Upregulation of the Renin–Angiotensin System Pathways and SARS-CoV-2 Infection: The Rationale for the Administration of Zinc-Chelating Agents in COVID-19 Patients
Abstract
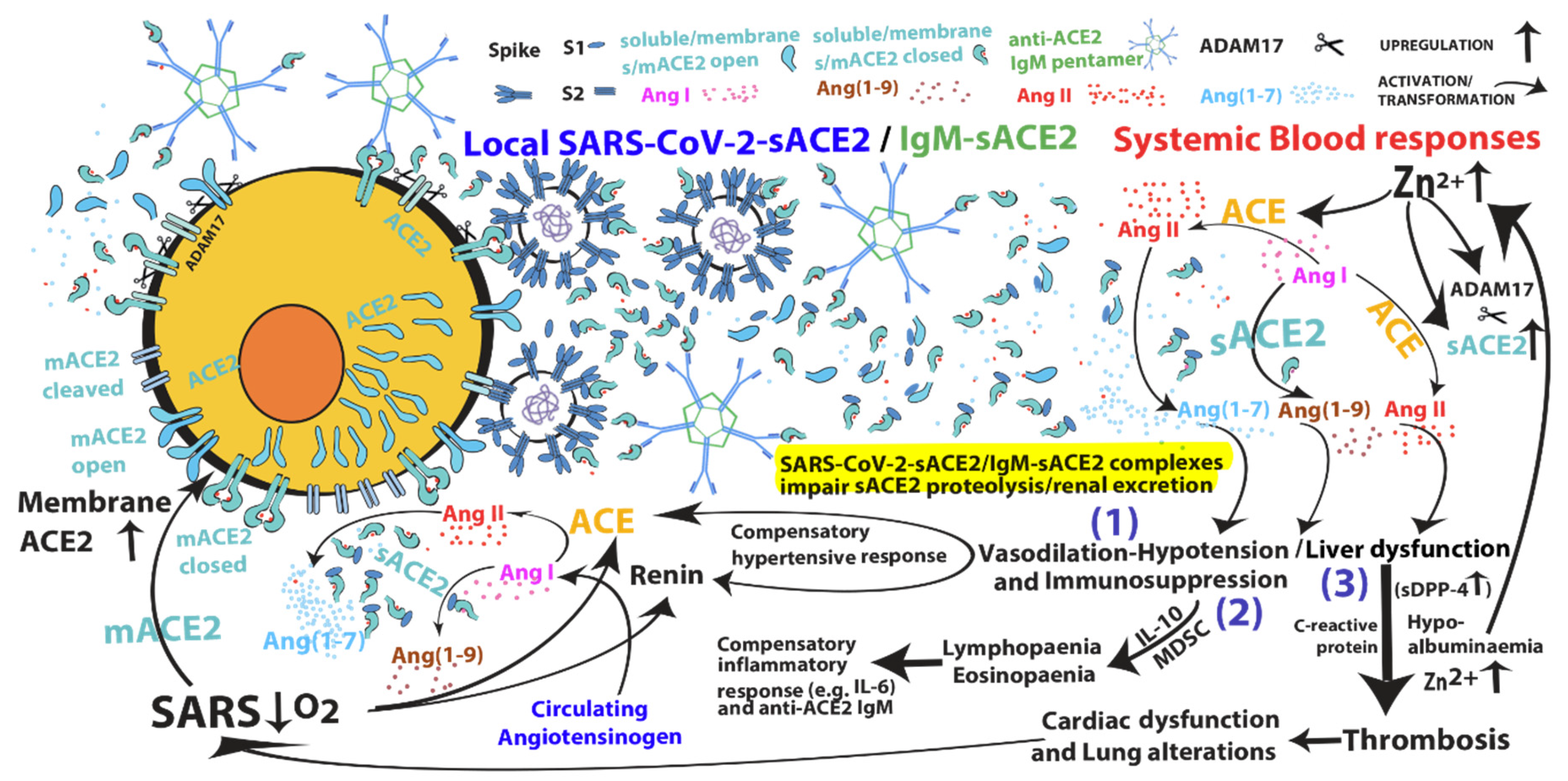
:1. Introduction
2. Anti-SARS-CoV-2 Bismuth-Based Drugs
3. Crucial Role of IL-10 in COVID-19 Pathogenesis
4. Targeting of SARS-CoV-2-Upregulated Zinc-Dependent Pathways
5. Efficacy and Limits of Convalescent Plasma Transfusion and Auto-Immune Complications
6. SARS-CoV-1/2 and MERS-CoV Severe Infections: Is It a Matter of the Liver?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, S.; Zhang, X.; Yang, Y.; Hotez, P.J.; Du, L. Neutralizing antibodies for the treatment of COVID-19. Nat. Biomed. Eng. 2020, 4, 1134–1139. [Google Scholar] [CrossRef]
- Pizza, M.; Sala, C.; Andreano, E.; Pantano, E.; Rappuoli, R.; Troisi, M. Antibodies, epicenter of SARS-CoV-2 immunology. Cell Death Differ. 2021, 28, 821–824. [Google Scholar]
- Zamai, L. The Yin and Yang of ACE/ACE2 Pathways: The Rationale for the Use of Renin-Angiotensin System Inhibitors in COVID-19 Patients. Cells 2020, 9, 1704. [Google Scholar] [CrossRef]
- Perotti, C.; Baldanti, F.; Bruno, R.; Del Fante, C.; Seminari, E.; Casari, S.; Percivalle, E.; Glingani, C.; Musella, V.; Belliato, M.; et al. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica 2020, 105, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Wheatley, A.K.; Kent, S.J.; DeKosky, B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 2020, 5, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 11, 585354. [Google Scholar] [CrossRef] [PubMed]
- Sama, I.E.; Ravera, A.; Santema, B.T.; Van Goor, H.; Ter Maaten, J.M.; Cleland, J.G.F.; Rienstra, M.; Friedrich, A.W.; Samani, N.J.; Ng, L.L.; et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur. Heart J. 2020, 41, 1810–1817. [Google Scholar] [CrossRef]
- Wallentin, L.; Lindbäck, J.; Eriksson, N.; Hijazi, Z.; Eikelboom, J.W.; Ezekowitz, M.D.; Granger, C.B.; Lopes, R.D.; Yusuf, S.; Oldgren, J.; et al. Angiotensin-converting enzyme 2 (ACE2) levels in relation to risk factors for COVID-19 in two large cohorts of patients with atrial fibrillation. Eur. Heart J. 2020, 41, 4037–4046. [Google Scholar] [CrossRef]
- McMillan, P.; Uhal, B.D. COVID-19-A theory of autoimmunity to ACE-2. MOJ Immunol. 2020, 7, 17–19. [Google Scholar]
- Yuan, S.; Wang, R.; Chan, J.F.W.; Zhang, A.J.; Cheng, T.; Chik, K.K.H.; Ye, Z.W.; Wang, S.; Lee, A.C.Y.; Jin, L.; et al. Metallodrug ranitidine bismuth citrate suppresses SARS-CoV-2 replication and relieves virus-associated pneumonia in Syrian hamsters. Nat. Microbiol. 2020, 5, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Tanner, J.A.; Zheng, B.J.; Watt, R.M.; He, M.L.; Lu, L.Y.; Jiang, J.Q.; Shum, K.T.; Lin, Y.P.; Wong, K.L.; et al. Bismuth complexes inhibit the SARS coronavirus. Angew. Chem. Int. Ed. 2007, 46, 6464–6468. [Google Scholar] [CrossRef]
