Social Defeat Stress during Early Adolescence Confers Resilience against a Single Episode of Prolonged Stress in Adult Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Social Defeat Stress
2.3. Single Prolonged Stress Protocol
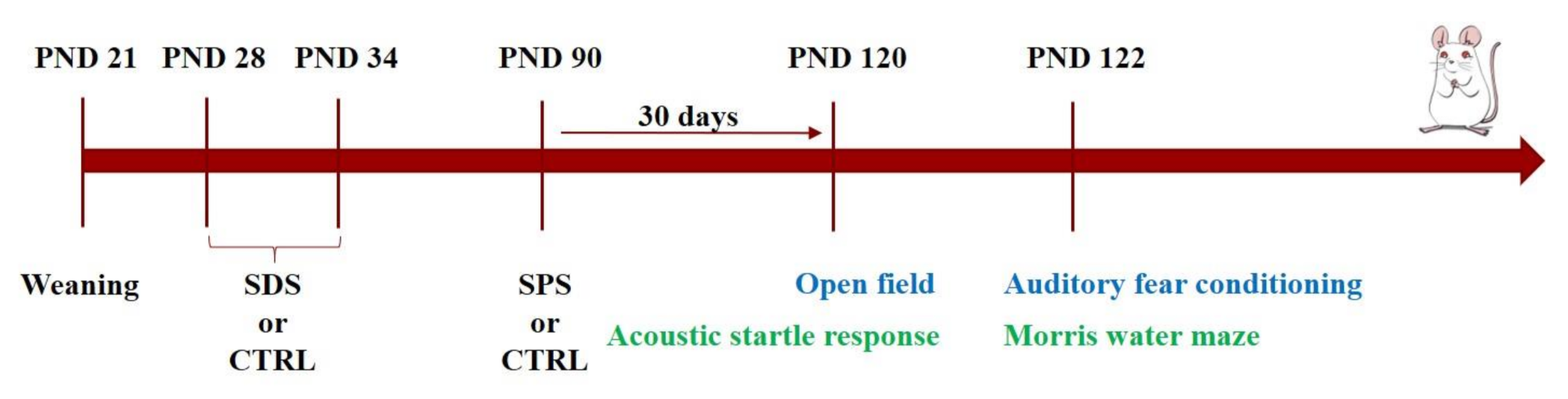
2.4. Experimental Design
- -
- CTRL-CTRL group: rats not exposed to any stressors
- -
- SDS-CTRL group: rats exposed to only SDS during the adolescence (PND 28-34)
- -
- CTRL-SPS group: rats exposed to only SPS at PND 90
- -
- SDS-SPS group: rats exposed to SDS during adolescence and SPS at PND 90
2.5. Behavioral Tests
2.5.1. Open Field
2.5.2. Acoustic Startle Response
2.5.3. Morris Water Maze
2.5.4. Auditory Fear Conditioning
2.6. Biochemical Analyses
2.6.1. Tissue Collection and Western Blotting Analysis
2.6.2. Plasma Corticosterone Levels
2.7. Statistical Analysis
3. Results
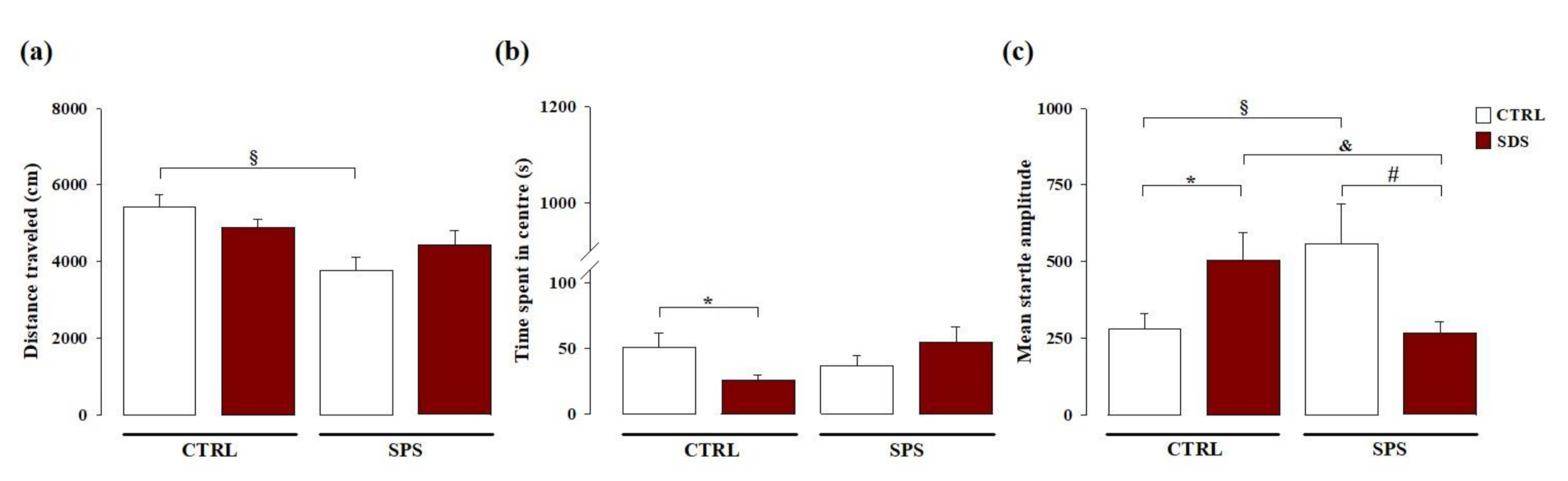
3.1. The Interaction Between Social Defeat Stress (SDS) during Early Adolescence and Single Prolonged Stress (SPS) in Adulthood Induces Resilience towards the Development of Hyperarousal and Anxiety Later in Life
3.1.1. Open Field Test
3.1.2. Acoustic Startle Response
3.2. The Interaction between SDS during Early Adolescence and SPS in Adulthood Induces Resilience towards Spatial Memory Deficits Later in Life
3.3. The Interaction Between SDS during Early Adolescence and SPS in Adulthood Induces Vulnerability towards Cued Fear Memory Deficits Later in Life
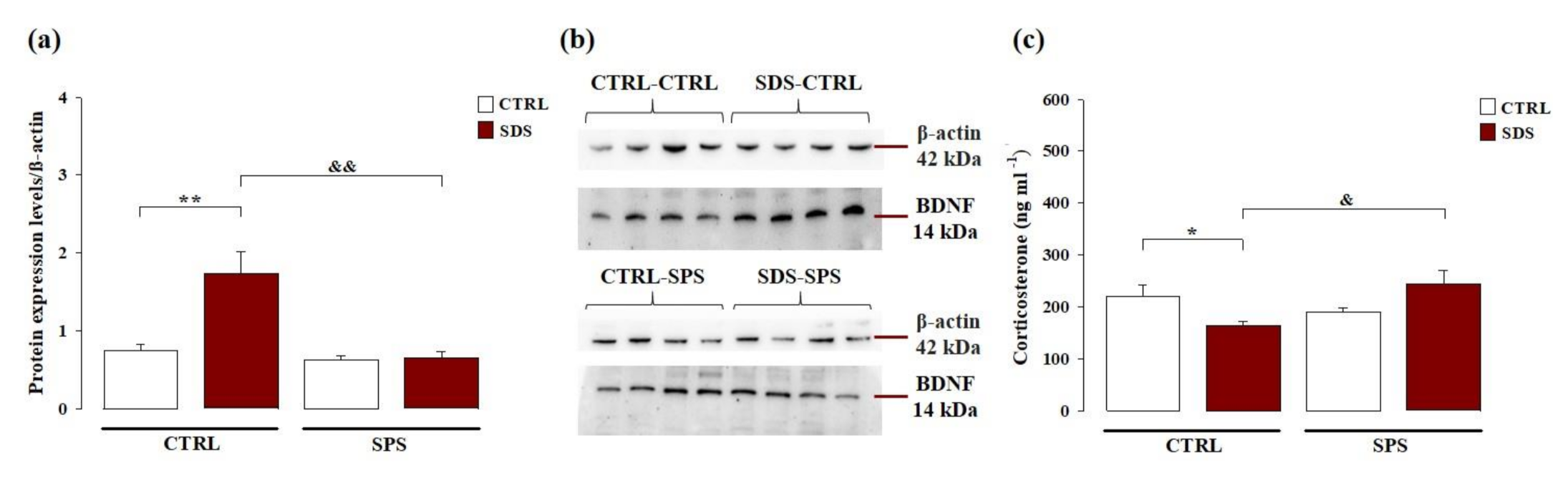
3.4. The Interaction Between SDS during Early Adolescence and SPS in Adulthood Normalizes Protein Levels of Brain-Derived Neurotrophic Factor (BDNF) Within the Hippocampus Later in Life
3.5. The Interaction between SDS during Early Adolescence and SPS in Adulthood Normalizes the Corticosterone Plasma Levels Later in Life
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McEwen, B.S. Mood disorders and allostatic load. Biol. Psychiatry 2003, 54, 200–207. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, S.; Lesuis, S.L.; Wang, X.D.; Wagner, K.V.; Hartmann, J.; Labermaier, C.; Scharf, S.H.; Müller, M.B.; Holsboer, F.; Schmidt, M.V. Evidence supporting the match/mismatch hypothesis of psychiatric disorders. Eur. Neuropsychopharmacol. 2014, 24, 907–918. [Google Scholar] [CrossRef]
- Champagne, D.L.; Ronald de Kloet, E.; Joëls, M. Fundamental aspects of the impact of glucocorticoids on the (immature) brain. Semin. Fetal Neonatal Med. 2009, 14, 136–142. [Google Scholar] [CrossRef]
- Krugers, H.J.; Arp, J.M.; Xiong, H.; Kanatsou, S.; Lesuis, S.L.; Korosi, A.; Joels, M.; Lucassen, P.J. Early life adversity: Lasting consequences for emotional learning. Neurobiol. Stress 2017, 6, 14–21. [Google Scholar] [CrossRef]
- Daskalakis, N.P.; Bagot, R.C.; Parker, K.J.; Vinkers, C.H.; de Kloet, E.R. The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 2013, 38, 1858–1873. [Google Scholar] [CrossRef] [Green Version]
