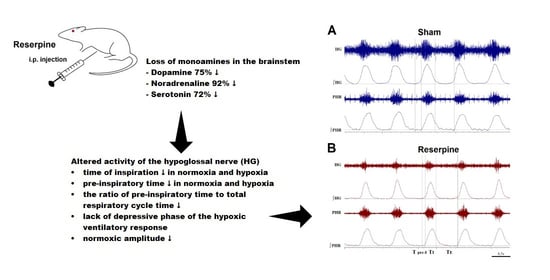
Deficiency of Biogenic Amines Modulates the Activity of Hypoglossal Nerve in the Reserpine Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Compounds Used to Induce Parkinsonism
2.2. Electrophysiological Experiments
2.3. High-Performance Liquid Chromatography (HPLC) Analysis. Assay of Dopamine, Serotonin, Noradrenaline, and Its Metabolites
2.4. Statistics
3. Results
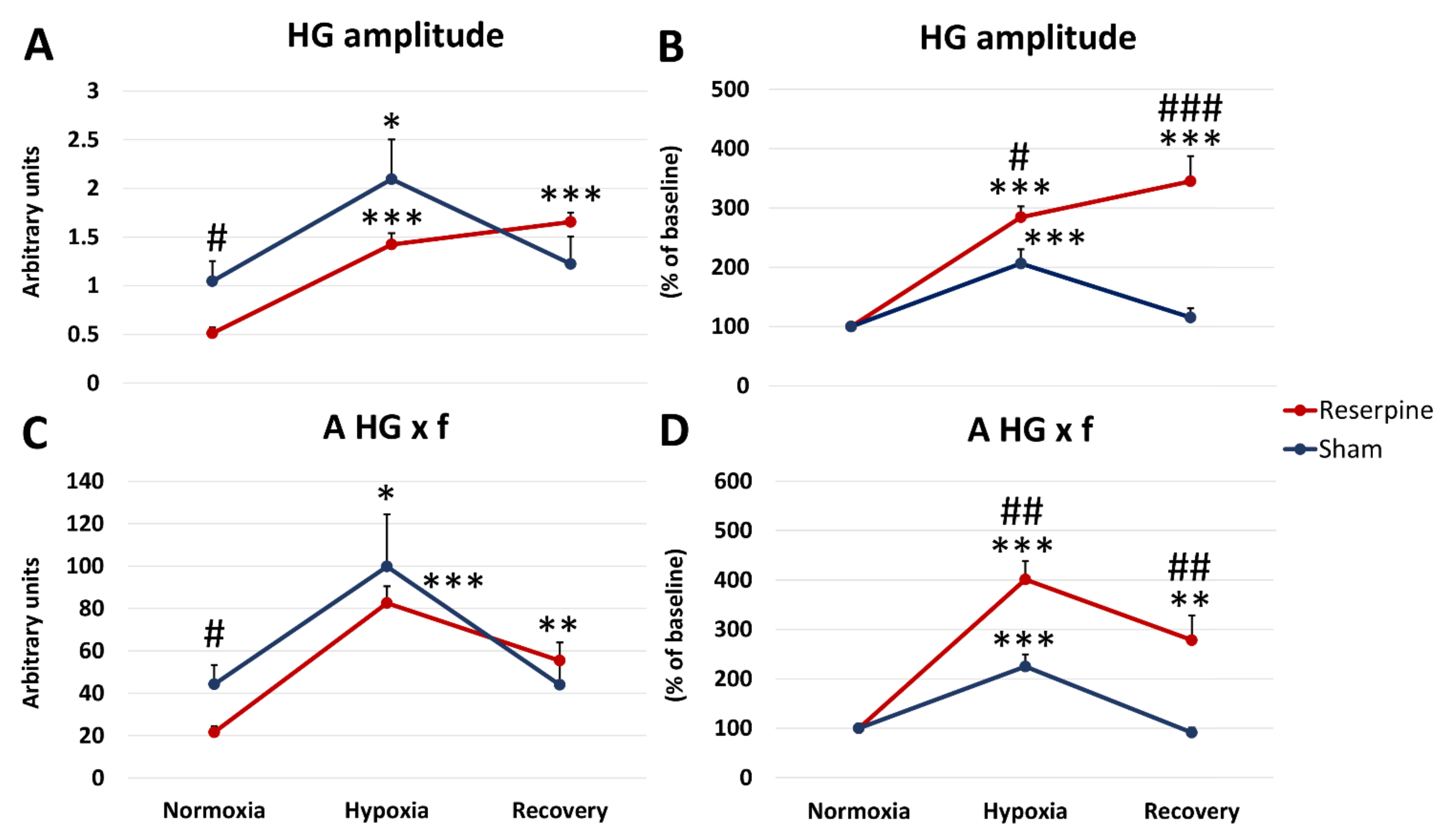
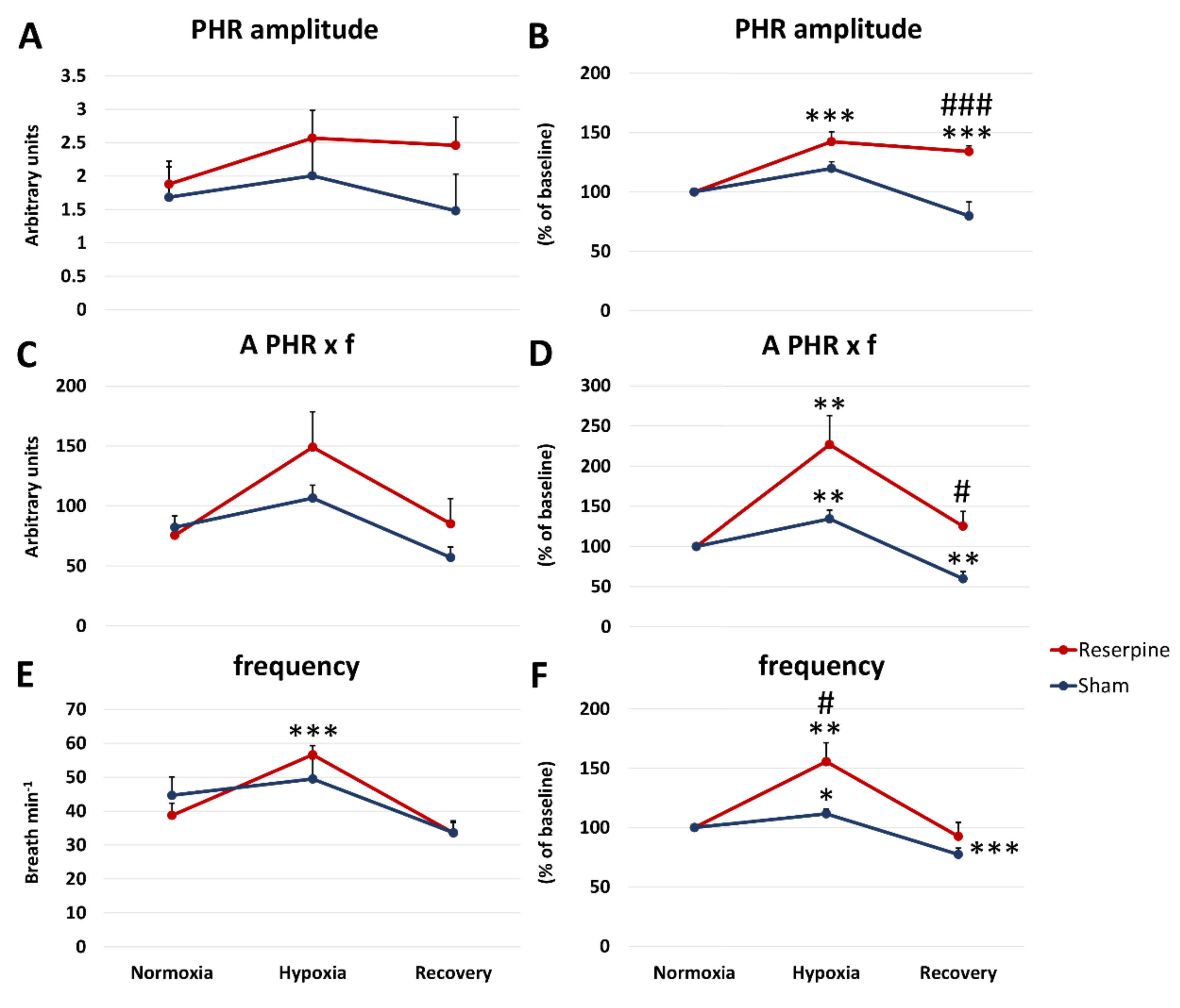
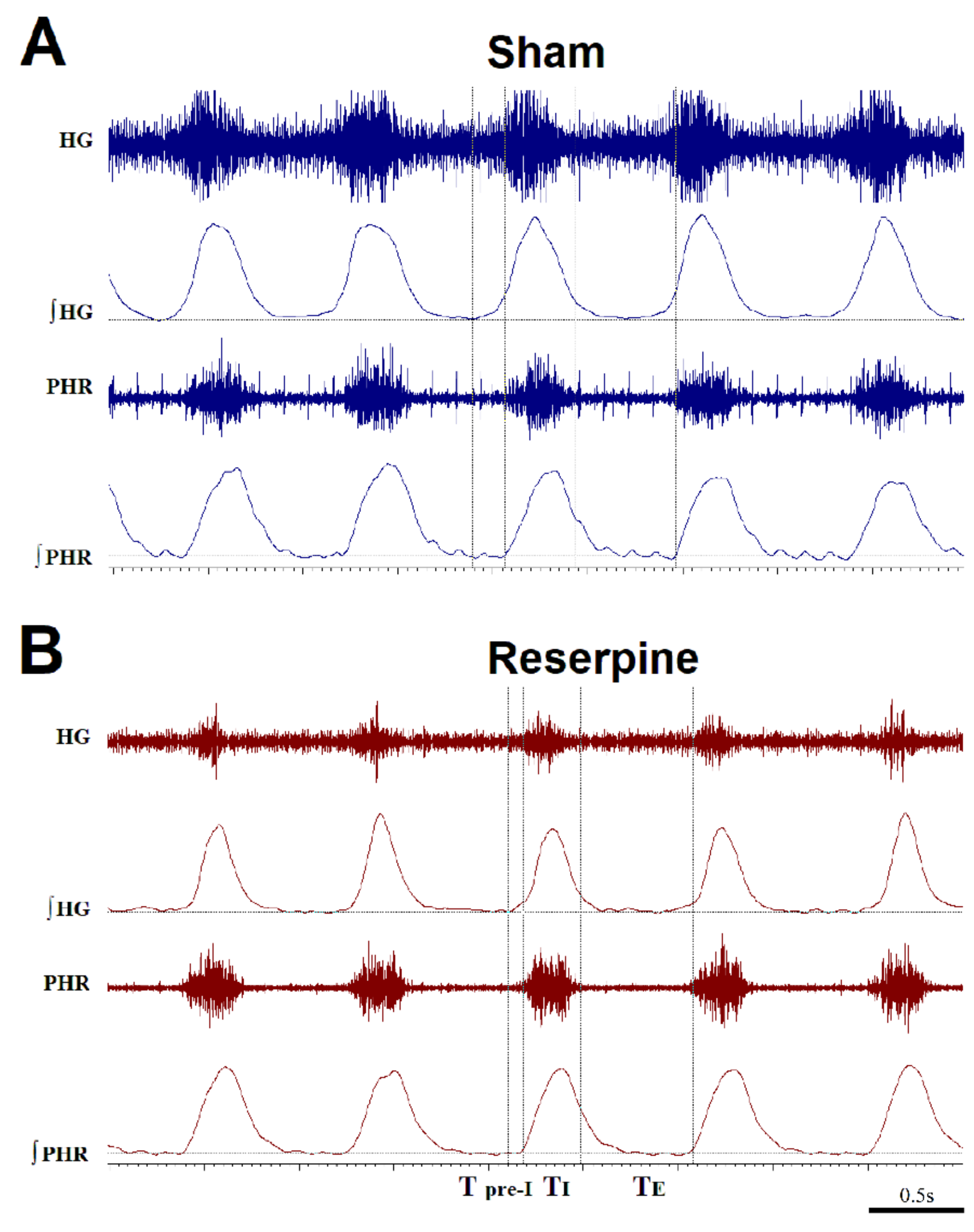
3.1. Changes in the Normoxic and Hypoxic Activity of the Hypoglossal (HG) and Pphrenic (PHR) Nerves in Reserpine and Sham Rats
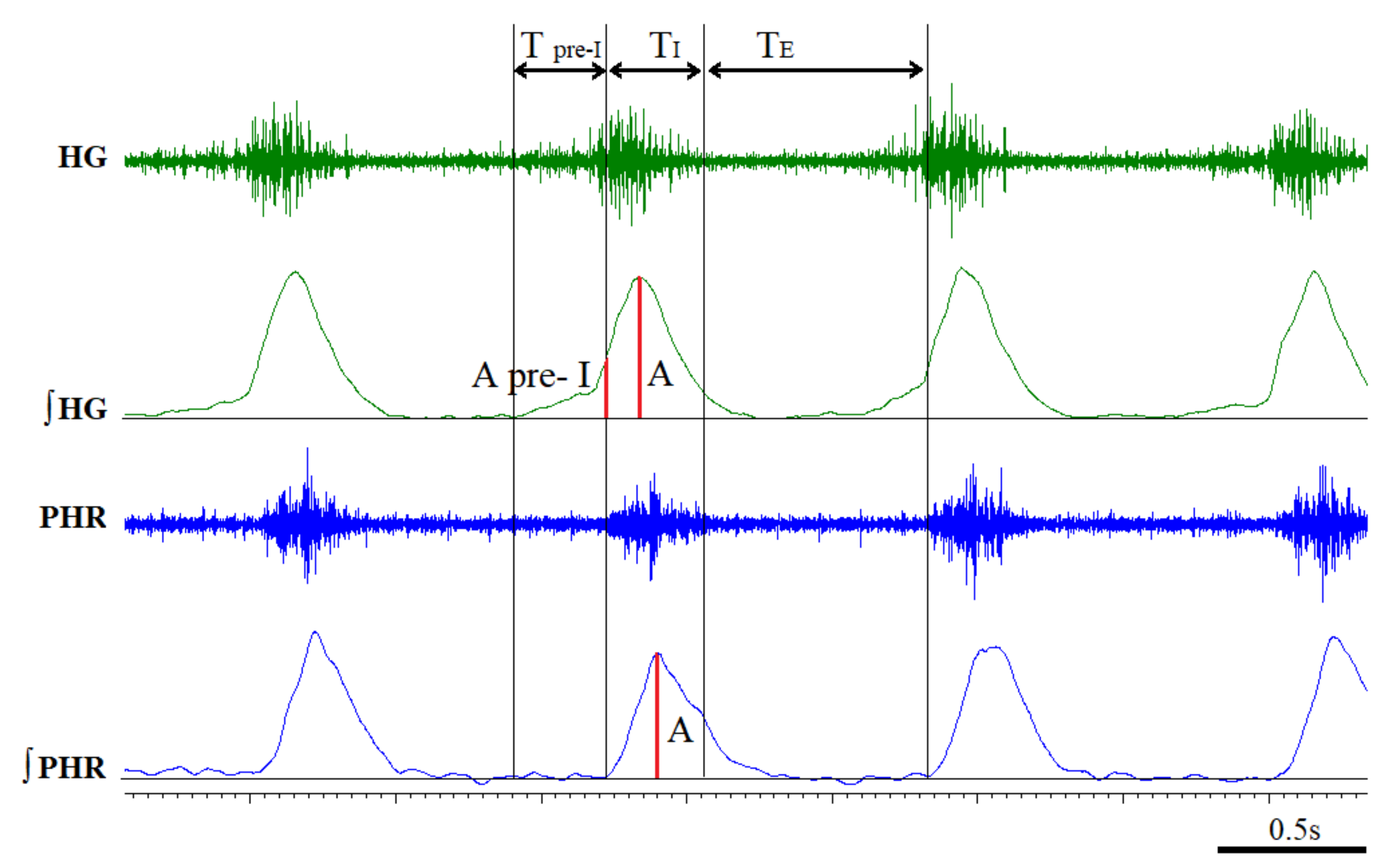
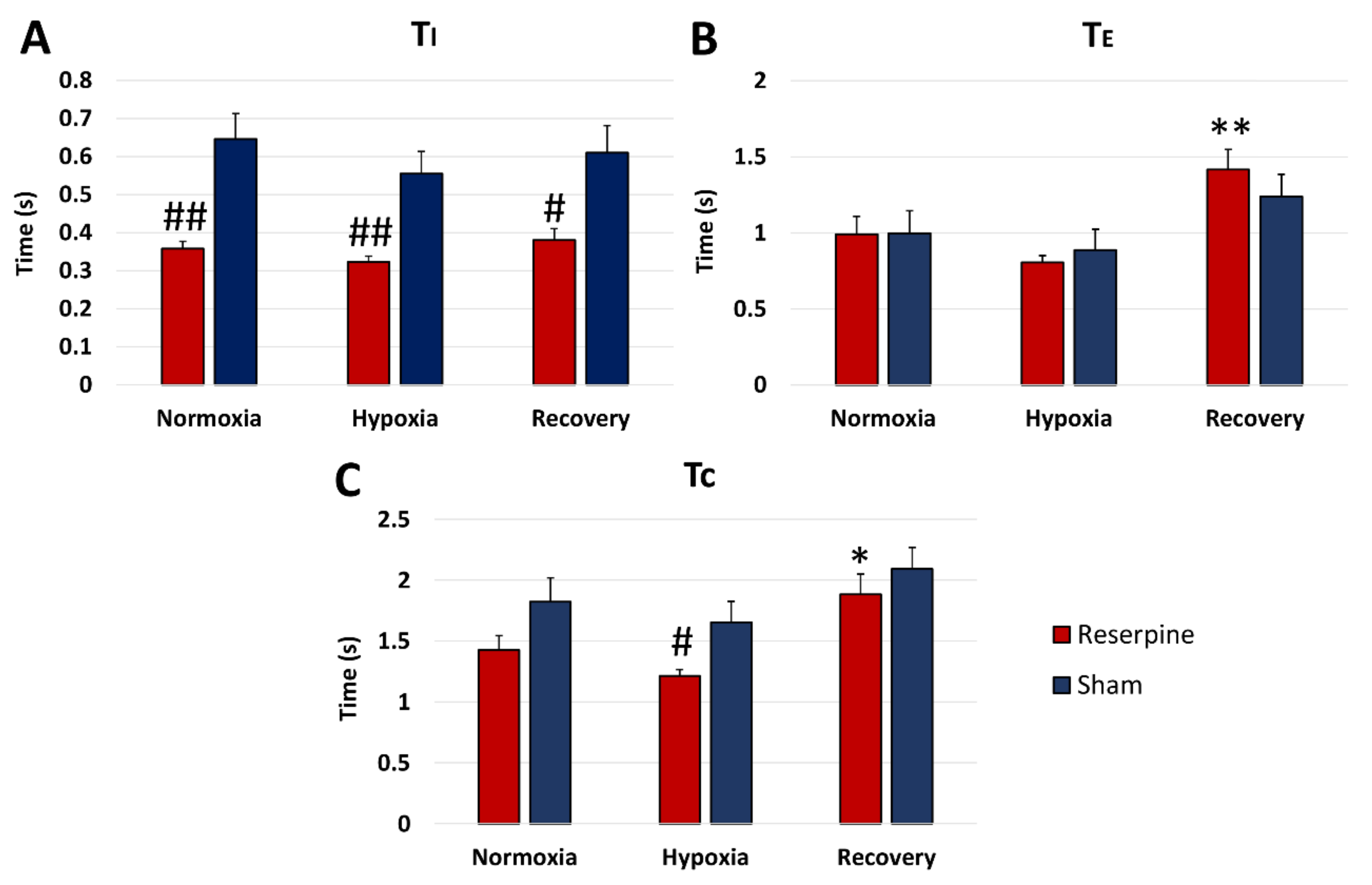
3.2. Time Components of HG and PHR Respiratory Activity during Normoxia and Hypoxia Response in Reserpine and Sham Rats
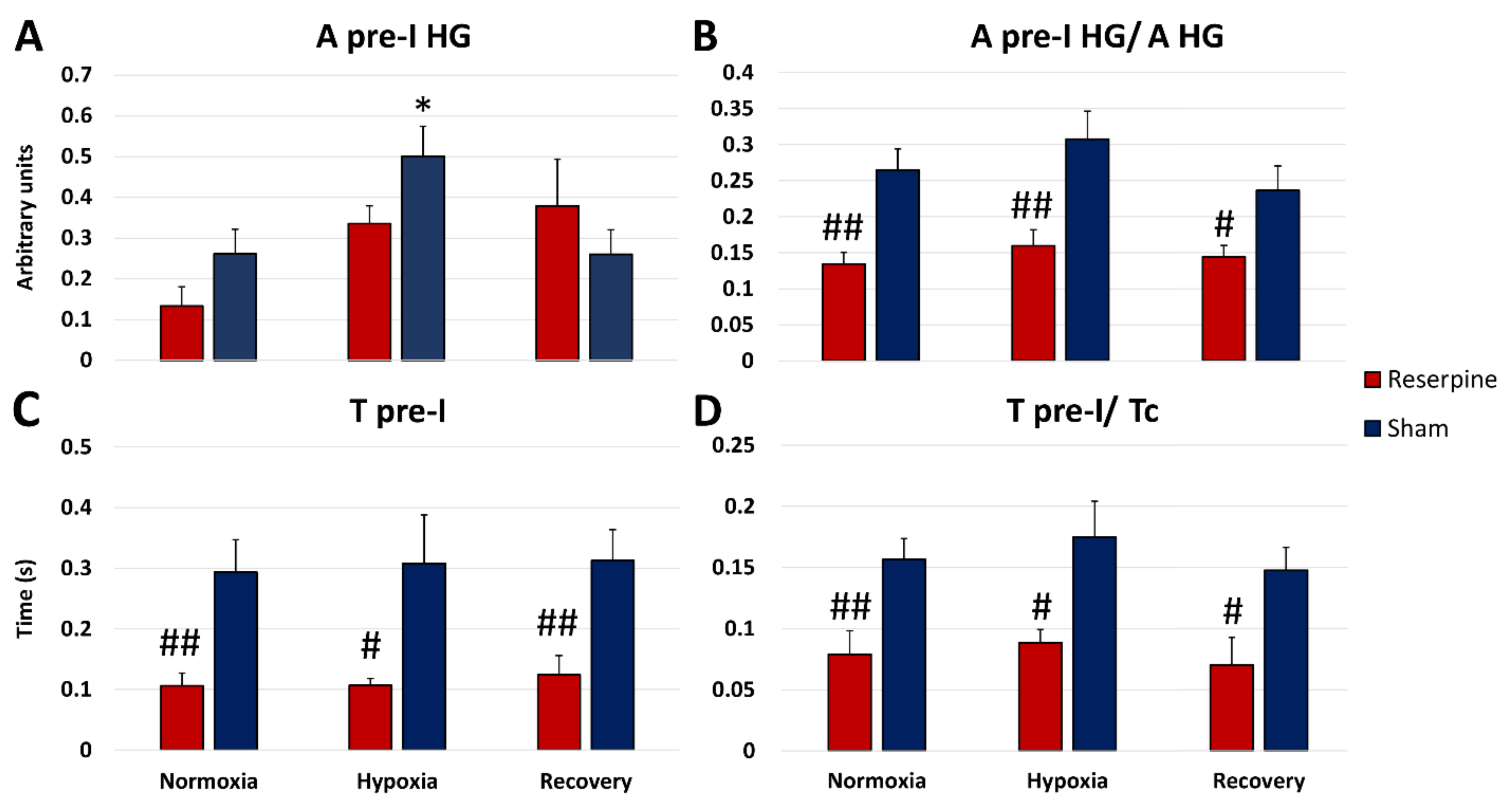
3.3. The Pre-Inspiratory Activity of the Hypoglossal Nerve in Reserpine and Sham Rats
3.4. Content of Monoamines and Metabolites in the Striatum and Brainstem
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirsch, E.; Graybiel, A.M.; Agid, Y.A. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature 1988, 334, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.; Morris, R.; Giritharan, A.; Quinn, J.; Nutt, J.G.; Mancini, M. Prefrontal Cortex Activity and Gait in Parkinson’s Disease With Cholinergic and Dopaminergic Therapy. Mov. Disord. 2020, 35, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- da Silva Córneo, E.; de Bem Silveira, G.; Scussel, R.; Borges Correa, M.E.A.; da Silva Abel, J.; Luiz, G.P.; Feuser, P.E.; Silveira, P.C.L.; Machado-de-Ávila, R.A. Effects of gold nanoparticles administration through behavioral and oxidative parameters in animal model of Parkinson’s disease. Colloids Surf. B Biointerfaces 2020, 196, 111302. [Google Scholar] [CrossRef] [PubMed]
- Baille, G.; De Jesus, A.M.; Perez, T.; Devos, D.; Dujardin, K.; Charley, C.M.; Defebvre, L.; Moreau, C. Ventilatory Dysfunction in Parkinson’s Disease. J. Parkinsons Dis. 2016, 6, 463–471. [Google Scholar] [CrossRef] [Green Version]
- Pokusa, M.; Hajduchova, D.; Buday, T.; Kralova Trancikova, A. Respiratory Function and Dysfunction in Parkinson-Type Neurodegeneration. Physiol. Res. 2020, 69, S69–S79. [Google Scholar] [CrossRef]
