Emerging Role of Pericytes and Their Secretome in the Heart
Abstract
:1. Introduction
2. Characteristics of Pericytes
2.1. Origin
2.2. Diversity of Pericytes
2.3. Identification
2.4. The Secretome of Pericytes
3. Pericytes Cross-Talking with Adjacent Cells
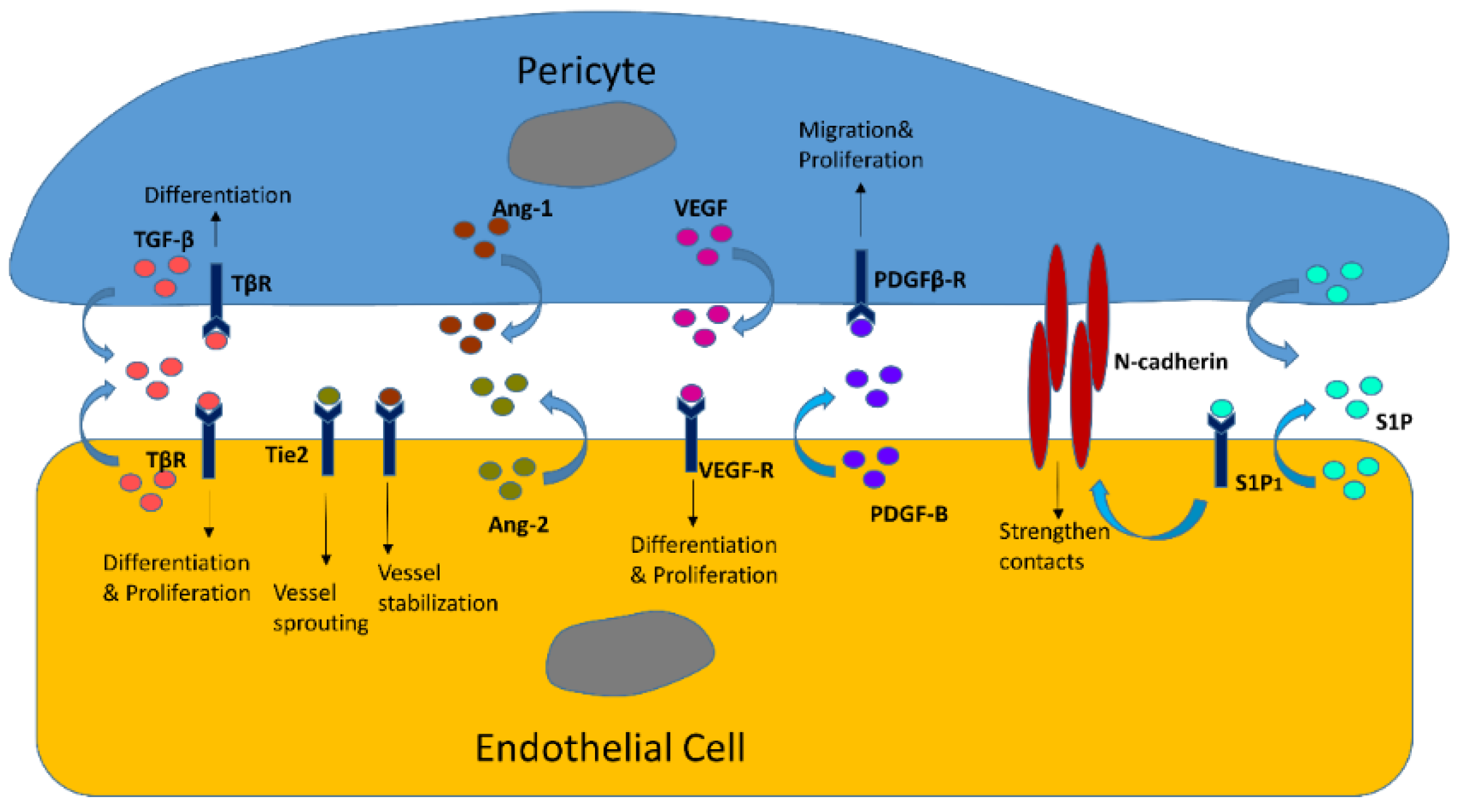
3.1. Endothelial Cells
3.1.1. Reciprocal Interactions in Structures and Functions
3.1.2. EC/Pericyte Interactions in the Heart
3.2. Vascular Smooth Muscle Cells (VSMCs)
3.2.1. Pericyte Coordination with VSMCs
3.2.2. SMC-Like Properties of Pericytes
3.2.3. Pericyte–VSMC Transition
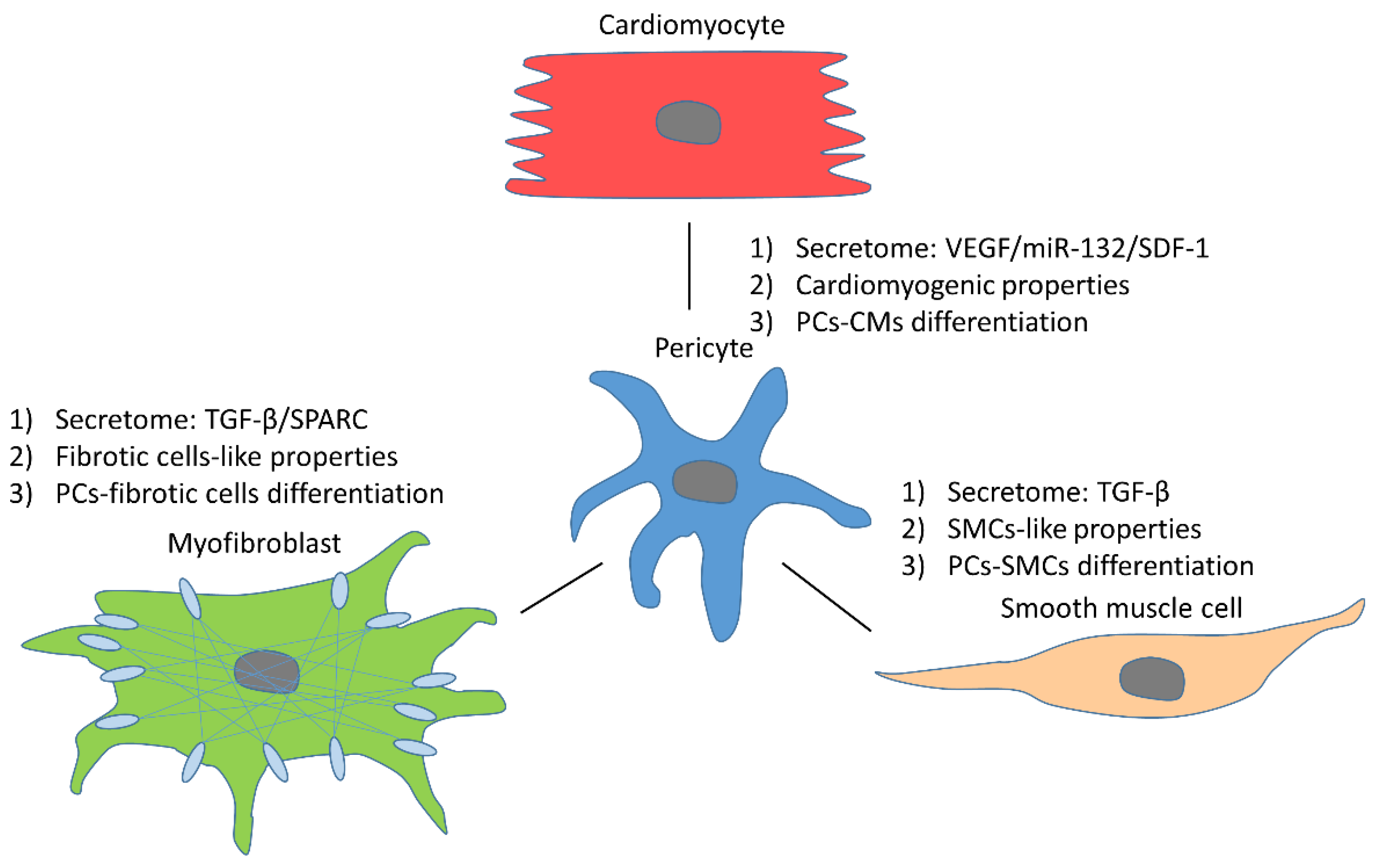
3.3. Pericyte Reciprocal Interactions with Other Cells
3.3.1. Cardiomyocytes
3.3.2. Fibrotic Cells
3.3.3. Telocytes
4. Pericytes and Blood Flow
4.1. Pericytes and VSMCs
4.2. Atherosclerosis
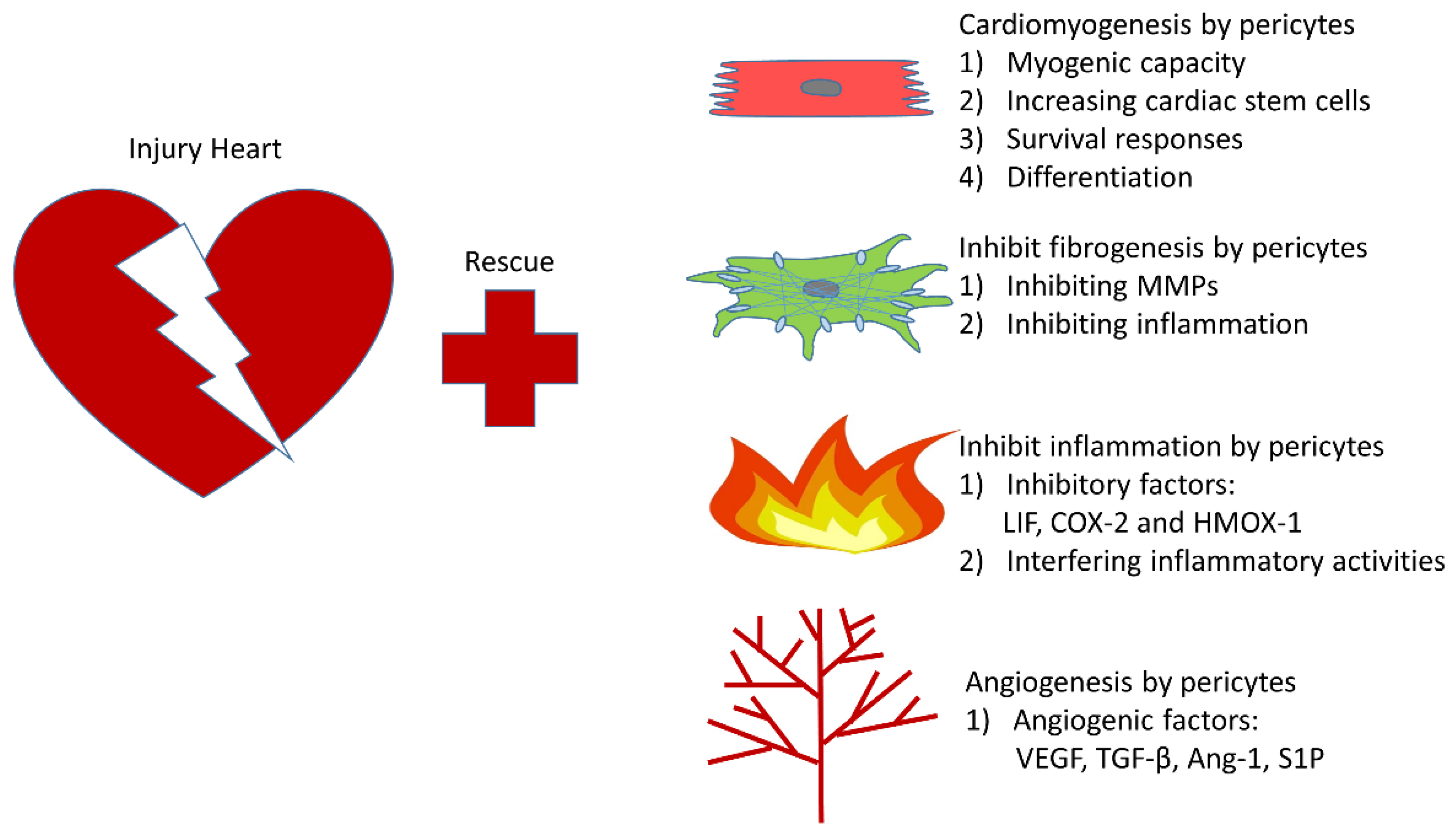
5. The Rapeutic Role of Pericytes in Infarcted Heart
5.1. Cardiomyogenesis
5.2. Cardiac Fibrosis
5.3. Inflammatory Response
5.4. Angiogenesis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Ang-1 | angiopoietins-1 |
| α-SMA | smooth muscle α-actin |
| BBB | blood–brain barrier |
| BMP | bone morphogenetic protein |
| CAD | coronary arterial disease |
| CBF | coronary blood flow |
| CFR | coronary flow reserve |
| COX-2 | cyclooxygenase-2 |
| cTn-T | cardiac troponin-T |
| DLK-1 | delta-like homolog 1 |
| EC | endothelial cells |
| ECM | extracellular matrix |
| EMT | mesenchymal transition |
| EndMT | endothelial–mesenchymal transition |
| hHP | human heart pericyte |
| HMOX-1 | heme oxygenase-1 |
| hPSC | human pluripotent stem cell |
| ICAM-1 | intercellular cell adhesion molecule-1 |
| IFNγ | interferon-γ |
| IL | interleukin |
| IP-10 | interferon gamma-induced protein 10 |
| MMP | matrix metalloproteinases |
| OPG | osteoprotegerin |
| PC | pericyte |
| PDGFR-β | platelet-derived growth factor receptor |
| RGS 5 | regulator of G protein signaling 5 |
| SIRT3 | Sirtuin3 |
| S1P | sphingosine-1-phosphate |
| SPARC | secreted protein acidic and cysteine-rich |
| TGF-β | transforming growth factor-β |
| TIMPs | endogenous inhibitors |
| TNF-α | tumor necrosis factor alpha |
| VEGF | vascular endothelial growth factor |
| VSMC | vascular smooth muscle cell |
