aging Acceleration Due to Metabolic Deregulation. Aging Cell 2019, 18, 12845. [Google Scholar] [CrossRef] [PubMed]
- Manola, M.S.; Tsakiri, E.N.; Trougakos, I.P. Alterations in Organismal Physiology, Impaired Stress Resistance, and Accelerated Aging in Drosophila Flies Adapted to Multigenerational Proteome Instability. Oxid. Med. Cell. Longev. 2019, 2019, 7823285. [Google Scholar] [CrossRef]
- Alayari, N.N.; Vogler, G.; Taghli-Lamallem, O.; Ocorr, K.; Bodmer, R.; Cammarato, A. Fluorescent Labeling of Drosophila Heart Structures. J. Vis. Exp. 2009, 32, 1432. [Google Scholar] [CrossRef]
- Tsakiri, E.N.; Gaboriaud-Kolar, N.; Iliaki, K.K.; Tchoumtchoua, J.; Papanagnou, E.-D.; Chatzigeorgiou, S.; Tallas, K.D.; Mikros, E.; Halabalaki, M.; Skaltsounis, A.-L.; et al. The Indirubin Derivative 6-Bromoindirubin-3′-Oxime Activates Proteostatic Modules, Reprograms Cellular Bioenergetic Pathways, and Exerts Antiaging Effects. Antioxid. Redox Signal. 2017, 27, 1027–1047. [Google Scholar] [CrossRef]
- Ayrinhac, A.; Debat, V.; Gibert, P.; Kister, A.G.; Legout, H.; Moreteau, B.; Vergilino, R.; David, J.R. Cold Adaptation in Geographical Populations of Drosophila melanogaster: Phenotypic Plasticity Is More Important than Genetic Variability. Funct. Ecol. 2004, 18, 700–706. [Google Scholar] [CrossRef]
- Gumeni, S.; Evangelakou, Z.; Tsakiri, E.N.; Scorrano, L.; Trougakos, I.P. Functional Wiring of Proteostatic and Mitostatic Modules Ensures Transient Organismal Survival during Imbalanced Mitochondrial Dynamics. Redox Biol. 2019, 24, 101219. [Google Scholar] [CrossRef]
- Barrio, L.; Dekanty, A.; Milán, M. MicroRNA-Mediated Regulation of Dp53 in the Drosophila Fat Body Contributes to Metabolic Adaptation to Nutrient Deprivation. Cell Rep. 2014, 8, 528–541. [Google Scholar] [CrossRef]
- Matsushita, R.; Nishimura, T. Trehalose Metabolism Confers Developmental Robustness and Stability in Drosophila by Regulating Glucose Homeostasis. Commun. Biol. 2020, 3, 170. [Google Scholar] [CrossRef]
- Grönke, S.; Mildner, A.; Fellert, S.; Tennagels, N.; Petry, S.; Müller, G.; Jäckle, H.; Kühnlein, R.P. Brummer Lipase Is an Evolutionary Conserved Fat Storage Regulator in Drosophila. Cell Metab. 2005, 1, 323–330. [Google Scholar] [CrossRef]
- Kim, M.S.; Pak, Y.K.; Jang, P.G.; Namkoong, C.; Choi, Y.S.; Won, J.C.; Kim, K.S.; Kim, S.W.; Kim, H.S.; Park, J.Y.; et al. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat. Neurosci. 2006, 9, 901–906. [Google Scholar] [CrossRef]
- Blice-Baum, A.C.; Kaushik, G.; Viswanathan, M.C.; Zambon, A.C.; Engler, A.J.; Bodmer, R.; Cammarato, A. Overexpression of Foxo in the Heart Ameliorates Performance Decline through Enhanced UPS Processing in Aging Drosophila. Biophys. J. 2015, 108, 361a. [Google Scholar] [CrossRef][Green Version]