- Jin, L.; Szeto, K.Y.; Zhang, L.; Du, W.; Sun, H. Inhibition of alcohol dehydrogenase by bismuth. J. Inorg. Biochem. 2004, 98, 1331–1337. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Cui, Y.; Zhu, Y. Recombinant human ACE2: Potential therapeutics of SARS-CoV-2 infection and its complication. Acta Pharmacol. Sin. 2020, 41, 1255–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; Niu, S. ACE2 and COVID-19 and the resulting ARDS. Postgrad. Med. J. 2020, 96, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Pucci, F.; Bogaerts, P.; Rooman, M. Modeling the Molecular Impact of SARS-CoV-2 Infection on the Renin-Angiotensin System. Viruses 2020, 12, 1367. [Google Scholar] [CrossRef] [PubMed]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913. [Google Scholar] [CrossRef]
- Zoufaly, A.; Poglitsch, M.; Aberle, J.H.; Hoepler, W.; Seitz, T.; Traugott, M.; Grieb, A.; Pawelka, E.; Laferl, H.; Wenisch, C.; et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020, 8, 1154–1158. [Google Scholar] [CrossRef]
- Anguiano, L.; Riera, M.; Pascual, J.; Valdivielso, J.M.; Barrios, C.; Betriu, A.; Mojal, S.; Fernández, E.; Soler, M.J.; Faura, A.; et al. Circulating angiotensin-converting enzyme 2 activity in patients with chronic kidney disease without previous history of cardiovascular disease. Nephrol. Dial. Transplant. 2015, 30, 1176–1185. [Google Scholar] [CrossRef] [Green Version]
- Epelman, S.; Shrestha, K.; Troughton, R.W.; Francis, G.S.; Sen, S.; Klein, A.L.; Wilson Tang, W.H. Soluble Angiotensin-Converting Enzyme 2 in Human Heart Failure: Relation With Myocardial Function and Clinical Outcomes. J. Card. Fail. 2009, 15, 565–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Pérez, J.T.; Riera, M.; Bosch, X.; De Caralt, T.M.; Perea, R.J.; Pascual, J.; Soler, M.J. Role of Circulating Angiotensin Converting Enzyme 2 in Left Ventricular Remodeling following Myocardial Infarction: A Prospective Controlled Study. PLoS ONE 2013, 8, e61695. [Google Scholar] [CrossRef] [Green Version]
- Ramchand, J.; Patel, S.K.; Srivastava, P.M.; Farouque, O.; Burrell, L.M. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS ONE 2018, 13, e0198144. [Google Scholar] [CrossRef] [PubMed]
- Walters, T.E.; Kalman, J.M.; Patel, S.K.; Mearns, M.; Velkoska, E.; Burrell, L.M. Angiotensin converting enzyme 2 activity and human atrial fibrillation: Increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace 2017, 19, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Úri, K.; Fagyas, M.; Siket, I.M.; Kertész, A.; Csanádi, Z.; Sándorfi, G.; Clemens, M.; Fedor, R.; Papp, Z.; Édes, I.; et al. New perspectives in the renin-angiotensin-aldosterone system (RAAS) IV: Circulating ACE2 as a biomarker of systolic dysfunction in human hypertension and heart failure. PLoS ONE 2014, 9, e0198144. [Google Scholar] [CrossRef] [PubMed]
- Uri, K.; Fagyas, M.; Kertesz, A.; Borbely, A.; Jenei, C.; Bene, O.; Csanadi, Z.; Paulus, W.J.; Edes, I.; Papp, Z.; et al. Circulating ACE2 activity correlates with cardiovascular disease development. J. Renin Angiotensin Aldosterone Syst. 2016, 17, 1470320316668435. [Google Scholar] [CrossRef] [Green Version]
- Soro-Paavonen, A.; Gordin, D.; Forsblom, C.; Rosengard-Barlund, M.; Waden, J.; Thorn, L.; Sandholm, N.; Thomas, M.C.; Groop, P.H. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J. Hypertens. 2012, 30, 375–383. [Google Scholar] [CrossRef]