- Casey, B.J.; Jones, R.M.; Hare, T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008, 1124, 111–126. [Google Scholar] [CrossRef]
- Spear, L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000. [Google Scholar] [CrossRef]
- Romeo, R.D.; McEwen, B.S. Stress and the adolescent brain. Ann. N. Y. Acad. Sci. 2006, 1094, 202–214. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.M.; Mathews, I.Z.; Thomas, C.; Waters, P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010, 72, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.; Winget, C.; Hollingshead, G.W.; Levine, S. Postweaning development of negative feedback in the pituitary adrenal system of the rat. Neuroendocrinology 1973, 12, 199–211. [Google Scholar] [CrossRef]
- Andersen, S.L. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003, 27, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Haynes, L.E.; Barber, D.; Mitchell, I.J. Chronic antidepressant medication attenuates dexamethasone-induced neuronal death and sublethal neuronal damage in the hippocampus and striatum. Brain Res. 2004. [Google Scholar] [CrossRef]
- Schoenfeld, T.J.; Gould, E. Stress, stress hormones, and adult neurogenesis. Exp. Neurol. 2012, 233, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlides, C.; Watanabe, Y.; McEwen, B.S. Effects of glucocorticoids on hippocampal long-term potentiation. Hippocampus 1993. [Google Scholar] [CrossRef] [PubMed]
- Pavlides, C.; Ogawa, S.; Kimura, A.; McEwen, B.S. Role of adrenal steroid mineralocorticoid and glucocorticoid receptors in long-term potentiation in the CA1 field of hippocampal slices. Brain Res. 1996. [Google Scholar] [CrossRef]
- Jeanneteau, F.; Chao, M.V. Are BDNF and glucocorticoid activities calibrated? Neuroscience 2013, 239, 173–195. [Google Scholar] [CrossRef] [Green Version]
- Notaras, M.; van den Buuse, M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol. Psychiatry 2020, 25, 2251–2274. [Google Scholar] [CrossRef]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef]
- Suri, D.; Vaidya, V.A. Glucocorticoid regulation of brain-derived neurotrophic factor: Relevance to hippocampal structural and functional plasticity. Neuroscience 2013, 10, 1089–1093. [Google Scholar] [CrossRef]
- Gray, J.D.; Milner, T.A.; McEwen, B.S. Dynamic plasticity: The role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience 2013, 239, 214–227. [Google Scholar] [CrossRef] [Green Version]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef] [Green Version]
- Bremner, J.D.; Narayan, M.; Anderson, E.R.; Staib, L.H.; Miller, H.L.; Charney, D.S. Hippocampal volume reduction in major depression. Am. J. Psychiatry 2000. [Google Scholar] [CrossRef]
