Short-Term Amygdala Low-Frequency Stimulation Does not Influence Hippocampal Interneuron Changes Observed in the Pilocarpine Model of Epilepsy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Lithium-Pilocarpine Model
2.3. Electrode Implantation Protocol
2.4. Stimulation Protocol
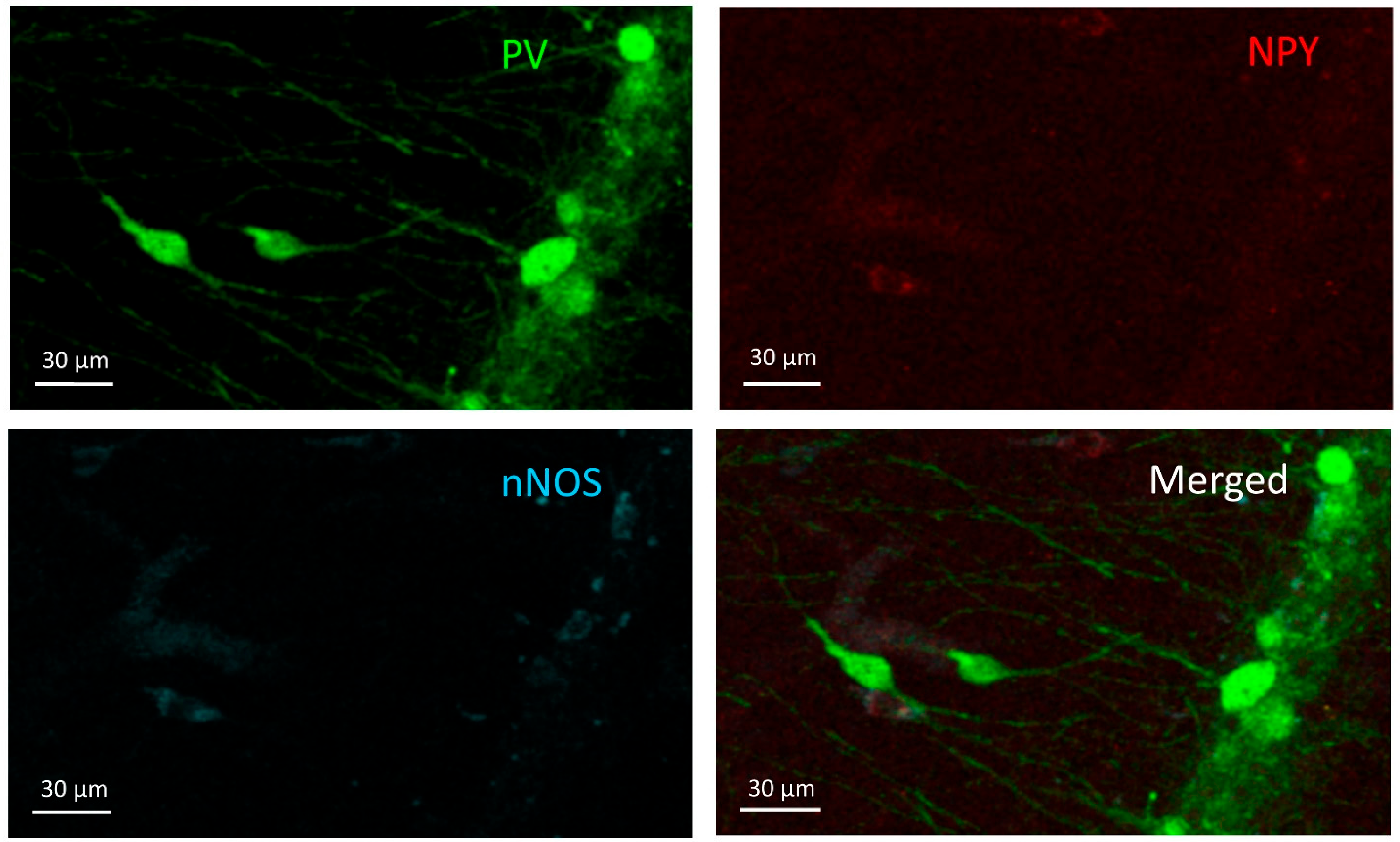
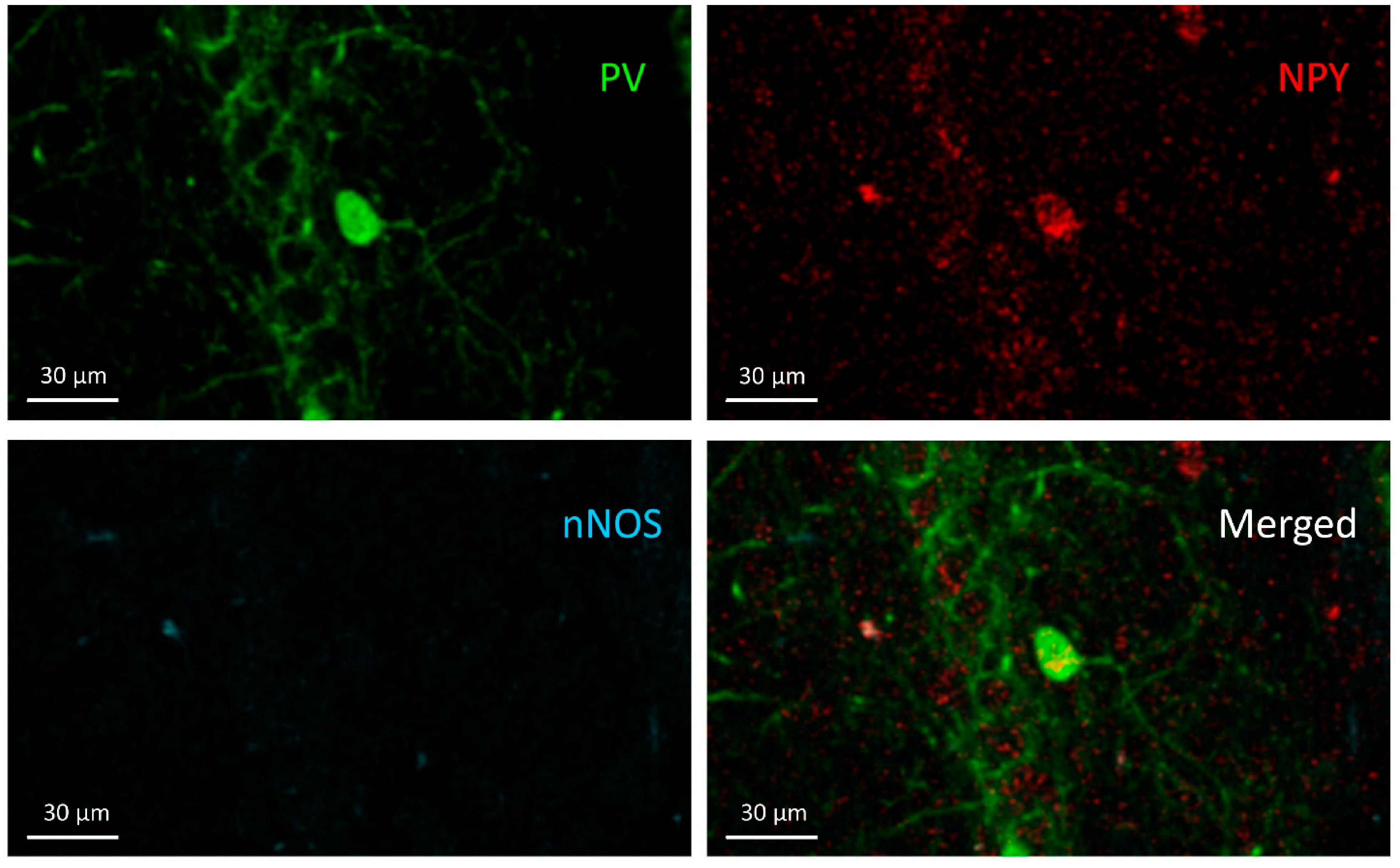
2.5. Histology and Immunocytochemistry
2.6. Statistics
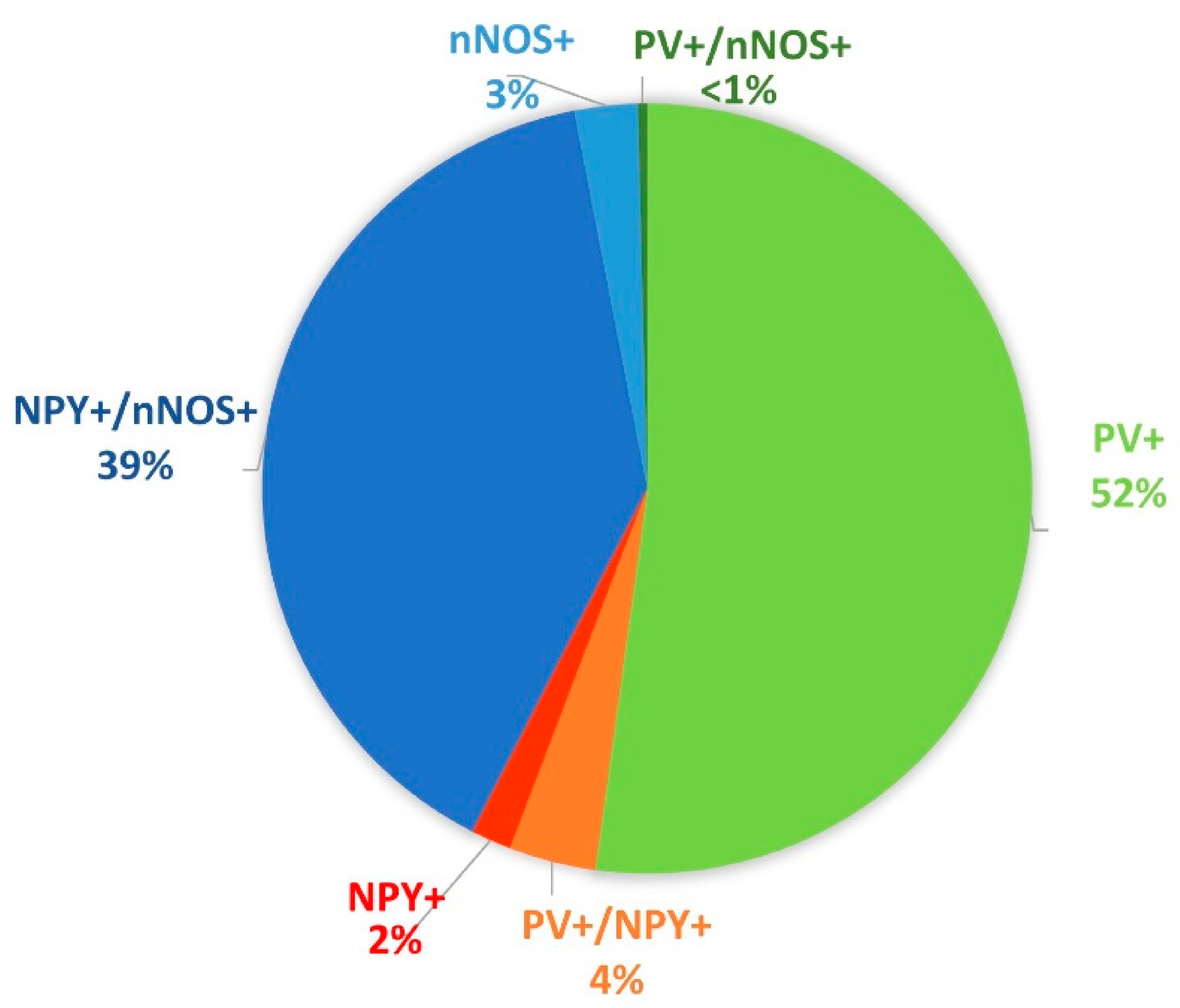
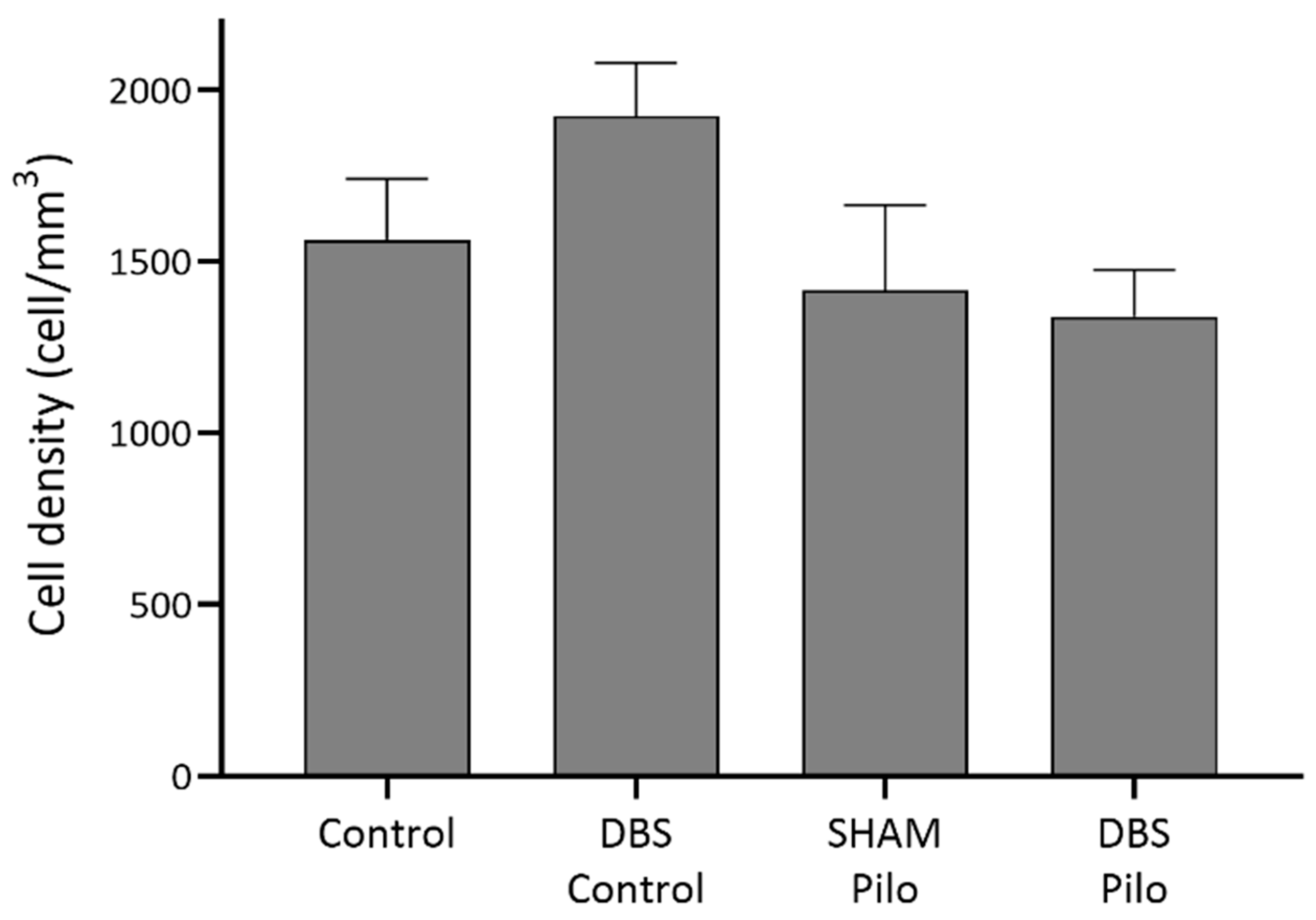
3. Results
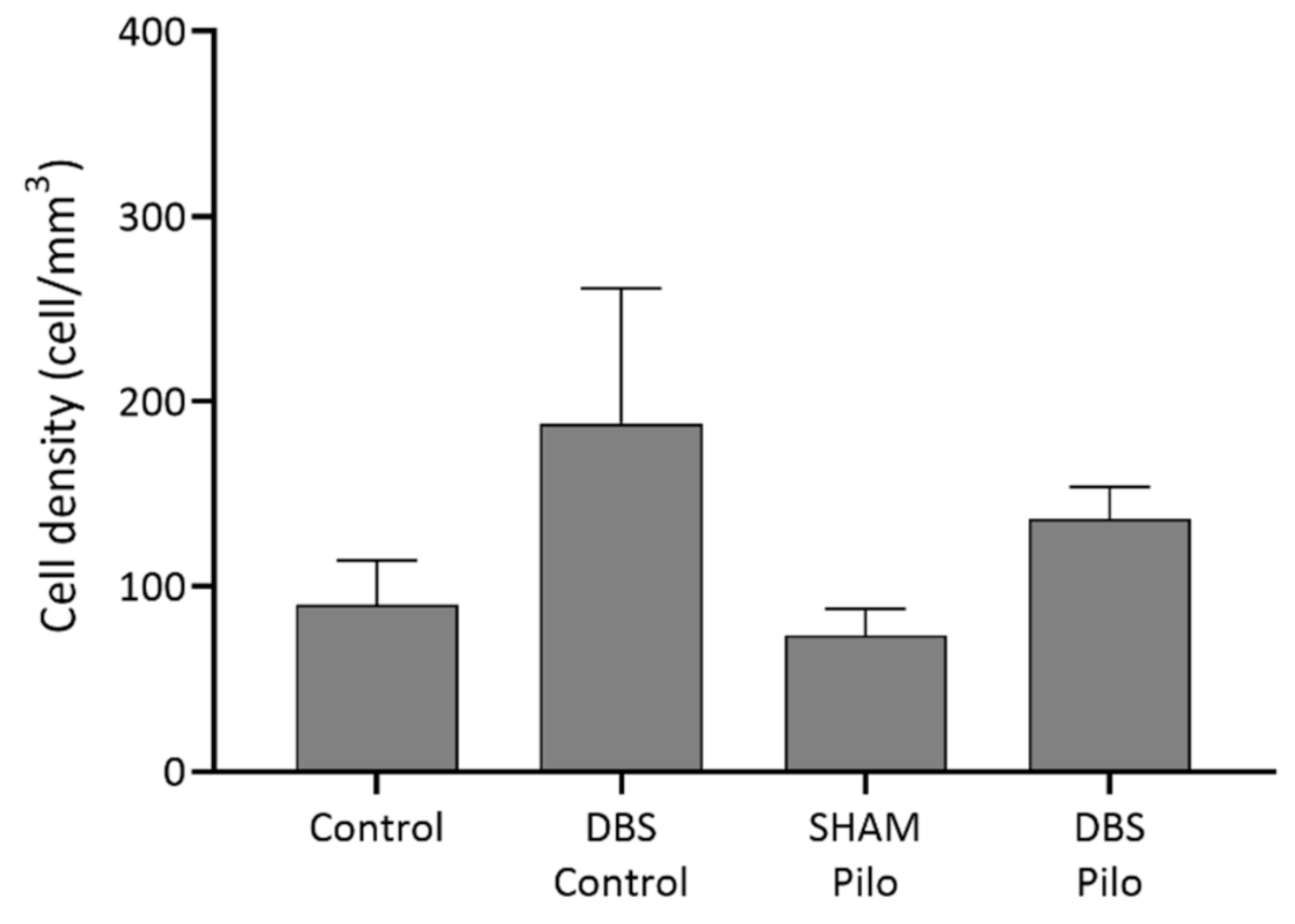
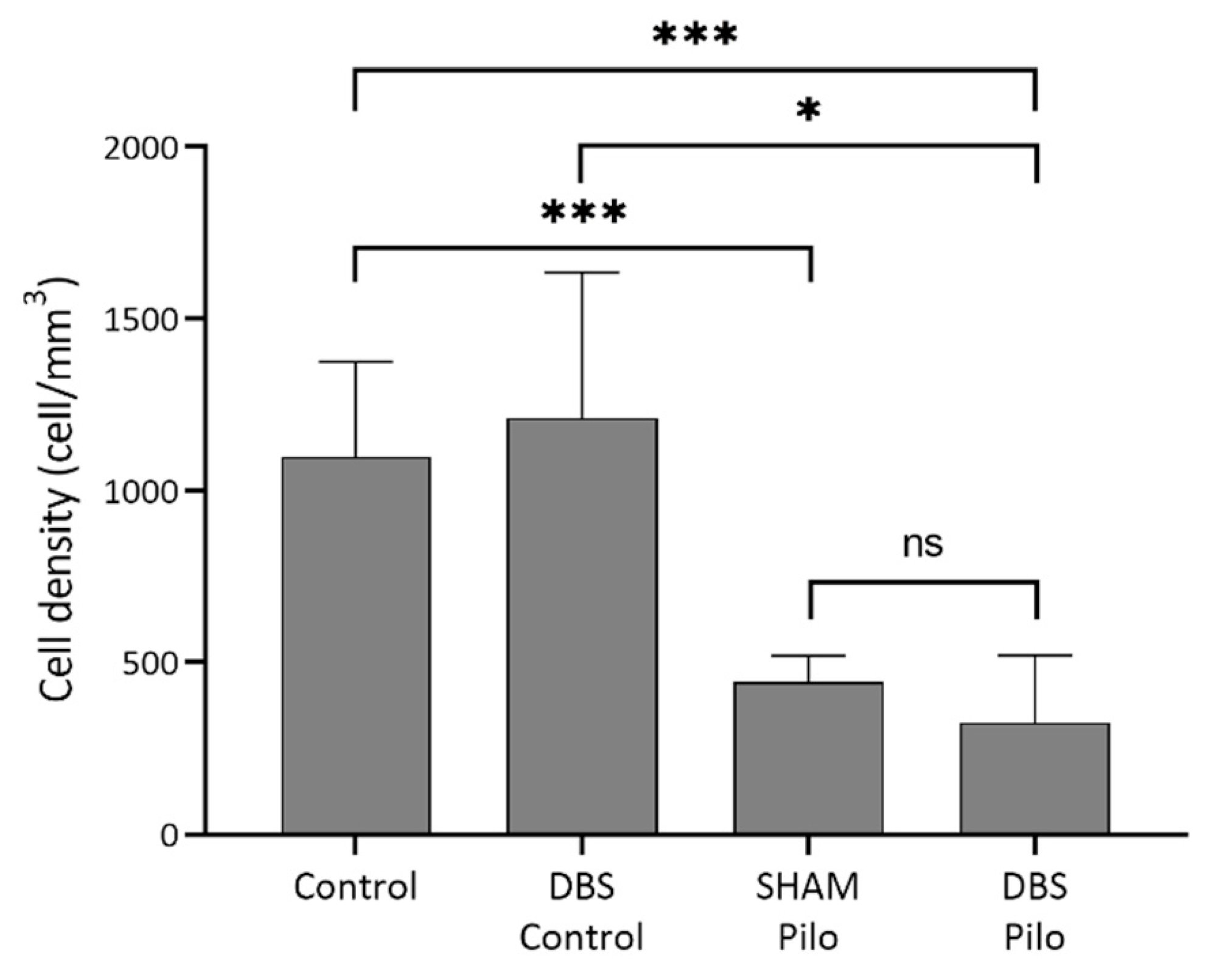
3.1. PV+ Cells
3.2. PV+/NPY+ Cells
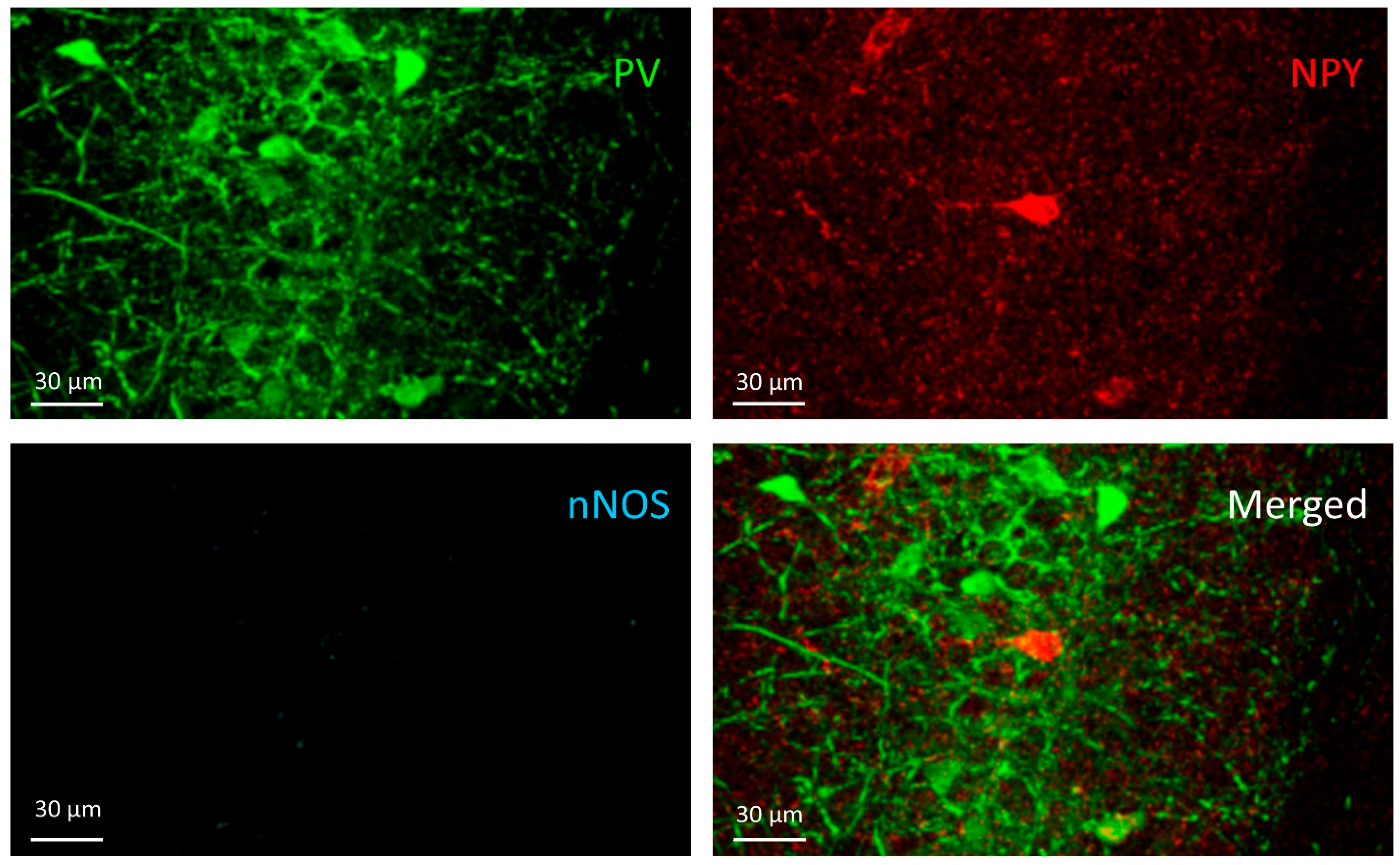
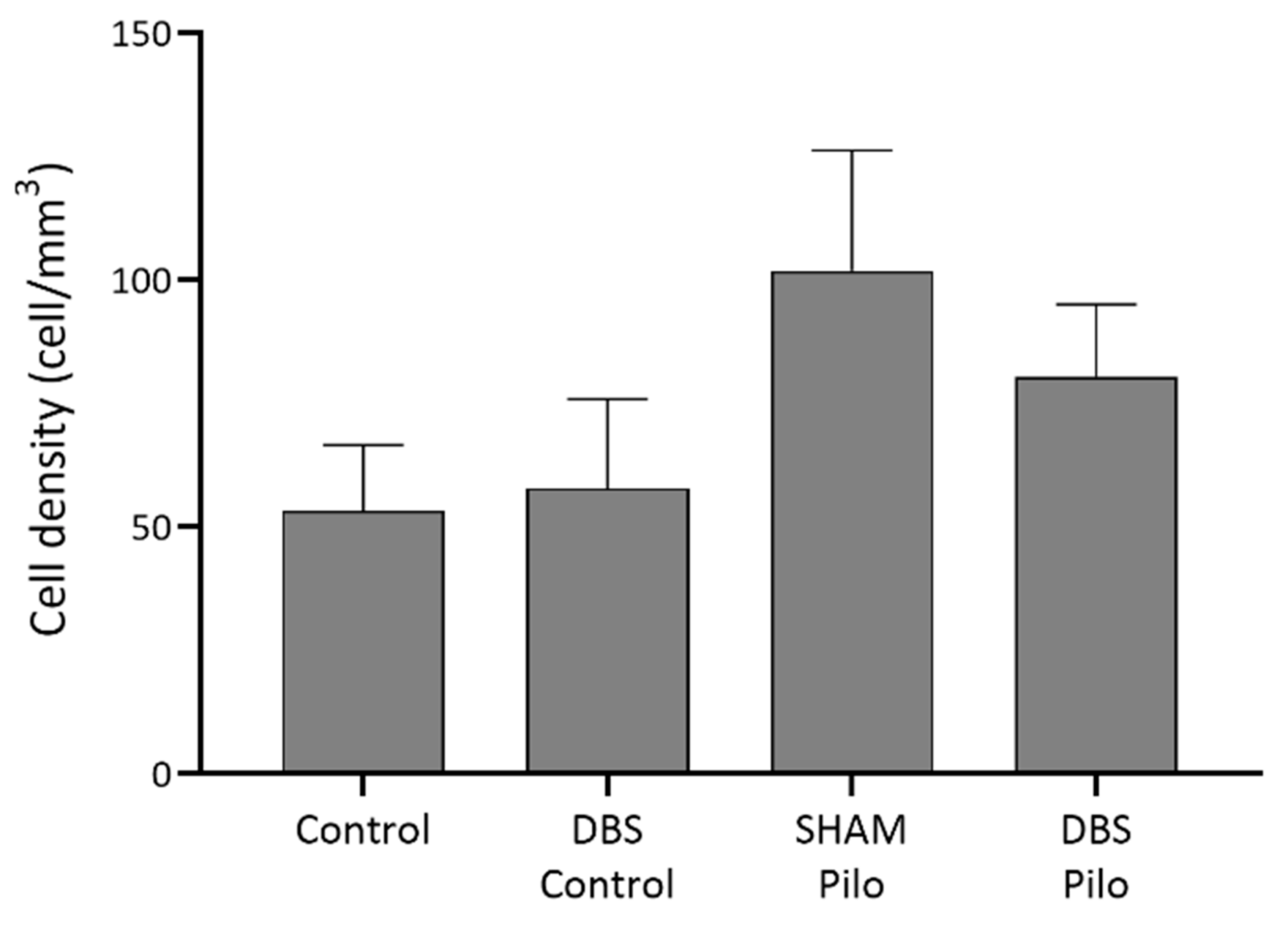
3.3. NPY+ Cells
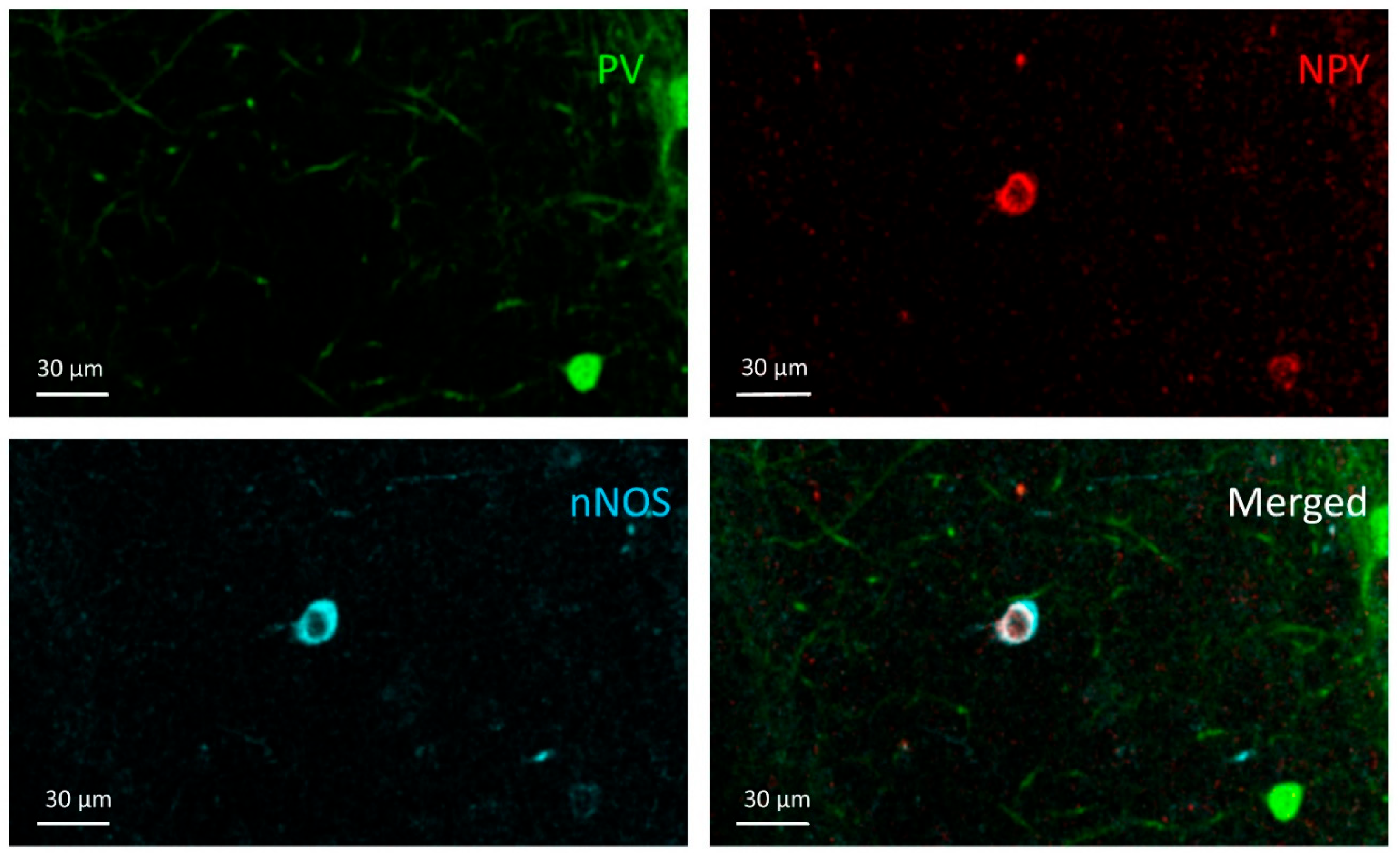
3.4. NPY+/nNOS+ Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BLA | Basolateral amygdala |
| CA1 | Cornu ammonis 1 |
| DBS | Deep brain stimulation |
| EEG | Electroencephalography |
| GABA | Gamma-aminobutyric acid |
| HFS | High-frequency stimulation |
| LFS | Low-frequency stimulation |
| NHS | Normal horse serum |
| nNOS | Neuronal nitric oxide synthase |
| NPY | Neuropeptide y |
| O-LM | Oriens-lacunoscum moleculare |
| PB | Phosphate buffer |
| Pilo | Pilocarpine |
| PTZ | Pentylenetetrazole |
| PV | Parvalbumin |
| SE | Status epilepticus |
| SEM | Standard error of the mean |
| SRSs | Spontaneous recurrent seizures |
| TBS | Tris-buffered saline |
| TLE | Temporal lobe epilepsy |
References
- Sander, J.W.; Shorvon, S.D. Epidemiology of the Epilepsies. J. Neurol. Neurosurg. Psychiatry 1996, 61, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Wilner, A.N.; Sharma, B.K.; Soucy, A.; Krueger, A. Health Plan Paid Cost of Epilepsy in 2009 in the U.S. Epilepsy Behav. 2012, 25, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, S. Epidemiology of Temporal Lobe Epilepsy. Can. J. Neurol. Sci. 2000, 27, 6–21. [Google Scholar] [CrossRef]
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; Van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W.; et al. Revised Terminology and Concepts for Organization of Seizures and Epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010, 51, 676–685. [Google Scholar] [CrossRef]
- Mohanraj, R.; Brodie, M.J. Diagnosing Refractory Epilepsy: Response to Sequential Treatment Schedules. Eur. J. Neurol. 2006, 13, 277–282. [Google Scholar] [CrossRef]
- Thom, M. Hippocampal Sclerosis: Progress since Sommer. Brain Pathol. 2009, 19, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Buckmaster, P.S. Mossy Fiber Sprouting in the Dentate Gyrus. Epilepsia 2010, 51 Suppl., 39. [Google Scholar] [CrossRef]