- Haga, S.; Yamamoto, N.; Nakai-Murakami, C.; Osawa, Y.; Tokunaga, K.; Sata, T.; Yamamoto, N.; Sasazuki, T.; Ishizaka, Y. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc. Natl. Acad. Sci. USA 2008, 105, 7809–7814. [Google Scholar] [CrossRef] [Green Version]
- Glowacka, I.; Bertram, S.; Herzog, P.; Pfefferle, S.; Steffen, I.; Muench, M.O.; Simmons, G.; Hofmann, H.; Kuri, T.; Weber, F.; et al. Differential Downregulation of ACE2 by the Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus and Human Coronavirus NL63. J. Virol. 2010, 84, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Li, W.; Peng, G.; Li, F. Crystal structure of NL63 respiratory coronavirus receptor-binding domain complexed with its human receptor. Proc. Natl. Acad. Sci. USA 2009, 106, 19970–19974. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.P.; Look, D.C.; Tan, P.; Shi, L.; Hickey, M.; Gakhar, L.; Chappell, M.C.; Wohlford-Lenane, C.; McCray, P.B. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, C.; Sui, J.; Kuhn, J.H.; Moore, M.J.; Luo, S.; Wong, S.K.; Huang, I.C.; Xu, K.; Vasilieva, N.; et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005, 24, 1634–1643. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.K.; Juno, J.A.; Lee, W.S.; Wragg, K.M.; Hogarth, P.M.; Kent, S.J.; Burrell, L.M. Plasma ACE2 activity is persistently elevated following SARS-CoV-2 infection: Implications for COVID-19 pathogenesis and consequences. medRxiv 2020. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Laing, A.G.; Lorenc, A.; del Molino del Barrio, I.; Das, A.; Fish, M.; Monin, L.; Muñoz-Ruiz, M.; McKenzie, D.R.; Hayday, T.S.; Francos-Quijorna, I.; et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 2020, 26, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Ansari, P.; Sakatsume, M.; Dickensheets, H.; Vazquez, N.; Donnelly, R.P.; Larner, A.C.; Finbloom, D.S. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma-induced genes by suppressing tyrosine phosphorylation of STAT1. Blood 1999, 93, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.M.; Abuharfeil, N.M.; Darmani, H.; Daoud, A. Mechanisms of immune suppression by myeloid-derived suppressor cells: The role of interleukin-10 as a key immunoregulatory cytokine. Open Biol. 2020, 10, 200111. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, H.; Dauphars, D.J.; He, Y.W. A Potential Role of Interleukin 10 in COVID-19 Pathogenesis. Trends Immunol. 2021, 42, 3–5. [Google Scholar] [CrossRef]
- Lauw, F.N.; Pajkrt, D.; Hack, C.E.; Kurimoto, M.; van Deventer, S.J.H.; van der Poll, T. Proinflammatory Effects of IL-10 During Human Endotoxemia. J. Immunol. 2000, 165, 2783–2789. [Google Scholar] [CrossRef]
- Agrati, C.; Sacchi, A.; Bordoni, V.; Cimini, E.; Notari, S.; Grassi, G.; Casetti, R.; Tartaglia, E.; Lalle, E.; D’Abramo, A.; et al. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19). Cell Death Differ. 2020, 27, 3196–3207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, L.; Zhang, P.; Li, K.; Liang, L.; Sun, J.; Xu, B.; Dai, Y.; Li, X.; Zhang, C.; et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight 2020, 5, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G. Hypoalbuminemia: An underestimated, vital characteristic of hospitalized COVID-19 positive patients? Hepatoma Res. 2020, 6, 28. [Google Scholar] [CrossRef]