- Vijayan, S.; Singh, B.; Ghosh, S.; Stell, R.; Mastaglia, F.L. Brainstem ventilatory dysfunction: A plausible mechanism for dyspnea in Parkinson’s Disease? Mov. Disord. 2020, 35, 379–388. [Google Scholar] [CrossRef]
- Halliday, G.M.; Blumbergs, P.C.; Cotton, R.G.; Blessing, W.W.; Geffen, L.B. Loss of brainstem serotonin- and substance P-containing neurons in Parkinson’s disease. Brain Res. 1990, 510, 104–107. [Google Scholar] [CrossRef]
- Goiny, M.H.; Lagercrantz, M.; Srinivasan, M.; Ungerstedt, U.; Yamamoto, Y. Hypoxia-mediated in vivo release of dopamine in nucleus tractus solitarii of rabbits. J. Appl. Physiol. 1991, 70, 2395–2400. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.; Jellinger, K. The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1991, 50, 743–755. [Google Scholar] [CrossRef]
- Hilaire, G.; Voituron, N.; Menuet, C.; Ichiyama, R.M.; Subramanian, H.H.; Dutschmann, M. The role of serotonin in respiratory function and dysfunction. Respir. Physiol. Neurobiol. 2010, 174, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Delaville, C.; Deurwaerdere, P.; Benazzouz, A. Noradrenaline and Parkinson’s diseases. Front. Syst. Neurosci. 2011, 18, 5–31. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.J.; Pilowsky, P.; Minson, J.; Arnolda, L.; Chalmers, J.; Llewellyn-Smith, I.J. Close appositions between tyrosine hydroxylase immunoreactive boutons and respiratory neurons in the rat ventrolateral medulla. J. Comp. Neurol. 1994, 340, 1–10. [Google Scholar] [CrossRef]
- Li, A.; Nattie, E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J. Physiol. 2006, 570, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Viemari, J.C.; Ramirez, J.M. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J. Neurophysiol. 2006, 95, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Lalley, P.M. Opioidergic and dopaminergic modulation of respiration. Respir. Physiol. Neurobiol. 2008, 164, 160–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcoran, A.E.; Milsom, W.K. Maturational changes in pontine and medullary alpha-adrenoceptor influences on respiratory rhythm generation in neonatal rats. Respir. Physiol. Neurobiol. 2009, 165, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Szereda-Przestaszewska, M.; Kaczyńska, K. Serotonin and substance P: Synergy or competition in the control of breathing. Auton. Neurosci. 2020, 225, 102658. [Google Scholar] [CrossRef]