| VCAM-1 | vascular cell adhesion molecule-1 |
References
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauch, S.; Reale, E. [The pericytes (Rouget cells) of the stria vascularis vessels]. Arch. Klin. Exp. Ohren Nasen Kehlkopfheilkd. 1968, 192, 82–90. [Google Scholar] [CrossRef]
- Stout, A.P.; Murray, M.R. Hemangiopericytoma: A vascular tumor featuring zimmermann’s pericytes. Ann. Surg. 1942, 116, 26–33. [Google Scholar] [CrossRef]
- Herman, I.M.; D’Amore, P.A. Microvascular pericytes contain muscle and nonmuscle actins. J. Cell Biol. 1985, 101, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyce, N.C.; Haire, M.F.; Palade, G.E. Contractile proteins in pericytes. I. Immunoperoxidase localization of tropomyosin. J. Cell Biol. 1985, 100, 1379–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyce, N.C.; Haire, M.F.; Palade, G.E. Contractile proteins in pericytes. II. Immunocytochemical evidence for the presence of two isomyosins in graded concentrations. J. Cell Biol. 1985, 100, 1387–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geevarghese, A.; Herman, I.M. Pericyte-endothelial crosstalk: Implications and opportunities for advanced cellular therapies. Transl. Res. J. Lab. Clin. Med. 2014, 163, 296–306. [Google Scholar] [CrossRef] [Green Version]
- Sims, D.E. The pericyte—A review. Tissue Cell 1986, 18, 153–174. [Google Scholar] [CrossRef]
- Kumar, A.; D’Souza, S.S.; Moskvin, O.V.; Toh, H.; Wang, B.; Zhang, J.; Swanson, S.; Guo, L.W.; Thomson, J.A.; Slukvin, I.I. Specification and Diversification of Pericytes and Smooth Muscle Cells from Mesenchymoangioblasts. Cell Rep. 2017, 19, 1902–1916. [Google Scholar] [CrossRef] [Green Version]
- Birbrair, A.; Zhang, T.; Files, D.C.; Mannava, S.; Smith, T.; Wang, Z.M.; Messi, M.L.; Mintz, A.; Delbono, O. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res. Ther. 2014, 5, 122. [Google Scholar] [CrossRef] [Green Version]
- Birbrair, A.; Zhang, T.; Wang, Z.M.; Messi, M.L.; Mintz, A.; Delbono, O. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am. J. Physiol. Cell Physiol. 2013, 305, C1098–C1113. [Google Scholar] [CrossRef]