- Derwand, R.; Scholz, M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win todays battle against COVID-19? Med. Hypotheses 2020, 142, 109815. [Google Scholar] [CrossRef]
- Skalny, A.V.; Rink, L.; Ajsuvakova, O.P.; Aschner, M.; Gritsenko, V.A.; Alekseenko, S.I.; Svistunov, A.A.; Petrakis, D.; Spandidos, D.A.; Aaseth, J.; et al. Zinc and respiratory tract infections: Perspectives for COVID-19 (Review). Int. J. Mol. Med. 2020, 46, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Arentz, S.; Hunter, J.; Yang, G.; Goldenberg, J.; Beardsley, J.; Myers, S.P.; Mertz, D.; Leeder, S. Zinc for the prevention and treatment of SARS-CoV-2 and other acute viral respiratory infections: A rapid review. Adv. Integr. Med. 2020, 7, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Squitti, R.; Picozza, M.; Pawar, A.; Rongioletti, M.; Dutta, A.K.; Sahoo, S.; Goswami, K.; Sharma, P.; Prasad, R. Zinc and COVID-19: Basis of Current Clinical Trials. Biol. Trace Elem. Res. 2020, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Joachimiak, M.P. Zinc against COVID-19? Symptom surveillance and deficiency risk groups. PLoS Negl. Trop. Dis. 2021, 15, e0008895. [Google Scholar] [CrossRef]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection. JAMA Netw. Open 2021, 4, e210369. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Hajo, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [Green Version]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Asadzadeh, F.; Maleki-Kaklar, M.; Soiltanalinejad, N.; Shabani, F. Central composite design optimization of zinc removal from contaminated soil, using citric acid as biodegradable chelant. Sci. Rep. 2018, 8, 2633. [Google Scholar] [CrossRef] [Green Version]
- Córdova, A.; Navas, F.J. Effect of Training on Zinc Metabolism: Changes in Serum and Sweat Zinc Concentrations in Sportsmen. Ann. Nutr. Metab. 1998, 42, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.W.; Anderson, R.A. Effects of Aerobic Exercise and Training on the Trace Minerals Chromium, Zinc and Copper. Sport. Med. Int. J. Appl. Med. Sci. Sport Exerc. 1987, 4, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Coverdale, J.P.C.; Khazaipoul, S.; Arya, S.; Stewart, A.J.; Blindauer, C.A. Crosstalk between zinc and free fatty acids in plasma. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Riccelli, M.G.; Goldoni, M.; Poli, D.; Mozzoni, P.; Cavallo, D.; Corradi, M. Welding fumes, a risk factor for lung diseases. Int. J. Environ. Res. Public Health 2020, 17, 2552. [Google Scholar] [CrossRef] [Green Version]