- Bremner, J.D.; Randall, P.; Vermetten, E.; Staib, L.; Bronen, R.A.; Mazure, C.; Capelli, S.; McCarthy, G.; Innis, R.B.; Charney, D.S. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—A preliminary report. Biol. Psychiatry 1997. [Google Scholar] [CrossRef]
- Rettew, D.C.; Pawlowski, S. Bullying. Child. Adolesc. Psychiatr. Clin. N. Am. 2016, 25, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Menesini, E.; Salmivalli, C. Bullying in schools: The state of knowledge and effective interventions. Psychol. Heal. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, G.L.; Parker, G.B.; Malhi, G.S. Do Bullied Children Become Anxious and Depressed Adults? J. Nerv. Ment. Dis. 2006, 194, 201–208. [Google Scholar] [CrossRef]
- Miczek, K.A. A new test for aggression in rats without aversive stimulation: Differential effects of d-amphetamine and cocaine. Psychopharmacology 1979, 60, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Björkqvist, K. Social defeat as a stressor in humans. Physiol. Behav. 2001, 73, 435–442. [Google Scholar] [CrossRef]
- Golden, S.A.; Covington, H.E.; Berton, O.; Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef]
- Andolina, D.; Puglisi-Allegra, S.; Ventura, R. Strain-dependent differences in corticolimbic processing of aversive or rewarding stimuli. Front. Syst. Neurosci. 2015, 8. [Google Scholar] [CrossRef] [Green Version]
- Daskalakis, N.P.; Binder, E.B. Schizophrenia in the Spectrum of Gene-Stress Interactions: The FKBP5 Example. Schizophr. Bull. 2015, 41, 323–329. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S. In pursuit of resilience: Stress, epigenetics, and brain plasticity. Ann. N. Y. Acad. Sci. 2016. [Google Scholar] [CrossRef]
- McEWEN, B.S. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Corniquel, M.B.; Koenigsberg, H.W.; Likhtik, E. Toward an animal model of borderline personality disorder. Psychopharmacology 2019, 236, 2485–2500. [Google Scholar] [CrossRef] [PubMed]
- Faraji, J.; Soltanpour, N.; Ambeskovic, M.; Zucchi, F.C.R.; Beaumier, P.; Kovalchuk, I.; Metz, G.A.S. Evidence for ancestral programming of resilience in a two-hit stress model. Front. Behav. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, R.A.; Kiss Von Soly, S.; Ratnayake, U.; Klug, M.; Binder, M.D.; Hannan, A.J.; van den Buuse, M. Long-term effects of combined neonatal and adolescent stress on brain-derived neurotrophic factor and dopamine receptor expression in the rat forebrain. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 2126–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peña, C.J.; Kronman, H.G.; Walker, D.M.; Cates, H.M.; Bagot, R.C.; Purushothaman, I.; Issler, O.; Eddie Loh, Y.H.; Leong, T.; Kiraly, D.D.; et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 2017, 356, 1185–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoory, M.; Cohen, H.; Richter-Levin, G. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur. Neuropsychopharmacol. 2007. [Google Scholar] [CrossRef]