- Birbrair, A.; Zhang, T.; Wang, Z.M.; Messi, M.L.; Olson, J.D.; Mintz, A.; Delbono, O. Type-2 pericytes participate in normal and tumoral angiogenesis. Am. J. Physiol. Cell Physiol. 2014, 307, C25–C38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asahina, K.; Zhou, B.; Pu, W.T.; Tsukamoto, H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology 2011, 53, 983–995. [Google Scholar] [CrossRef] [Green Version]
- Wilm, B.; Ipenberg, A.; Hastie, N.D.; Burch, J.B.; Bader, D.M. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 2005, 132, 5317–5328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, I.R.; Baily, J.E.; Chen, W.C.W.; Dar, A.; Gonzalez, Z.N.; Jensen, A.R.; Petrigliano, F.A.; Deb, A.; Henderson, N.C. Skeletal and cardiac muscle pericytes: Functions and therapeutic potential. Pharmacol. Ther. 2017, 171, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Zhang, H.; Liu, Y.; Adams, S.; Eilken, H.; Stehling, M.; Corada, M.; Dejana, E.; Zhou, B.; Adams, R.H. Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nat. Commun. 2016, 7, 12422. [Google Scholar] [CrossRef] [PubMed]
- Etchevers, H.C.; Vincent, C.; Le Douarin, N.M.; Couly, G.F. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 2001, 128, 1059–1068. [Google Scholar] [PubMed]
- Bergwerff, M.; Verberne, M.E.; DeRuiter, M.C.; Poelmann, R.E.; Gittenberger-de Groot, A.C. Neural crest cell contribution to the developing circulatory system: Implications for vascular morphology? Circ. Res. 1998, 82, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef] [Green Version]
- Nees, S.; Weiss, D.R.; Senftl, A.; Knott, M.; Förch, S.; Schnurr, M.; Weyrich, P.; Juchem, G. Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: The second most frequent myocardial cell type in vitro. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H69–H84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armulik, A.; Mäe, M.; Betsholtz, C. Pericytes and the blood-brain barrier: Recent advances and implications for the delivery of CNS therapy. Ther. Deliv. 2011, 2, 419–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishioku, T.; Dohgu, S.; Takata, F.; Eto, T.; Ishikawa, N.; Kodama, K.B.; Nakagawa, S.; Yamauchi, A.; Kataoka, Y. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood-brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell. Mol. Neurobiol. 