- Bleidorn, J.; Alamzad-Krabbe, H.; Gerhards, B.; Kraus, T.; Brand, P.; Krabbe, J.; Martin, C. The pro-inflammatory stimulus of zinc- and copper-containing welding fumes in whole blood assay via protein tyrosine phosphatase 1B inhibition. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, F.; Zhang, X.; Xie, L. Prognostic value of serum zinc levels in patients with acute HC/zinc chloride smoke inhalation. Medicine 2017, 96, e8156. [Google Scholar] [CrossRef] [PubMed]
- Hjortsø, E.; Ovist, J.; Bud, M.I.; Thomsen, J.L.; Andersen, J.B.; Wiberg-Jørgensen, F.; Jensen, N.K.; Jones, R.; Reid, L.M.; Zapol, W.M. ARDS after accidental inhalation of zinc chloride smoke. Intensive Care Med. 1988, 14, 17–24. [Google Scholar] [CrossRef]
- Towler, P.; Staker, B.; Prasad, S.G.; Menon, S.; Tang, J.; Parsons, T.; Ryan, D.; Fisher, M.; Williams, D.; Dales, N.A.; et al. ACE2 X-Ray Structures Reveal a Large Hinge-bending Motion Important for Inhibitor Binding and Catalysis. J. Biol. Chem. 2004, 279, 17996–18007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi-Alijanvand, H.; Rouhani, M. Studying the Effects of ACE2 Mutations on the Stability, Dynamics, and Dissociation Process of SARS-CoV-2 S1/hACE2 Complexes. J. Proteome Res. 2020, 19, 4609–4623. [Google Scholar] [CrossRef] [PubMed]
- Fernández, D.; Boix, E.; Pallarès, I.; Avilés, F.X.; Vendrell, J. Structural and functional analysis of the complex between citrate and the zinc peptidase carboxypeptidase A. Enzym. Res. 2011, 2011, 128676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sul, J.W.; Kim, T.Y.; Yoo, H.J.; Kim, J.; Suh, Y.A.; Hwang, J.J.; Koh, J.Y. A novel mechanism for the pyruvate protection against zinc-induced cytotoxicity: Mediation by the chelating effect of citrate and isocitrate. Arch. Pharm. Res. 2016, 39, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Kiran Gotru, S.; van Geffen, J.P.; Nagy, M.; Mammadova-Bach, E.; Eilenberger, J.; Volz, J.; Manukjan, G.; Schulze, H.; Wagner, L.; Eber, S.; et al. Defective Zn2+ homeostasis in mouse and human platelets with α- and δ-storage pool diseases. Sci. Rep. 2019, 9, 8333. [Google Scholar] [CrossRef]
- Belizna, C.; Pregnolato, F.; Abad, S.; Alijotas-Reig, J.; Amital, H.; Amoura, Z.; Andreoli, L.; Andres, E.; Aouba, A.; Apras Bilgen, S.; et al. HIBISCUS: Hydroxychloroquine for the secondary prevention of thrombotic and obstetrical events in primary antiphospholipid syndrome. Autoimmun. Rev. 2018, 17, 1153–1168. [Google Scholar] [CrossRef]
- Xue, J.; Moyer, A.; Peng, B.; Wu, J.; Hannafon, B.N.; Ding, W.Q. Chloroquine is a zinc ionophore. PLoS ONE 2014, 9, e109180. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.K.; Desikan, R.; Dixit, N.M. Preferential Presentation of High-Affinity Immune Complexes in Germinal Centers Can Explain How Passive Immunization Improves the Humoral Response. Cell Rep. 2019, 29, 3946–3957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody Responses to SARS-CoV-2 in Patients with Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Liu, F.; Zheng, N.S.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse Functional Autoantibodies in Patients with COVID-19. medRxiv 2020. Version Posted 19 December 2020. [Google Scholar] [CrossRef]