- Zalosnik, M.I.; Pollano, A.; Trujillo, V.; Suárez, M.M.; Durando, P.E. Effect of maternal separation and chronic stress on hippocampal-dependent memory in young adult rats: Evidence for the match-mismatch hypothesis. Stress 2014. [Google Scholar] [CrossRef]
- Buwalda, B.; Stubbendorff, C.; Zickert, N.; Koolhaas, J.M. Adolescent social stress does not necessarily lead to a compromised adaptive capacity during adulthood: A study on the consequences of social stress in rats. Neuroscience 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohda, K.; Harada, K.; Kato, K.; Hoshino, A.; Motohashi, J.; Yamaji, T.; Morinobu, S.; Matsuoka, N.; Kato, N. Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single-prolonged stress rats: A putative post-traumatic stress disorder model. Neuroscience 2007, 148, 22–33. [Google Scholar] [CrossRef]
- Liberzon, I.; Krstov, M.; Young, E.A. Stress-restress: Effects on ACTH and fast feedback. Psychoneuroendocrinology 1997, 22, 443–453. [Google Scholar] [CrossRef]
- Liberzon, I.; López, J.F.; Flagel, S.B.; Vázquez, D.M.; Young, E.A. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: Relevance to post-traumatic stress disorder. J. Neuroendocrinol. 1999. [Google Scholar] [CrossRef] [PubMed]
- Mümüşoğlu, S.; Hacıvelioğlu, S.; Sökmensüer, L.K.; Karataylı, R.; Süzer, A.; Kaymaz, F. The comparison of the degree of apoptosis in ovaries and fallopian tubes between two different surgical interventions for tubal ligation: A rat model. J. Turkish Ger. Gynecol. Assoc. 2018, 19, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Bingham, B.; McFadden, K.; Zhang, X.; Bhatnagar, S.; Beck, S.; Valentino, R. Early adolescence as a critical window during which social stress distinctly alters behavior and brain norepinephrine activity. Neuropsychopharmacology 2011, 36, 896–909. [Google Scholar] [CrossRef]
- Ganon-Elazar, E.; Akirav, I. Cannabinoids prevent the development of behavioral and endocrine alterations in a rat model of intense stress. Neuropsychopharmacology 2012, 37, 456–466. [Google Scholar] [CrossRef]
- Mancini, G.F.; Marchetta, E.; Riccardi, E.; Trezza, V.; Morena, M.; Campolongo, P. Sex-divergent long-term effects of single prolonged stress in adult rats. Behav. Brain Res. 2020, 113096. [Google Scholar] [CrossRef]
- Colucci, P.; Marchetta, E.; Mancini, G.F.; Alva, P.; Chiarotti, F.; Hasan, M.T.; Campolongo, P. Predicting susceptibility and resilience in an animal model of post-traumatic stress disorder (PTSD). Transl. Psychiatry 2020, 10. [Google Scholar] [CrossRef]