2009, 29, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Nuttall, A.; Yang, Y.; Shi, X. Visualization and contractile activity of cochlear pericytes in the capillaries of the spiral ligament. Hear. Res. 2009, 254, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avolio, E.; Rodriguez-Arabaolaza, I.; Spencer, H.L.; Riu, F.; Mangialardi, G.; Slater, S.C.; Rowlinson, J.; Alvino, V.V.; Idowu, O.O.; Soyombo, S.; et al. Expansion and characterization of neonatal cardiac pericytes provides a novel cellular option for tissue engineering in congenital heart disease. J. Am. Heart Assoc. 2015, 4, e002043. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, H.; Betsholtz, C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003, 314, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hellström, M.; Kalén, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126, 3047–3055. [Google Scholar] [PubMed]
- Morikawa, S.; Baluk, P.; Kaidoh, T.; Haskell, A.; Jain, R.K.; McDonald, D.M. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 2002, 160, 985–1000. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Zeng, H.; Liu, B.; Chen, J.X. Sirtuin 3 is essential for hypertension-induced cardiac fibrosis via mediating pericyte transition. J. Cell Mol. Med. 2020, 24, 8057–8068. [Google Scholar] [CrossRef]
- Berger, M.; Bergers, G.; Arnold, B.; Hämmerling, G.J.; Ganss, R. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood 2005, 105, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, P.; Johansson, B.R.; Levéen, P.; Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997, 277, 242–245. [Google Scholar] [CrossRef]
- Huang, F.J.; You, W.K.; Bonaldo, P.; Seyfried, T.N.; Pasquale, E.B.; Stallcup, W.B. Pericyte deficiencies lead to aberrant tumor vascularizaton in the brain of the NG2 null mouse. Dev. Biol. 2010, 344, 1035–1046. [Google Scholar] [CrossRef] [Green Version]
- Ozerdem, U.; Grako, K.A.; Dahlin-Huppe, K.; Monosov, E.; Stallcup, W.B. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2001, 222, 218–227. [Google Scholar] [CrossRef]