- Casciola-Rosen, L.; Thiemann, D.R.; Andrade, F.; Zambrano, M.I.T.; Hooper, J.E.; Leonard, E.K.; Spangler, J.B.; Cox, A.L.; Machamer, C.E.; Sauer, L.; et al. IgM autoantibodies recognizing ACE2 are associated with severe COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Stork, A.C.J.; Rijkers, G.T.; Vlam, L.; Cats, E.A.; de Jong, B.A.W.; Fritsch-Stork, R.D.E.; Veldink, J.H.; van den Berg, L.H.; Notermans, N.C.; van der Pol, W.L. Serum cytokine patterns in immunoglobulin m monoclonal gammopathy-associated polyneuropathy. Muscle Nerve 2019, 59, 694–698. [Google Scholar] [CrossRef]
- Amiral, J. Can COVID-19 Induce an Autoimmune Disease Associated with Long-Lasting Symptoms and Delayed Complications? Ann. Clin. Immunol. Microbiol. 2020, 2, 1014. [Google Scholar]
- Simonovich, V.A.; Burgos Pratx, L.D.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vázquez, C.; Savoy, N.; Giunta, D.H.; Pérez, L.G.; Sánchez, M. del L.; et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N. Engl. J. Med. 2020, 384, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, C.; Bayry, J. Autoimmune and inflammatory diseases following COVID-19. Nat. Rev. Rheumatol. 2020, 16, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Zamai, L. Unveiling Human Non-Random Genome Editing Mechanisms Activated in Response to Chronic Environmental Changes: I. Where Might These Mechanisms Come from and What Might They Have Led To? Cells 2020, 9, 2362. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Z.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Bigio, B.; Yang, R.; Arias, A.A.; Zhou, Q.; Han, J.E.; et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020, 370, eabd4570. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, E.C. The type I interferon response in COVID-19: Implications for treatment. Nat. Rev. Immunol. 2020, 20, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Park, G.E.; Lee, J.Y.; Lee, J.Y.; Cho, S.Y.; Ha, Y.E.; Kang, C.I.; Kang, J.M.; Kim, Y.J.; Huh, H.J.; et al. Predictive factors for pneumonia development and progression to respiratory failure in MERS-CoV infected patients. J. Infect. 2016, 73, 468–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, M.; Omrani, A.S.; Baig, K.; Bahloul, A.; Elzein, F.; Matin, M.A.; Selim, M.A.A.; Al Mutairi, M.; Al Nakhli, D.; Aidaroos, A.Y.A.; et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: A single-center experience in Saudi Arabia. Int. J. Infect. Dis. 2014, 29, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS: Are they closely related? Clin. Microbiol. Infect. 2020, 26, 729–734. [Google Scholar] [CrossRef]
- Zanza, C.; Racca, F.; Longhitano, Y.; Piccioni, A.; Franceschi, F.; Artico, M.; Abenavoli, L.; Maiese, A.; Passaro, G.; Volonnino, G.; et al. Risk management and treatment of coagulation disorders related to COVID-19 infection. Int. J. Environ. Res. Public Health 2021, 18, 1268. [Google Scholar] [CrossRef]
- Itou, M.; Kawaguchi, T.; Taniguchi, E.; Sata, M. Dipeptidyl peptidase-4: A key player in chronic liver disease. World J. Gastroenterol. 2013, 19, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Röhrborn, D.; Eckel, J.; Sell, H. Shedding of dipeptidyl peptidase 4 is mediated by metalloproteases and up-regulated by hypoxia in human adipocytes and smooth muscle cells. FEBS Lett. 2014, 588, 3870–3877. [Google Scholar] [CrossRef] [Green Version]