- Scuderi, C.; Stecca, C.; Valenza, M.; Ratano, P.; Bronzuoli, M.R.; Bartoli, S.; Steardo, L.; Pompili, E.; Fumagalli, L.; Campolongo, P.; et al. Palmitoylethanolamide controls reactive gliosis and exerts neuroprotective functions in a rat model of Alzheimer’s disease. Cell Death Dis. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Morena, M.; De Castro, V.; Gray, J.M.; Palmery, M.; Trezza, V.; Roozendaal, B.; Hill, M.N.; Campolongo, P. Training-associated emotional arousal shapes endocannabinoid modulation of spatial memory retrieval in rats. J. Neurosci. 2015, 35, 13962–13974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Romaguera, J.; Sotres-Bayon, F.; Mueller, D.; Quirk, G.J. Systemic Propranolol Acts Centrally to Reduce Conditioned Fear in Rats Without Impairing Extinction. Biol. Psychiatry 2009, 65, 887–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atsak, P.; Hauer, D.; Campolongo, P.; Schelling, G.; Fornari, R.V.; Roozendaal, B. Endocannabinoid Signaling within the Basolateral Amygdala Integrates Multiple Stress Hormone Effects on Memory Consolidation. Neuropsychopharmacology 2015, 40, 1485–1494. [Google Scholar] [CrossRef]
- Blanchard, D.C.; Blanchard, R.J. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J. Comp. Physiol. Psychol. 1972, 81, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Ratano, P.; Petrella, C.; Forti, F.; Passeri, P.P.; Morena, M.; Palmery, M.; Trezza, V.; Severini, C.; Campolongo, P. Pharmacological inhibition of 2-arachidonoilglycerol hydrolysis enhances memory consolidation in rats through CB2 receptor activation and mTOR signaling modulation. Neuropharmacology 2018, 138, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Kanoski, S.E.; Meisel, R.L.; Mullins, A.J.; Davidson, T.L. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav. Brain Res. 2007. [Google Scholar] [CrossRef] [Green Version]
- Santori, A.; Colucci, P.; Mancini, G.F.; Morena, M.; Palmery, M.; Trezza, V.; Puglisi-Allegra, S.; Hill, M.N.; Campolongo, P. Anandamide modulation of circadian- and stress-dependent effects on rat short-term memory. Psychoneuroendocrinology 2019. [Google Scholar] [CrossRef]
- Fletcher, K.; Xiong, Y.; Fletcher, E.; Gustafsson, L. Glucocorticoid response to both predictable and unpredictable challenges detected as corticosterone metabolites in collared flycatcher droppings. PLoS ONE 2018. [Google Scholar] [CrossRef]
- McCormick, C.M.; Green, M.R. From the stressed adolescent to the anxious and depressed adult: Investigations in rodent models. Neuroscience 2013, 249, 242–257. [Google Scholar] [CrossRef]
- Buwalda, B.; Geerdink, M.; Vidal, J.; Koolhaas, J.M. Social behavior and social stress in adolescence: A focus on animal models. Neurosci. Biobehav. Rev. 2011, 35, 1713–1721. [Google Scholar] [CrossRef]
- Mukherjee, S.; Clouston, S.; Bromet, E.; Leibowitz, G.S.; Scott, S.B.; Bernard, K.; Kotov, R.; Luft, B. Past Experiences of Getting Bullied and Assaulted and Posttraumatic Stress Disorder (PTSD) after a Severe Traumatic Event in Adulthood: A Study of World Trade Center (WTC) Responders. J. Aggress. Maltreatment Trauma 2020. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.V. Animal models for depression and the mismatch hypothesis of disease. Psychoneuroendocrinology 2011. [Google Scholar] [CrossRef]