- Nehls, V.; Drenckhahn, D. The versatility of microvascular pericytes: From mesenchyme to smooth muscle? Histochemistry 1993, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guimarães-Camboa, N.; Cattaneo, P.; Sun, Y.; Moore-Morris, T.; Gu, Y.; Dalton, N.D.; Rockenstein, E.; Masliah, E.; Peterson, K.L.; Stallcup, W.B.; et al. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell 2017, 20, 345–359.e5. [Google Scholar] [CrossRef] [Green Version]
- Volz, K.S.; Jacobs, A.H.; Chen, H.I.; Poduri, A.; McKay, A.S.; Riordan, D.P.; Kofler, N.; Kitajewski, J.; Weissman, I.; Red-Horse, K. Pericytes are progenitors for coronary artery smooth muscle. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Su, H.; He, X.; Chen, J.X.; Zeng, H. SIRT3 Deficiency Sensitizes Angiotensin-II-Induced Renal Fibrosis. Cells 2020, 9, 2510. [Google Scholar] [CrossRef]
- Milesi, S.; Boussadia, B.; Plaud, C.; Catteau, M.; Rousset, M.C.; De Bock, F.; Schaeffer, M.; Lerner-Natoli, M.; Rigau, V.; Marchi, N. Redistribution of PDGFRβ cells and NG2DsRed pericytes at the cerebrovasculature after status epilepticus. Neurobiol. Dis. 2014, 71, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Gaceb, A.; Barbariga, M.; Özen, I.; Paul, G. The pericyte secretome: Potential impact on regeneration. Biochimie 2018, 155, 16–25. [Google Scholar] [CrossRef]
- Gaceb, A.; Paul, G. Pericyte Secretome. Adv. Exp. Med. Biol. 2018, 1109, 139–163. [Google Scholar] [CrossRef]
- Alcendor, D.J.; Charest, A.M.; Zhu, W.Q.; Vigil, H.E.; Knobel, S.M. Infection and upregulation of proinflammatory cytokines in human brain vascular pericytes by human cytomegalovirus. J. Neuroinflammation 2012, 9, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.M.; Graham, E.S.; Feng, S.X.; Oldfield, R.L.; Bergin, P.M.; Mee, E.W.; Faull, R.L.; Curtis, M.A.; Dragunow, M. Adult human glia, pericytes and meningeal fibroblasts respond similarly to IFNy but not to TGFβ1 or M-CSF. PLoS ONE 2013, 8, e80463. [Google Scholar] [CrossRef] [PubMed]
- Guijarro-Muñoz, I.; Compte, M.; Álvarez-Cienfuegos, A.; Álvarez-Vallina, L.; Sanz, L. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-κB signaling pathway and proinflammatory response in human pericytes. J. Biol. Chem. 2014, 289, 2457–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehmé, A.; Edelman, J. Dexamethasone inhibits high glucose-, TNF-alpha-, and IL-1beta-induced secretion of inflammatory and angiogenic mediators from retinal microvascular pericytes. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2030–2038. [Google Scholar] [CrossRef]