- Chan, E.; Steenland, H.W.; Liu, H.; Horner, R.L. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am. J. Respir. Crit. Care Med. 2006, 174, 1264–1273. [Google Scholar] [CrossRef] [Green Version]
- Kubin, L. Sleep-wake control of the upper airway by noradrenergic neurons, with and without intermittent hypoxia. In The Central Nervous System Control of Respiration; Holstege, G., Beers, C.M., Subramanian, H.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 209, pp. 255–274. [Google Scholar]
- Kubin, L. Neural control of the upper airway: Respiratory and state-dependent mechanisms. Compr. Physiol. 2016, 6, 1801–1850. [Google Scholar]
- Boyle, C.E.; Parkar, A.; Barror, A.; Kubin, L. Noradrenergic terminal density varies among different groups of hypoglossal premotor neurons. J. Chem. Neuroanat. 2019, 100, 101651. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef]
- Maria, B.; Sophia, S.; Michalis, M.; Charalampos, L.; Andreas, P.; John, M.E.; Nikolaos, S.M. Sleep breathing disorders in patients with idiopathic Parkinson’s disease. Respir. Med. 2003, 97, 1151–1157. [Google Scholar] [CrossRef] [Green Version]
- Crosta, F.; Desideri, G.; Marini, C. Obstructive sleep apnea syndrome in Parkinson’s disease and other parkinsonisms. Funct. Neurol. 2017, 32, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Sobreira-Neto, M.A.; Pena-Pereira, M.A.; Sobreira, E.S.T.; Chagas, M.H.N.; Fernandes, R.M.F.; Tumas, V.; Eckeli, A.L. High Frequency of Sleep Disorders in Parkinson’s Disease and Its Relationship with Quality of Life. Eur. Neurol. 2017, 78, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Colpaert, F.C. Pharmacological characteristics of tremor, rigidity and hypokinesia induced by reserpine in rat. Neuropharmacology 1987, 26, 1431–1440. [Google Scholar] [CrossRef]
- Skalisz, L.L.; Beijamini, V.; Joca, S.L.; Vital, M.A.B.F.; Da Cunha, C.; Andreatini, R. Evaluation of the face validity of reserpine administration as an animal model of depression—Parkinson’s disease association. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 879–883. [Google Scholar] [CrossRef]