- Zhang, S.; Khanna, S.; Tang, F.R. Patterns of Hippocampal Neuronal Loss and Axon Reorganization of the Dentate Gyrus in the Mouse Pilocarpine Model of Temporal Lobe Epilepsy. J. Neurosci. Res. 2009, 87, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Botterill, J.J.; Brymer, K.J.; Caruncho, H.J.; Kalynchuk, L.E. Aberrant Hippocampal Neurogenesis after Limbic Kindling: Relationship to BDNF and Hippocampal-Dependent Memory. Epilepsy Behav. 2015, 47, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Freund, T.F.; Buzsaki, G. Interneurons of the Hippocampus. Hippocampus 1996, 5, 347–470. [Google Scholar] [CrossRef]
- Somogyi, P.; Klausberger, T. Defined Types of Cortical Interneurone Structure Space and Spike Timing in the Hippocampus. J. Physiol. 2005, 562, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, P. Hippocampus: Intrinsic Organization. In Handbook of Brain Microcircuits; Oxford University Press: New York, NY, USA, 2010; pp. 148–164. ISBN 13:9780195389883. [Google Scholar]
- Liu, Y.Q.; Yu, F.; Liu, W.H.; He, X.H.; Peng, B.W. Dysfunction of Hippocampal Interneurons in Epilepsy. Neurosci. Bull. 2014, 30, 985–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maglóczky, Z.; Freund, T.F. Impaired and Repaired Inhibitory Circuits in the Epileptic Human Hippocampus. Trends Neurosci. 2005, 28, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Sloviter, R.S. Permanently Altered Hippocampal Structure, Excitability, and Inhibition after Experimental Status Epilepticus in the Rat: The “Dormant Basket Cell” Hypothesis and Its Possible Relevance to Temporal Lobe Epilepsy. Hippocampus 1991, 1, 41–66. [Google Scholar] [CrossRef]
- Szilagyi, T.; Szava, I.; Metz, E.J.; Mihaly, I.; Orban-Kis, K. Untangling the Pathomechanisms of Temporal Lobe Epilepsy-The Promise of Epileptic Biomarkers and Novel Therapeutic Approaches. Brain Res. Bull. 2014, 109, 1–12. [Google Scholar] [CrossRef]
- Gáll, Z.; Kelemen, K.; Mihály, I.; Salamon, P.; Miklóssy, I.; Zsigmond, B.; Kolcsár, M. Role of Lacosamide in Preventing Pentylenetetrazole Kindling-Induced Alterations in the Expression of the Gamma-2 Subunit of the GABAA Receptor in Rats. Curr. Mol. Pharmacol. 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Harris, K.D.; Hochgerner, H.; Skene, N.G.; Magno, L.; Katona, L.; Bengtsson Gonzales, C.; Somogyi, P.; Kessaris, N.; Linnarsson, S.; Hjerling-Leffler, J. Classes and Continua of Hippocampal CA1 Inhibitory Neurons Revealed by Single-Cell Transcriptomics. PLoS Biol. 2018, 16, e2006387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelkey, K.A.; Chittajallu, R.; Craig, M.T.; Tricoire, L.; Wester, J.C.; McBain, C.J. Hippocampal Gabaergic Inhibitory Interneurons. Physiol. Rev. 2017, 97, 1619–1747. [Google Scholar] [CrossRef]