- Ghannam, S.; Bouffi, C.; Djouad, F.; Jorgensen, C.; Noël, D. Immunosuppression by mesenchymal stem cells: Mechanisms and clinical applications. Stem Cell Res. Ther. 2010, 1, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Hu, G.; Su, J.; Li, W.; Chen, Q.; Shou, P.; Xu, C.; Chen, X.; Huang, Y.; Zhu, Z.; et al. Mesenchymal stem cells: A new strategy for immunosuppression and tissue repair. Cell Res. 2010, 20, 510–518. [Google Scholar] [CrossRef]
- Luo, M.; Li, J.F.; Yang, Q.; Zhang, K.; Wang, Z.W.; Zheng, S.; Zhou, J.J. Stem cell quiescence and its clinical relevance. World J. Stem Cells 2020, 12, 1307–1326. [Google Scholar] [CrossRef]
- Singh, A.; Veeriah, V.; Xi, P.; Labella, R.; Chen, J.; Romeo, S.G.; Ramasamy, S.K.; Kusumbe, A.P. Angiocrine signals regulate quiescence and therapy resistance in bone metastasis. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Von Tell, D.; Armulik, A.; Betsholtz, C. Pericytes and vascular stability. Exp. Cell Res. 2006, 312, 623–629. [Google Scholar] [CrossRef]
- Brown, L.A.; Cox, C.; Baptiste, J.; Summers, H.; Button, R.; Bahlow, K.; Spurrier, V.; Kyser, J.; Luttge, B.G.; Kuo, L.; et al. NMR structure of the myristylated feline immunodeficiency virus matrix protein. Viruses 2015, 7, 2210–2229. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Avolio, E.; Mangialardi, G.; Slater, S.C.; Alvino, V.V.; Gu, Y.; Cathery, W.; Beltrami, A.P.; Katare, R.; Heesom, K.; Caputo, M.; et al. Secreted Protein Acidic and Cysteine Rich Matricellular Protein Is Enriched in the Bioactive Fraction of the Human Vascular Pericyte Secretome. Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef] [PubMed]
- Eilken, H.M.; Diéguez-Hurtado, R.; Schmidt, I.; Nakayama, M.; Jeong, H.W.; Arf, H.; Adams, S.; Ferrara, N.; Adams, R.H. Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat. Commun. 2017, 8, 1574. [Google Scholar] [CrossRef] [Green Version]
- Cuevas, P.; Gutierrez-Diaz, J.A.; Reimers, D.; Dujovny, M.; Diaz, F.G.; Ausman, J.I. Pericyte endothelial gap junctions in human cerebral capillaries. Anat. Embryol. 1984, 170, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Tilton, R.G.; Kilo, C.; Williamson, J.R. Pericyte-endothelial relationships in cardiac and skeletal muscle capillaries. Microvasc. Res. 1979, 18, 325–335. [Google Scholar] [CrossRef]