- Santos, J.R.; Cunha, J.A.; Dierschnabel, A.L.; Campêlo, C.L.; Leão, A.H.; Silva, A.F.; Engelberth, R.C.; Izídio, G.S.; Cavalcante, J.S.; Abílio, V.C.; et al. Cognitive, motor and tyrosine hydroxylase temporal impairment in a model of parkinsonism induced by reserpine. Behav. Brain Res. 2013, 253, 68–77. [Google Scholar] [CrossRef]
- Leao, A.H.; Sarmento-Silva, A.J.; Santos, J.R.; Ribeiro, A.M.; Silva, R.H. Molecular, Neurochemical, and Behavioral Hallmarks of Reserpine as a Model for Parkinson’s Disease. New Perspect. Long Standing Model. Brain Pathol. 2015, 25, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, V.S.; Santos, J.R.; Leao, A.H.; Medeiros, A.M.; Melo, T.G.; Izídio, G.S.; Cabral, A.; Ribeiro, R.A.; Abílio, V.C.; Ribeiro, A.M.; et al. Repeated treatment with a low dose of reserpine as a progressive model of Parkinson’s disease. Behavioural. Brain Res. 2012, 231, 154–163. [Google Scholar] [CrossRef]
- Andrzejewski, K.; Budzińska, K.; Kaczyńska, K. Phrenic and hypoglossal nerve activity during respiratory response to hypoxia in 6-OHDA unilateral model of Parkinson’s disease. Life Sci. 2017, 180, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, K.; Jampolska, M.; Zaremba, M.; Joniec-Maciejak, I.; Boguszewski, P.M.; Kaczyńska, K. Respiratory pattern and phrenic and hypoglossal nerve activity during normoxia and hypoxia in 6-OHDA induced bilateral model of Parkinson’s disease. J. Physiol. Sci. 2020, 70, 16. [Google Scholar] [CrossRef] [Green Version]
- McClung, J.R.; Goldberg, S.J. Functional anatomy of the hypoglossal innervated muscles of the rat tongue: A model for elongation and protrusion of the mammalian tongue. Anat. Rec. 2000, 260, 378–386. [Google Scholar] [CrossRef]
- Fregosi, R.F. Respiratory related control of hypoglossal motoneurons—Knowing what we do not know. Respir. Physiol. Neurobiol. 2011, 179, 43–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinney, H.C. Brainstem mechanisms underlying the sudden infant death syndrome: Evidence from human pathologic studies. Dev. Psychobiol. 2009, 51, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Strollo, P.J., Jr.; Soose, R.J.; Maurer, J.T.; de Vries, N.; Cornelius, J.; Froymovich, O.; Hanson, R.D.; Padhya, T.A.; Steward, D.L.; Gillespie, M.B.; et al. Upper-Airway Stimulation for Obstructive Sleep Apnea. N. Engl. J. Med. 2014, 370, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Schiefer, M.; Gamble, J.; Baskin, J.; Strohl, K. Hypoglossal nerve stimulation in a rabbit model of obstructive sleep apnea reduces apneas and improves oxygenation. J. Appl. Physiol. 