- Bezaire, M.J.; Soltesz, I. Quantitative Assessment of CA1 Local Circuits: Knowledge Base for Interneuron-Pyramidal Cell Connectivity. Hippocampus 2013, 23, 751–785. [Google Scholar] [CrossRef] [Green Version]
- Buhl, E.H.; Szilágyi, T.; Halasy, K.; Somogyi, P. Physiological Properties of Anatomically Identified Basket and Bistratified Cells in the CA1 Area of the Rat Hippocampus in Vitro. Hippocampus 1996, 6, 294–305. [Google Scholar] [CrossRef]
- Dinocourt, C.; Petanjek, Z.; Freund, T.F.; Ben-Ari, Y.; Esclapez, M. Loss of Interneurons Innervating Pyramidal Cell Dendrites and Axon Initial Segments in the CA1 Region of the Hippocampus Following Pilocarpine-Induced Seizures. J. Comp. Neurol. 2003, 459, 407–425. [Google Scholar] [CrossRef]
- Andrioli, A.; Alonso-Nanclares, L.; Arellano, J.I.; DeFelipe, J. Quantitative Analysis of Parvalbumin-Immunoreactive Cells in the Human Epileptic Hippocampus. Neuroscience 2007, 149, 131–143. [Google Scholar] [CrossRef]
- Orbán-Kis, K.; Szabadi, T.; Szilágyi, T. The Loss of Ivy Cells and the Hippocampal Input Modulatory O-LM Cells Contribute to the Emergence of Hyperexcitability in the Hippocampus. Rom. J. Morphol. Embryol. 2015, 56, 155–161. [Google Scholar]
- Colmers, W.F.; El Bahh, B. Neuropeptide Y and Epilepsy. Epilepsy Curr. 2003, 3, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Somogyi, J.; Szabo, A.; Somogyi, P.; Lamsa, K. Molecular Analysis of Ivy Cells of the Hippocampal CA1 Stratum Radiatum Using Spectral Identification of Immunofluorophores. Front. Neural Circuits 2012, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, C.; Krook-Magnuson, E.; Soltesz, I. Neurogliaform and Ivy Cells: A Major Family of NNOS Expressing GABAergic Neurons. Front. Neural Circuits 2012, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite, J.P.; Chimelli, L.; Terra-Bustamante, V.C.; Costa, E.T.; Assirati, J.A.; de Nucci, G.; Martins, A.R. Loss and Sprouting of Nitric Oxide Synthase Neurons in the Human Epileptic Hippocampus. Epilepsia 2002, 43, 235–242. [Google Scholar] [CrossRef]
- Wyeth, M.; Nagendran, M.; Buckmaster, P.S. Ictal Onset Sites and γ-Aminobutyric Acidergic Neuron Loss in Epileptic Pilocarpine-Treated Rats. Epilepsia 2020, 61, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Engel, J. Surgery for Seizures. N. Engl. J. Med. 1996, 334, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, S.; Blume, W.T.; Girvin, J.P.; Eliasziw, M. A Randomized, Controlled Trial of Surgery for Temporal-Lobe Epilepsy. N. Engl. J. Med. 2001, 345, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.L.; Velasco, M.; Velasco, F.; Menes, D.; Gordon, F.; Rocha, L.; Briones, M.; Márquez, I. Subacute and Chronic Electrical Stimulation of the Hippocampus on Intractable Temporal Lobe Seizures: Preliminary Report. Arch. Med. Res. 2000, 31, 316–328. [Google Scholar] [CrossRef]