- Duvnjak, L.; Perković, M.N.; Blaslov, K. Dipeptidyl peptidase-4 activity is associated with urine albumin excretion in type 1 diabetes. J. Diabetes Complicat. 2017, 31, 218–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Or, D.; Slone, D.S.; Mains, C.W.; Rael, L.T. Dipeptidyl peptidase IV activity in commercial solutions of human serum albumin. Anal. Biochem. 2013, 441, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Naicker, S.; Yang, C.W.; Hwang, S.J.; Liu, B.C.; Chen, J.H.; Jha, V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020, 97, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Solerte, S.B.; D’Addio, F.; Trevisan, R.; Lovati, E.; Rossi, A.; Pastore, I.; Dell’Acqua, M.; Ippolito, E.; Scaranna, C.; Bellante, R.; et al. Sitagliptin Treatment at the Time of Hospitalization Was Associated With Reduced Mortality in Patients With Type 2 Diabetes and COVID-19: A Multicenter, Case-Control, Retrospective, Observational Study. Diabetes Care 2020, 57, 779–783. [Google Scholar]
- Takahashi, Y.; Haga, S.; Ishizaka, Y.; Mimori, A. Autoantibodies to angiotensin-converting enzyme 2 in patients with connective tissue diseases. Arthritis Res. Ther. 2010, 12, R85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattle, D.; Hess, B. Preventive treatment of nephrolithiasis with alkali citrate—A critical review. Urol. Res. 2005, 33, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegmüller, R.; Tay, F.; Zeder, C.; Brnić, M.; Hurrell, R.F. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J. Nutr. 2014, 144, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Longhitano, Y.; Racca, F.; Zanza, C.; Muncinelli, M.; Guagliano, A.; Peretti, E.; Minerba, A.C.; Mari, M.; Boverio, R.; Salio, M.; et al. Venous thrombo-embolism in hospitalized sars-cov-2 patients treated with three different anticoagulation protocols: Prospective observational study. Biology 2020, 9, 310. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamai, L. Upregulation of the Renin–Angiotensin System Pathways and SARS-CoV-2 Infection: The Rationale for the Administration of Zinc-Chelating Agents in COVID-19 Patients. Cells 2021, 10, 506. https://doi.org/10.3390/cells10030506
Zamai L. Upregulation of the Renin–Angiotensin System Pathways and SARS-CoV-2 Infection: The Rationale for the Administration of Zinc-Chelating Agents in COVID-19 Patients. Cells. 2021; 10(3):506. https://doi.org/10.3390/cells10030506
Chicago/Turabian StyleZamai, Loris. 2021. "Upregulation of the Renin–Angiotensin System Pathways and SARS-CoV-2 Infection: The Rationale for the Administration of Zinc-Chelating Agents in COVID-19 Patients" Cells 10, no. 3: 506. https://doi.org/10.3390/cells10030506
APA StyleZamai, L. (2021). Upregulation of the Renin–Angiotensin System Pathways and SARS-CoV-2 Infection: The Rationale for the Administration of Zinc-Chelating Agents in COVID-19 Patients. Cells, 10(3), 506. https://doi.org/10.3390/cells10030506