2020, 129, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.D.; Sarber, K.M.; Epperson, M.V.; Tabangin, M.; Altaye, M.; Mesa, F.; Ishman, S.L. Hypoglossal Nerve Stimulation: Outcomes in Veterans with Obstructive Sleep Apnea and Common Comorbid Post-traumatic Stress Disorder. Laryngoscope 2020, 1–11. [Google Scholar] [CrossRef]
- Budzinska, K.; Andrzejewski, K. Respiratory activity in the 6-hydroxydopamine model of Parkinson’s disease in the rat. Acta Neurobiol. Exp. 2014, 74, 67–81. [Google Scholar]
- Andrzejewski, K.; Budzinska, K.; Zaremba, M.; Kaczyńska, K. Hypoxic ventilatory response after dopamine D2 receptor blockade in unilateral rat model of Parkinson’s disease. Neuroscience 2016, 316, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, K.; Zaremba, M.; Kaczyńska, K. Serotonergic system in hypoxic ventilatory response in unilateral rat model of Parkinson’s disease. J. Biomed. Sci. 2017, 24, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Białkowska, M.; Boguszewski, P.; Pokorski, M. Breathing in parkinsonism in the rat. Adv. Exp. Med. Biol. Neurosci. Respir. 2016, 18, 1–11. [Google Scholar]
- Guner, I.; Yelmen, N.; Sahin, G.; Oruc, T. The effect of intracerebroventricular dopamine administration on the respiratory response to hypoxia. Tohoku J. Exp. Med. 2002, 196, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, C.; Lahiri, S.; Mokashi, A. Peripheral and central dopamine receptors in respiratory control. Respir. Physiol. 1989, 76, 327–336. [Google Scholar] [CrossRef]
- Kitahama, K.; Nagatsu, I.; Geffard, M.; Maeda, T. Distribution of dopamine-immunoreactive fibers in the rat brainstem. J. Chem. Neuroanat. 2000, 18, 1–9. [Google Scholar] [CrossRef]
- Granata, A.R.; Woodruff, G.N. Dopaminergic mechanisms in the nucleus tractus solitarius and effects on blood pressure. Brain Res. Bull. 1982, 8, 483–488. [Google Scholar] [CrossRef]
- Yokoyama, C.; Okamura, H.; Nakajima, T.; Taguchi, J.; Ibata, Y. Autoradiographic distribution of (3H)YM-09151-2, a high-affinity and selective antagonist ligand for the dopamine D2 receptor group, in the rat brain and spinal cord. J. Comp. Neurol. 1994, 344, 121–136. [Google Scholar] [CrossRef]