- Tullet, J.M.A.; Hertweck, M.; An, J.H.; Baker, J.; Hwang, J.Y.; Liu, S.; Oliveira, R.P.; Baumeister, R.; Blackwell, T.K. Direct Inhibition of the Longevity-Promoting Factor SKN-1 by Insulin-like Signaling in C. elegans. Cell 2008, 132, 1025–1038. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Milman, S.; Lin, J.-R.; Wierbowski, S.; Yu, H.; Barzilai, N.; Gorbunova, V.; Ladiges, W.C.; Niedernhofer, L.J.; Suh, Y.; et al. Genetics of Extreme Human Longevity to Guide Drug Discovery for Healthy Ageing. Nat. Metab. 2020, 2, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Alic, N.; Andrews, T.D.; Giannakou, M.E.; Papatheodorou, I.; Slack, C.; Hoddinott, M.P.; Cochemé, H.M.; Schuster, E.F.; Thornton, J.M.; Partridge, L. Genome-wide DFOXO Targets and Topology of the Transcriptomic Response to Stress and Insulin Signalling. Mol. Syst. Biol. 2011, 7, 502. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, T.; Huang, P. Post-translational modifications of FOXO family proteins (Review). Mol. Med. Rep. 2016, 14, 4931–4941. [Google Scholar] [CrossRef]
- Gui, T.; Burgering, B. FOXOs: Masters of the equilibrium. FEBS J. 2021, 10, febs.16221. [Google Scholar] [CrossRef]
- Hannenhalli, S.; Kaestner, K.H. The Evolution of Fox Genes and Their Role in Development and Disease. Nat. Rev. Genet. 2009, 10, 233–240. [Google Scholar] [CrossRef]
- Daitoku, H.; Kaneko, Y.; Yoshimochi, K.; Matsumoto, K.; Araoi, S.; Sakamaki, J.I.; Takahashi, Y.; Fukamizu, A. Non-transcriptional Function of FOXO1/DAF-16 Contributes to Translesion DNA Synthesis. Mol. Cell Biol. 2016, 36, 2755–2766. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Liao, W.; Liu, X.; Zhang, H.; Wang, S.; Wang, D.; Feng, J.; Yu, L.; Zhu, W.G. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat. Cell Biol. 2012, 12, 665–675. [Google Scholar] [CrossRef]
- van der Vos, K.E.; Coffer, P.J. The extending network of FOXO transcriptional target genes. Antioxid. Redox Signal. 2011, 14, 579–592. [Google Scholar] [CrossRef]
- Tsai, W.-B.; Chung, Y.M.; Takahashi, Y.; Xu, Z.; Hu, M.C.T. Functional interaction between FOXO3a and ATM regulates DNA damage response. Nat. Cell Biol. 2008, 10, 460–467. [Google Scholar] [CrossRef]
- Cao, Y.; Kamioka, Y.; Yokoi, N.; Kobayashi, T.; Hino, O.; Onodera, M.; Mochizuki, N.; Nakae, J. Interaction of FoxO1 and TSC2 induces insulin resistance through activation of the mammalian target of rapamycin/p70 S6K pathway. J. Biol. Chem. 2006, 281, 40242–40251. [Google Scholar] [CrossRef] [PubMed]
- Gross, D.N.; van den Heuvel, A.P.J.; Birnbaum, M.J. The Role of FoxO in the Regulation of Metabolism. Oncogene 2008, 27, 2320–2336. [Google Scholar] [CrossRef]
- Kramer, J.M.; Davidge, J.T.; Lockyer, J.M.; Staveley, B.E. Expression of Drosophila FOXO Regulates Growth and Can Phenocopy Starvation. BMC Dev. Biol. 2003, 3, 5. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef]
- Kamei, Y.; Miura, S.; Suzuki, M.; Kai, Y.; Mizukami, J.; Taniguchi, T.; Mochida, K.; Hata, T.; Matsuda, J.; Aburatani, H.; et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J. Biol. Chem. 2004, 279, 41114–41123. [Google Scholar] [CrossRef]