- Velasco, A.L.; Velasco, F.; Velasco, M.; Trejo, D.; Castro, G.; Carrillo-Ruiz, J.D. Electrical Stimulation of the Hippocampal Epileptic Foci for Seizure Control: A Double-Blind, Long-Term Follow-up Study. Epilepsia 2007, 48, 1895–1903. [Google Scholar] [CrossRef]
- Cukiert, A.; Cukiert, C.M.; Burattini, J.A.; Mariani, P.P.; Bezerra, D.F. Seizure Outcome after Hippocampal Deep Brain Stimulation in Patients with Refractory Temporal Lobe Epilepsy: A Prospective, Controlled, Randomized, Double-Blind Study. Epilepsia 2017, 58, 1728–1733. [Google Scholar] [CrossRef] [Green Version]
- Tyrand, R.; Seeck, M.; Spinelli, L.; Pralong, E.; Vulliémoz, S.; Foletti, G.; Rossetti, A.O.; Allali, G.; Lantz, G.; Pollo, C.; et al. Effects of Amygdala-Hippocampal Stimulation on Interictal Epileptic Discharges. Epilepsy Res. 2012, 99, 87–93. [Google Scholar] [CrossRef]
- Asgari, A.; Semnanian, S.; Atapour, N.; Shojaei, A.; Moradi, H.; Mirnajafi-Zadeh, J. Combined Sub-Threshold Dosages of Phenobarbital and Low-Frequency Stimulation Effectively Reduce Seizures in Amygdala-Kindled Rats. Neurol. Sci. 2014, 35, 1255–1260. [Google Scholar] [CrossRef]
- Zhong, K.; Wu, D.C.; Jin, M.M.; Xu, Z.H.; Wang, Y.; Hou, W.W.; Li, X.M.; Zhang, S.H.; Chen, Z. Wide Therapeutic Time-Window of Low-Frequency Stimulation at the Subiculum for Temporal Lobe Epilepsy Treatment in Rats. Neurobiol. Dis. 2012, 48, 20–26. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.C.; de Castro Medeiros, D.; e Rezende, G.H.; Moraes, M.F.; Cota, V.R. Temporally Unstructured Electrical Stimulation to the Amygdala Suppresses Behavioral Chronic Seizures of the Pilocarpine Animal Model. Epilepsy Behav. 2014, 36, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Mihály, I.; Orbán-Kis, K.; Gáll, Z.; Berki, Á.J.; Bod, R.B.; Szilágyi, T. Amygdala Low-Frequency Stimulation Reduces Pathological Phase-Amplitude Coupling in the Pilocarpine Model of Epilepsy. Brain Sci. 2020, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Ikeda, A.; Kinoshita, M.; Matsumoto, R.; Satow, T.; Takeshita, K.; Matsuhashi, M.; Mikuni, N.; Miyamoto, S.; Hashimoto, N.; et al. Low-Frequency Electric Cortical Stimulation Decreases Interictal and Ictal Activity in Human Epilepsy. Seizure 2006, 15, 520–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrington, T.M.; Cheng, J.J.; Eskandar, E.N. Mechanisms of Deep Brain Stimulation. J. Neurophysiol. 2016, 115, 19–38. [Google Scholar] [CrossRef] [Green Version]