- Lee, K.Z.; Fuller, D.D. Preinspiratory and inspiratory hypoglossal motor output during hypoxia-induced plasticity in the rat. J. Appl. Physiol. 2010, 108, 1187–1198. [Google Scholar] [CrossRef] [Green Version]
- Ezure, K.; Tanaka, I.; Saito, Y. Activity of brainstem respiratory neurones just before the expiration-inspiration transition in the rat. J. Physiol. 2003, 547, 629–640. [Google Scholar] [CrossRef]
- Leiter, J.C.; St-John, W.M. Phrenic, vagal and hypoglossal activities in rat, pre-inspiratory, inspiratory, expiratory components. Respir. Physiol. Neurobiol. 2004, 142, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Z.; Fuller, D.D. Neural control of phrenic motoneuron discharge. Respir. Physiol. Neurobiol. 2011, 179, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Bautista, T.G.; Dutschmann, M. Inhibition of the pontine Kölliker-Fuse nucleus abolishes eupneic inspiratory hypoglossal motor discharge in rat. Neuroscience 2014, 267, 22–29. [Google Scholar] [CrossRef]
- Nomura, T.; Inoue, Y.; Kobayashi, M.; Namba, K.; Nakashima, K. Characteristics of obstructive sleep apnea in patients with Parkinson’s disease. J. Neurol. Sci. 2013, 327, 22–24. [Google Scholar] [CrossRef] [PubMed]
- da Silva-Júnior, F.P.; do Prado, G.F.; Barbosa, E.R.; Tufik, S.; Togeiro, S.M. Sleep disordered breathing in Parkinson’s disease: A critical appraisal. Sleep Med. Rev. 2014, 18, 173–178. [Google Scholar] [CrossRef]
- Ehringer, H.; Hornykiewicz, O. Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Parkinsonism Relat. Disord. 1998, 4, 53–57. [Google Scholar] [CrossRef]
- Zarow, C.; Lyness, S.A.; Mortimer, J.A.; Chui, H.C. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch. Neurol. 2003, 60, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. Invited article: Nervous system pathology in sporadic Parkinson disease. Neurology 2008, 70, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Marie, R.M.; Barre, L.; Rioux, P.; Allain, P.; Lechevalier, B.; Baron, J.C. PET imaging of neocortical monoaminergic terminals in Parkinson’s disease. J. Neural Transm. Park. Dis. Dement. Sect. 1995, 9, 55–71. [Google Scholar] [CrossRef]
- Milsom, W.K.; Sadig, T. Interaction between norepinephrine and hypoxia on carotid body chemoreception in rabbits. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 1893–1898. [Google Scholar] [CrossRef]