- Ellis, B.J.; Boyce, W.T.; Belsky, J.; Bakermans-Kranenburg, M.J.; Van Ijzendoorn, M.H. Differential susceptibility to the environment: An evolutionary- neurodevelopmental theory. Dev. Psychopathol. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selcher, J.C.; Nekrasova, T.; Paylor, R.; Landreth, G.E.; Sweatt, J.D. Mice lacking the ERK1 isoform of MAP kinase are unimpaired in emotional learning. Learn. Mem. 2001. [Google Scholar] [CrossRef] [Green Version]
- Shumyatsky, G.P.; Malleret, G.; Shin, R.M.; Takizawa, S.; Tully, K.; Tsvetkov, E.; Zakharenko, S.S.; Joseph, J.; Vronskaya, S.; Yin, D.Q.; et al. stathmin, a gene enriched in the amygdala, controls both learned and innate fear. Cell 2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehner, J.M.; Radcliffe, R.A. Cued and contextual fear conditioning in mice. Curr. Protoc. Neurosci. 2004. [Google Scholar] [CrossRef]
- Novick, A.M.; Mears, M.; Forster, G.L.; Lei, Y.; Tejani-Butt, S.M.; Watt, M.J. Adolescent social defeat alters N-methyl-d-aspartic acid receptor expression and impairs fear learning in adulthood. Behav. Brain Res. 2016, 304, 51–59. [Google Scholar] [CrossRef] [Green Version]
- MacKay, J.C.; Kent, P.; James, J.S.; Cayer, C.; Merali, Z. Ability of palatable food consumption to buffer against the short- and long-term behavioral consequences of social defeat exposure during juvenility in rats. Physiol. Behav. 2017. [Google Scholar] [CrossRef]
- Patki, G.; Solanki, N.; Atrooz, F.; Ansari, A.; Allam, F.; Jannise, B.; Maturi, J.; Salim, S. Novel mechanistic insights into treadmill exercise based rescue of social defeat-induced anxiety-like behavior and memory impairment in rats. Physiol. Behav. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patki, G.; Solanki, N.; Atrooz, F.; Allam, F.; Salim, S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013. [Google Scholar] [CrossRef] [Green Version]
- Riga, D.; Theijs, J.T.; De Vries, T.J.; Smit, A.B.; Spijker, S. Social defeat-induced anhedonia: Effects on operant sucrose-seeking behavior. Front. Behav. Neurosci. 2015. [Google Scholar] [CrossRef]
- Verbitsky, A.; Dopfel, D.; Zhang, N. Rodent models of post-traumatic stress disorder: Behavioral assessment. Transl. Psychiatry 2020, 10, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Lisieski, M.J.; Eagle, A.L.; Conti, A.C.; Liberzon, I.; Perrine, S.A. Single-prolonged stress: A review of two decades of progress in a rodent model of post-traumatic stress disorder. Front. Psychiatry 2018, 9, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, R.R.; Noble, L.J.; McIntyre, C.K. Using the single prolonged stress model to examine the pathophysiology of PTSD. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Morinobu, S.; Takahashi, T.; Yamawaki, S. Single prolonged stress increases contextual freezing and the expression of glycine transporter 1 and vesicle-associated membrane protein 2 mRNA in the hippocampus of rats. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2007, 31, 642–651. [Google Scholar] [CrossRef]