- Ashkan, K.; Rogers, P.; Bergman, H.; Ughratdar, I. Insights into the Mechanisms of Deep Brain Stimulation. Nat. Rev. Neurol. 2017, 13, 548–554. [Google Scholar] [CrossRef]
- Reddy, D.S.; Kuruba, R. Experimental Models of Status Epilepticus and Neuronal Injury for Evaluation of Therapeutic Interventions. Int. J. Mol. Sci. 2013, 14, 18284–18318. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, C.; Moraes, J.; Battapady, H.; Tannus, A.; Federal, U.; Paulo, D.S.; Paulo, S. The Neural Response to Deep Brain Stimulation of the Anterior Nucleus of the Thalamus: A MEMRI and c-Fos Study. Brain Res. Bull. 2019, 147, 133–139. [Google Scholar] [CrossRef]
- Ghafouri, S.; Fathollahi, Y.; Semnanian, S.; Shojaei, A.; Asgari, A.; Amini, A.E.; Mirnajafi-Zadeh, J. Deep Brain Stimulation Restores the Glutamatergic and GABAergic Synaptic Transmission and Plasticity to Normal Levels in Kindled Rats. PLoS ONE 2019, 14, e0224834. [Google Scholar] [CrossRef] [Green Version]
- Ladas, T.P.; Chiang, C.C.; Gonzalez-Reyes, L.E.; Nowak, T.; Durand, D.M. Seizure Reduction through Interneuron-Mediated Entrainment Using Low Frequency Optical Stimulation. Exp. Neurol. 2015, 269, 120–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amorim, B.O.; Covolan, L.; Ferreira, E.; Brito, J.G.; Nunes, D.P.; de Morais, D.G.; Nobrega, J.N.; Rodrigues, A.M.; de Almeida, A.C.; Hamani, C. Deep Brain Stimulation Induces Antiapoptotic and Anti-Inflammatory Effects in Epileptic Rats. J. Neuroinflamm. 2015, 12, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, M.F.; Hamani, C.; de Almeida, A.C.; Amorim, B.O.; Macedo, C.E.; Fernandes, M.J.; Nobrega, J.N.; Aarão, M.C.; Madureira, A.P.; Rodrigues, A.M.; et al. Role of Adenosine in the Antiepileptic Effects of Deep Brain Stimulation. Front. Cell. Neurosci. 2014, 8, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, G.; Meng, D.; Chen, Y.; Du, T.; Liu, Y.; Liu, D.; Shi, L.; Jiang, Y.; Zhang, X.; Zhang, J. Anterior Nucleus of Thalamus Stimulation Inhibited Abnormal Mossy Fiber Sprouting in Kainic Acid-Induced Epileptic Rats. Brain Res. 2018, 1701, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; He, X.; Feng, L.; Liu, D.; Yang, Z.; Zhang, J.; Xiao, B.; Yang, Z. The Role of Hippocampal Neurogenesis in ANT-DBS for LiCl-Pilocarpine-Induced Epileptic Rats. Stereotact. Funct. Neurosurg. 2020, 10, 1–10. [Google Scholar]
- Lüttjohann, A.; Fabene, P.F.; van Luijtelaar, G. A Revised Racine’s Scale for PTZ-Induced Seizures in Rats. Physiol. Behav. 2009, 98, 579–586. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Paxinos, G., Watson, C., Eds.; Academic Press: Cambridge, MA, USA, 2007; ISBN 9780125476126. [Google Scholar]