- Soulage, C.; Perrin, D.; Cottet-Emard, J.M.; Pequignot, J.M. A6 noradrenergic cell group modulates the hypoxic ventilatory response. Adv. Exp. Med. Biol. 2003, 536, 481–487. [Google Scholar] [PubMed]
- Malheiros-Lima, M.R.; Totola, L.T.; Takakura, A.C.; Moreira, T.S. Impaired chemosensory control of breathing after depletion of bulbospinal catecholaminergic neurons in rats. Pflugers Arch. Eur. J. Physiol 2018, 470, 277–293. [Google Scholar] [CrossRef]
- Rukhadze, I.; Kubin, L. Differential pontomedullary catecholaminergic projections to hypoglossal motor nucleus and viscerosensory nucleus of the solitary tract. J. Chem. Neuroanat. 2007, 33, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Volgin, D.V.; Mackiewicz, M.; Kubin, L. a1B receptors are the main postsynaptic mediators of adrenergic excitation in brainstem motoneurons, a single-cell RT-PCR study. J. Chem. Neuroanat. 2001, 22, 157–166. [Google Scholar] [CrossRef]
- Huot, P.; Fox, S.H.; Brotchie, J.M. The serotonergic system in Parkinson’s disease. Prog. Neurobiol. 2011, 95, 163–212. [Google Scholar] [CrossRef]
- Miguelez, C.; Morera-Herreras, T.; Torrecilla, M.; Ruiz-Ortega, J.A.; Ugedo, L. Interaction between the 5-HT system and the basal ganglia: Functional implication and therapeutic perspective in Parkinson’s disease. Front. Neural Circuits 2014, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Ohno, Y.; Shimizu, S.; Tokudome, K.; Kunisawa, N.; Sasa, M. New insight into the therapeutic role of the serotonergic system in Parkinson’s disease. Prog. Neurobiol. 2015, 134, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Kanamaru, M.; Homma, I. Dorsomedial medullary 5-HT2 receptors mediate immediate onset of initial hyperventilation, airway dilation, and ventilatory decline during hypoxia in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R34–R41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubin, L.; Tojima, H.; Davies, R.O.; Pack, A.I. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci. Lett. 1992, 139, 243–248. [Google Scholar] [CrossRef]
- Fenik, P.; Veasey, S.C. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am. J. Respir. Crit. Care Med. 2003, 167, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Brandes, I.F.; Zuperku, E.J.; Stucke, A.G.; Jakovcevic, D.; Hopp, F.A.; Stuth, E.A. Serotonergic modulation of inspiratory hypoglossal motoneurons in decerebrate dogs. J. Neurophysiol. 2006, 95, 3449–3459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| DA | DOPAC | HVA | DOPAC/DA | HVA/DA | NA | 5HT | 5HIAA | 5HIAA/5HT | |