- Knox, D.; George, S.A.; Fitzpatrick, C.J.; Rabinak, C.A.; Maren, S.; Liberzon, I. Single prolonged stress disrupts retention of extinguished fear in rats. Learn. Mem. 2012, 19, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Tian, Q.; Li, F.; Gao, J.; Liu, Y.; Mao, M.; Liu, J.; Wang, S.; Li, G.; Ge, D.; et al. Behavioral changes over time in post-traumatic stress disorder: Insights from a rat model of single prolonged stress. Behav. Processes 2016, 124, 123–129. [Google Scholar] [CrossRef]
- Jovanovic, T.; Blanding, N.Q.; Norrholm, S.D.; Duncan, E.; Bradley, B.; Ressler, K.J. Childhood abuse is associated with increased startle reactivity in adulthood. Depress. Anxiety 2009, 26, 1018–1026. [Google Scholar] [CrossRef]
- Jovanovic, T.; Norrholm, S.D.; Fennell, J.E.; Keyes, M.; Fiallos, A.M.; Myers, K.M.; Davis, M.; Duncan, E.J. Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Res. 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant, R.A. Post-traumatic stress disorder: A state-of-the-art review of evidence and challenges. World Psychiatry 2019. [Google Scholar] [CrossRef] [Green Version]
- Vollmayr, B.; Faust, H.; Lewicka, S.; Henn, F.A. Brain-derived-neurotrophic-factor (BDNF) stress response in rats bred for learned helplessness. Mol. Psychiatry 2001. [Google Scholar] [CrossRef] [Green Version]
- Coppens, C.M.; Sipornmongcolchai, T.; Wibrand, K.; Alme, M.N.; Buwalda, B.; de Boer, S.F.; Koolhaas, J.M.; Bramham, C.R. Social defeat during adolescence and adulthood differentially induce BDNF-regulated immediate early genes. Front. Behav. Neurosci. 2011. [Google Scholar] [CrossRef] [Green Version]
- Toth, E.; Gersner, R.; Wilf-Yarkoni, A.; Raizel, H.; Dar, D.E.; Richter-Levin, G.; Levit, O.; Zangen, A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J. Neurochem. 2008, 107, 522–532. [Google Scholar] [CrossRef]
- Montaron, M.F.; Piazza, P.V.; Aurousseau, C.; Urani, A.; Le Moal, M.; Abrous, D.N. Implication of corticosteroid receptors in the regulation of hippocampal structural plasticity. Eur. J. Neurosci. 2003. [Google Scholar] [CrossRef]
- Nacher, J.; Lanuza, E.; McEwen, B.S. Distribution of PSA-NCAM expression in the amygdala of the adult rat. Neuroscience 2002. [Google Scholar] [CrossRef]
- Tzanoulinou, S.; Gantelet, E.; Sandi, C.; Márquez, C. Programming effects of peripubertal stress on spatial learning. Neurobiol. Stress 2020. [Google Scholar] [CrossRef] [PubMed]