- Khan, A.A.; Shekh-Ahmad, T.; Khalil, A.; Walker, M.C.; Ali, A.B. Cannabidiol Exerts Antiepileptic Effects by Restoring Hippocampal Interneuron Functions in a Temporal Lobe Epilepsy Model. Br. J. Pharmacol. 2018, 175, 2097–2115. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, R.M.; Rogawski, M.A.; Klitgaard, H. The Potential of Antiseizure Drugs and Agents that Act on Novel Molecular Targets as Antiepileptogenic Treatments. Neurotherapeutics 2014, 11, 385–400. [Google Scholar] [CrossRef] [Green Version]
- Fisher, R.S.; Velasco, A.L. Electrical Brain Stimulation for Epilepsy. Nat. Rev. Neurol. 2014, 10, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Sun, H.L.; Fang, Q.; Zhong, K.; Wu, D.C.; Wang, S.; Chen, Z. Low-Frequency Stimulation of the Hippocampal CA3 Subfield Is Anti-Epileptogenic and Anti-Ictogenic in Rat Amygdaloid Kindling Model of Epilepsy. Neurosci. Lett. 2009, 455, 51–55. [Google Scholar] [CrossRef]
- André, V.; Marescaux, C.; Nehlig, A.; Fritschy, J.M. Alterations of Hippocampal GABAergic System Contribute to Development of Spontaneous Recurrent Seizures in the Rat Lithium-Pilocarpine Model of Temporal Lobe Epilepsy. Hippocampus 2001, 11, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Marx, M.; Haas, C.A.; Häussler, U.; Wierenga, C.J. Differential Vulnerability of Interneurons in the Epileptic Hippocampus. Front. Cell. Neurosci. 2013, 7, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuruba, R.; Hattiangady, B.; Parihar, V.K.; Shuai, B.; Shetty, A.K. Differential Susceptibility of Interneurons Expressing Neuropeptide Y or Parvalbumin in the Aged Hippocampus to Acute Seizure Activity. PLoS ONE 2011, 6, e24493. [Google Scholar] [CrossRef] [Green Version]
- Long, L.; Xiao, B.; Feng, L.; Yi, F.; Li, G.; Li, S.; Mutasem, M.A.; Chen, S.; Bi, F.; Li, Y. Selective Loss and Axonal Sprouting of GABAergic Interneurons in the Sclerotic Hippocampus Induced by LiClPilocarpine. Int. J. Neurosci. 2011, 121, 69–85. [Google Scholar] [CrossRef]
- Wittner, L.; Erőss, L.; Szabó, Z.; Tóth, S.; Czirják, S.; Halász, P.; Freund, T.F.; Maglóczky, Z.S. Synaptic Reorganization of Calbindin-Positive Neurons in the Human Hippocampal CA1 Region in Temporal Lobe Epilepsy. Neuroscience 2002, 115, 961–978. [Google Scholar] [CrossRef]
- Wittner, L.; Maglóczky, Z. Synaptic Reorganization of the Perisomatic Inhibitory Network in Hippocampi of Temporal Lobe Epileptic Patients. BioMed Res. Int. 2017, 2017, 7154295. [Google Scholar] [CrossRef] [Green Version]