|---|---|---|---|---|---|---|---|---|---|
| STRIATUM | |||||||||
| Sham | 11301 | 2791 | 1486 | 0.250 | 0.132 | 107.2 | 294.4 | 333.2 | 1.168 |
| ± 678.3 | ± 206.5 | ± 119.7 | ± 0.019 | ± 0.008 | ± 4.172 | ± 28.19 | ± 38.70 | ± 0.140 | |
| Reserpine | 764.24 | 201.61 | 85.24 | 0.372 | 0.214 | 44.01 | 197.3 | 407.6 | 2.309 |
| ± 341.72 | ± 63.00 | ± 34.07 | ± 0.068 | ± 0.102 | ± 6.710 | ± 20.13 | ± 36.59 | ± 0.366 | |
| Student’s t-test | *** | *** | *** | *** | ** | ** | |||
| BRAINSTEM | |||||||||
| Sham | 72.57 | 34.42 | 11.64 | 0.466 | 0.157 | 389.02 | 464.25 | 261.33 | 0.562 |
| ± 3.289 | ± 4.708 | ± 1.751 | ± 0.050 | ± 0.019 | ± 16.03 | ± 13.18 | ± 15.530 | ± 0.028 | |
| Reserpine | 17.64 | 6.015 | 1.294 | 0.318 | 0.040 | 30.30 | 127.43 | 334.22 | 3.775 |
| ± 6.561 | ± 2.919 | ± 1.074 | ± 0.067 | ± 0.018 | ± 8.400 | ± 26.617 | ± 28.312 | ± 0.801 | |
| Student’s t-test | *** | *** | *** | *** | *** | *** | * | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jampolska, M.; Andrzejewski, K.; Zaremba, M.; Joniec-Maciejak, I.; Kaczyńska, K. Deficiency of Biogenic Amines Modulates the Activity of Hypoglossal Nerve in the Reserpine Model of Parkinson’s Disease. Cells 2021, 10, 531. https://doi.org/10.3390/cells10030531
Jampolska M, Andrzejewski K, Zaremba M, Joniec-Maciejak I, Kaczyńska K. Deficiency of Biogenic Amines Modulates the Activity of Hypoglossal Nerve in the Reserpine Model of Parkinson’s Disease. Cells. 2021; 10(3):531. https://doi.org/10.3390/cells10030531
Chicago/Turabian StyleJampolska, Monika, Kryspin Andrzejewski, Małgorzata Zaremba, Ilona Joniec-Maciejak, and Katarzyna Kaczyńska. 2021. "Deficiency of Biogenic Amines Modulates the Activity of Hypoglossal Nerve in the Reserpine Model of Parkinson’s Disease" Cells 10, no. 3: 531. https://doi.org/10.3390/cells10030531
APA StyleJampolska, M., Andrzejewski, K., Zaremba, M., Joniec-Maciejak, I., & Kaczyńska, K. (2021). Deficiency of Biogenic Amines Modulates the Activity of Hypoglossal Nerve in the Reserpine Model of Parkinson’s Disease. Cells, 10(3), 531. https://doi.org/10.3390/cells10030531