- Sandri, M.; Barberi, L.; Bijlsma, A.Y.; Blaauw, B.; Dyar, K.A.; Milan, G.; Mammucari, C.; Meskers, C.G.M.; Pallafacchina, G.; Paoli, A.; et al. Signalling Pathways Regulating Muscle Mass in Ageing Skeletal Muscle. the Role of the IGF1-Akt-MTOR-FoxO Pathway. Biogerontology 2013, 14, 303–323. [Google Scholar] [CrossRef]
- Hariharan, N.; Ikeda, Y.; Hong, C.; Alcendor, R.R.; Usui, S.; Gao, S.; Maejima, Y.; Sadoshima, J. Autophagy Plays an Essential Role in Mediating Regression of Hypertrophy during Unloading of the Heart. PLoS ONE 2013, 8, e51632. [Google Scholar] [CrossRef]
- Cao, D.J.; Jiang, N.; Blagg, A.; Johnstone, J.L.; Gondalia, R.; Oh, M.; Luo, X.; Yang, K.; Shelton, J.M.; Rothermel, B.A.; et al. Mechanical Unloading Activates FoxO3 to Trigger Bnip3-Dependent Cardiomyocyte Atrophy. J. Am. Heart Assoc. 2013, 2, e000016. [Google Scholar] [CrossRef]
- Schips, T.G.; Wietelmann, A.; Höhn, K.; Schimanski, S.; Walther, P.; Braun, T.; Wirth, T.; Maier, H.J. FoxO3 Induces Reversible Cardiac Atrophy and Autophagy in a Transgenic Mouse Model. Cardiovasc. Res. 2011, 91, 587–597. [Google Scholar] [CrossRef]
- Vilchez, D.; Morantte, I.; Liu, Z.; Douglas, P.M.; Merkwirth, C.; Rodrigues, A.P.C.; Manning, G.; Dillin, A. RPN-6 Determines C. elegans Longevity under Proteotoxic Stress Conditions. Nature 2012, 489, 263–268. [Google Scholar] [CrossRef]
- Vilchez, D.; Boyer, L.; Morantte, I.; Lutz, M.; Merkwirth, C.; Joyce, D.; Spencer, B.; Page, L.; Masliah, E.; Travis Berggren, W.; et al. Increased Proteasome Activity in Human Embryonic Stem Cells Is Regulated by PSMD11. Nature 2012, 489, 304–308. [Google Scholar] [CrossRef]
- Kapetanou, M.; Nespital, T.; Tain, L.S.; Pahl, A.; Partridge, L.; Gonos, E.S. FoxO1 Is a Novel Regulator of 20S Proteasome Subunits Expression and Activity. Front. Cell Dev. Biol. 2021, 9, 169. [Google Scholar] [CrossRef]
- Kocaturk, N.M.; Gozuacik, D. Crosstalk Between Mammalian Autophagy and the Ubiquitin-Proteasome System. Front. Cell Dev. Biol. 2018, 6, 128. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manola, M.S.; Gumeni, S.; Trougakos, I.P. Differential Dose- and Tissue-Dependent Effects of foxo on Aging, Metabolic and Proteostatic Pathways. Cells 2021, 10, 3577. https://doi.org/10.3390/cells10123577
Manola MS, Gumeni S, Trougakos IP. Differential Dose- and Tissue-Dependent Effects of foxo on Aging, Metabolic and Proteostatic Pathways. Cells. 2021; 10(12):3577. https://doi.org/10.3390/cells10123577
Chicago/Turabian StyleManola, Maria S., Sentiljana Gumeni, and Ioannis P. Trougakos. 2021. "Differential Dose- and Tissue-Dependent Effects of foxo on Aging, Metabolic and Proteostatic Pathways" Cells 10, no. 12: 3577. https://doi.org/10.3390/cells10123577
APA StyleManola, M. S., Gumeni, S., & Trougakos, I. P. (2021). Differential Dose- and Tissue-Dependent Effects of foxo on Aging, Metabolic and Proteostatic Pathways. Cells, 10(12), 3577. https://doi.org/10.3390/cells10123577