- Ellender, T.J.; Raimondo, J.V.; Irkle, A.; Lamsa, K.P.; Akerman, C.J. Excitatory Effects of Parvalbumin-Expressing Interneurons Maintain Hippocampal Epileptiform Activity via Synchronous Afterdischarges. J. Neurosci. 2014, 34, 15208–15222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittner, L.; Erőss, L.; Czirják, S.; Halász, P.; Freund, T.F.; Maglóczky, Z. Surviving CA1 Pyramidal Cells Receive Intact Perisomatic Inhibitory Input in the Human Epileptic Hippocampus. Brain 2005, 128, 138–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentealba, P.; Begum, R.; Capogna, M.; Jinno, S.; Márton, L.F.; Csicsvari, J.; Thomson, A.; Somogyi, P.; Klausberger, T. Ivy Cells: A Population of Nitric-Oxide-Producing, Slow-Spiking GABAergic Neurons and Their Involvement in Hippocampal Network Activity. Neuron 2008, 57, 917–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capogna, M. Neurogliaform Cells and Other Interneurons of Stratum Lacunosum-Moleculare Gate Entorhinal-Hippocampal Dialogue. J. Physiol. 2011, 589, 1875–1883. [Google Scholar] [CrossRef]
- Beamer, E.; Otahal, J.; Sills, G.J.; Thippeswamy, T. Nw-Propyl-l-Arginine (L-NPA) Reduces Status Epilepticus and Early Epileptogenic Events in a Mouse Model of Epilepsy: Behavioural, EEG and Immunohistochemical Analyses. Eur. J. Neurosci. 2012, 36, 3194–3203. [Google Scholar] [CrossRef]
- González-Hernández, T.; García-Marín, V.; Pérez-Delgado, M.M.; González-González, M.L.; Rancel-Torres, N.; González-Feria, L. Nitric Oxide Synthase Expression in the Cerebral Cortex of Patients with Epilepsy. Epilepsia 2000, 41, 1259–1268. [Google Scholar] [CrossRef]
- Sardo, P.; Ferraro, G. Modulatory Effects of Nitric Oxide-Active Drugs on the Anticonvulsant Activity of Lamotrigine in an Experimental Model of Partial Complex Epilepsy in the Rat. BMC Neurosci. 2007, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Carreira, B.P.; Santos, D.F.; Santos, A.I.; Carvalho, C.M.; Araujo, I.M. Nitric Oxide Regulates Neurogenesis in the Hippocampus Following Seizures. Oxid. Med. Cell. Longev. 2015, 2015, 451512. [Google Scholar] [CrossRef] [Green Version]
- Lumme, A.; Soinila, S.; Sadeniemi, M.; Halonen, T.; Vanhatalo, S. Nitric Oxide Synthase Immunoreactivity in the Rat Hippocampus after Status Epilepticus Induced by Perforant Pathway Stimulation. Brain Res. 2000, 871, 303–310. [Google Scholar] [CrossRef]
- Park, C.; Kang, M.; Kang, K.; Lee, J.; Kim, J.; Yoo, J.; Ahn, H.; Huh, Y. Differential Changes in Neuropeptide Y and Nicotinamide Adenine Dinucleotide Phosphate-Diaphorase-Positive Neurons in Rat Hippocampus after Kainic Acid-Induced Seizure. Neurosci. Lett. 2001, 298, 49–52. [Google Scholar] [CrossRef]