- Tsoory, M.; Guterman, A.; Richter-Levin, G. Exposure to stressors during juvenility disrupts development-related alterations in the PSA-NCAM to NCAM expression ratio: Potential relevance for mood and anxiety disorders. Neuropsychopharmacology 2008. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Afonso, P.M.; Salazar, I.L.; Duarte, C.B. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015, 1621, 82–101. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Cory, S.; Kidane, A.H.; Shirkey, N.J.; Marshak, S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev. Neurobiol. 2010, 70, 271–288. [Google Scholar] [CrossRef] [Green Version]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Liu, Q.; Guo, B.; Ye, F.; Ge, J.; Xue, L. BDNF activates postsynaptic TrkB receptors to induce endocannabinoid release and inhibit presynaptic calcium influx at a calyx-type synapse. J. Neurosci. 2020. [Google Scholar] [CrossRef]
- Daskalakis, N.P.; De Kloet, E.R.; Yehuda, R.; Malaspina, D.; Kranz, T.M. Early life stress effects on glucocorticoid—BDNF interplay in the hippocampus. Front. Mol. Neurosci. 2015, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santa Ana, E.J.; Saladin, M.E.; Back, S.E.; Waldrop, A.E.; Spratt, E.G.; McRae, A.L.; LaRowe, S.D.; Timmerman, M.A.; Upadhyaya, H.; Brady, K.T. PTSD and the HPA axis: Differences in response to the cold pressor task among individuals with child vs. adult trauma. Psychoneuroendocrinology 2006. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Poon, L.; Papadopoulos, A.S.; Kumari, V.; Cleare, A.J. Long term effects of childhood trauma on cortisol stress reactivity in adulthood and relationship to the occurrence of depression. Psychoneuroendocrinology 2014. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, G.F.; Marchetta, E.; Pignani, I.; Trezza, V.; Campolongo, P. Social Defeat Stress during Early Adolescence Confers Resilience against a Single Episode of Prolonged Stress in Adult Rats. Cells 2021, 10, 360. https://doi.org/10.3390/cells10020360
Mancini GF, Marchetta E, Pignani I, Trezza V, Campolongo P. Social Defeat Stress during Early Adolescence Confers Resilience against a Single Episode of Prolonged Stress in Adult Rats. Cells. 2021; 10(2):360. https://doi.org/10.3390/cells10020360
Chicago/Turabian StyleMancini, Giulia Federica, Enrico Marchetta, Irene Pignani, Viviana Trezza, and Patrizia Campolongo. 2021. "Social Defeat Stress during Early Adolescence Confers Resilience against a Single Episode of Prolonged Stress in Adult Rats" Cells 10, no. 2: 360. https://doi.org/10.3390/cells10020360
APA StyleMancini, G. F., Marchetta, E., Pignani, I., Trezza, V., & Campolongo, P. (2021). Social Defeat Stress during Early Adolescence Confers Resilience against a Single Episode of Prolonged Stress in Adult Rats. Cells, 10(2), 360. https://doi.org/10.3390/cells10020360