- Sloviter, R.S.; Zappone, C.A.; Harvey, B.D.; Bumanglag, A.V.; Bender, R.A.; Frotscher, M. Dormant Basket Cell Hypothesis Revisited: Relative Vulnerabilities of Dentate Gyrus Mossy Cells and Inhibitory Interneurons after Hippocampal Status Epilepticus in the Rat. J. Comp. Neurol. 2003, 459, 44–76. [Google Scholar] [CrossRef]
- Jinno, S.; Klausberger, T.; Marton, L.F.; Dalezios, Y.; Roberts, J.D.; Fuentealba, P.; Bushong, E.A.; Henze, D.; Buzsaki, G.; Somogyi, P. Neuronal Diversity in GABAergic Long-Range Projections from the Hippocampus. J. Neurosci. 2007, 27, 8790–8804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Mtchedlishvili, Z.; Bertram, E.H.; Erisir, A.; Kapur, J. Selective Loss of Dentate Hilar Interneurons Contributes to Reduced Synaptic Inhibition of Granule Cells in an Electrical Stimulation-Based Animal Model of Temporal Lobe Epilepsy. J. Comp. Neurol. 2007, 500, 876–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabadzisz, D.; Antal, K.; Parpan, F.; Emri, Z.; Fritschy, J.M. Epileptogenesis and Chronic Seizures in a Mouse Model of Temporal Lobe Epilepsy Are Associated with Distinct EEG Patterns and Selective Neurochemical Alterations in the Contralateral Hippocampus. Exp. Neurol. 2005, 194, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, X.; Wu, L.; Nadler, J.V.; Zhan, R.Z. Persistent Hyperactivity of Hippocampal Dentate Interneurons after a Silent Period in the Rat Pilocarpine Model of Epilepsy. Front. Cell. Neurosci. 2016, 10, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihály, I.; Molnár, T.; Berki, Á.-J.; Bod, R.-B.; Orbán-Kis, K.; Gáll, Z.; Szilágyi, T. Short-Term Amygdala Low-Frequency Stimulation Does not Influence Hippocampal Interneuron Changes Observed in the Pilocarpine Model of Epilepsy. Cells 2021, 10, 520. https://doi.org/10.3390/cells10030520
Mihály I, Molnár T, Berki Á-J, Bod R-B, Orbán-Kis K, Gáll Z, Szilágyi T. Short-Term Amygdala Low-Frequency Stimulation Does not Influence Hippocampal Interneuron Changes Observed in the Pilocarpine Model of Epilepsy. Cells. 2021; 10(3):520. https://doi.org/10.3390/cells10030520
Chicago/Turabian StyleMihály, István, Tímea Molnár, Ádám-József Berki, Réka-Barbara Bod, Károly Orbán-Kis, Zsolt Gáll, and Tibor Szilágyi. 2021. "Short-Term Amygdala Low-Frequency Stimulation Does not Influence Hippocampal Interneuron Changes Observed in the Pilocarpine Model of Epilepsy" Cells 10, no. 3: 520. https://doi.org/10.3390/cells10030520
APA StyleMihály, I., Molnár, T., Berki, Á.-J., Bod, R.-B., Orbán-Kis, K., Gáll, Z., & Szilágyi, T. (2021). Short-Term Amygdala Low-Frequency Stimulation Does not Influence Hippocampal Interneuron Changes Observed in the Pilocarpine Model of Epilepsy. Cells, 10(3), 520. https://doi.org/10.3390/cells10030520