- Gerhardt, H.; Wolburg, H.; Redies, C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2000, 218, 472–479. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; Madrid, J.F.; Varela, H.; Valladares, F.; Acosta, E.; Martín-Vasallo, P.; Díaz-Flores, L., Jr. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol. Histopathol. 2009, 24, 909–969. [Google Scholar] [CrossRef]
- Sieczkiewicz, G.J.; Herman, I.M. TGF-beta 1 signaling controls retinal pericyte contractile protein expression. Microvasc. Res. 2003, 66, 190–196. [Google Scholar] [CrossRef]
- Li, J.; Brown, L.F.; Hibberd, M.G.; Grossman, J.D.; Morgan, J.P.; Simons, M. VEGF, flk-1, and flt-1 expression in a rat myocardial infarction model of angiogenesis. Am. J. Physiol. 1996, 270, H1803–H1811. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Shamskhou, E.A.; Orcholski, M.E.; Nathan, A.; Reddy, S.; Honda, H.; Mani, V.; Zeng, Y.; Ozen, M.O.; Wang, L.; et al. Loss of Endothelium-Derived Wnt5a Is Associated With Reduced Pericyte Recruitment and Small Vessel Loss in Pulmonary Arterial Hypertension. Circulation 2019, 139, 1710–1724. [Google Scholar] [CrossRef]
- Syväranta, S.; Helske, S.; Laine, M.; Lappalainen, J.; Kupari, M.; Mäyränpää, M.I.; Lindstedt, K.A.; Kovanen, P.T. Vascular endothelial growth factor-secreting mast cells and myofibroblasts: A novel self-perpetuating angiogenic pathway in aortic valve stenosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1220–1227. [Google Scholar] [CrossRef] [Green Version]
- Kono, M.; Mi, Y.; Liu, Y.; Sasaki, T.; Allende, M.L.; Wu, Y.P.; Yamashita, T.; Proia, R.L. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 2004, 279, 29367–29373. [Google Scholar] [CrossRef] [Green Version]
- Teichert, M.; Milde, L.; Holm, A.; Stanicek, L.; Gengenbacher, N.; Savant, S.; Ruckdeschel, T.; Hasanov, Z.; Srivastava, K.; Hu, J.; et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat. Commun. 2017, 8, 16106. [Google Scholar] [CrossRef]
- Gaengel, K.; Genové, G.; Armulik, A.; Betsholtz, C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 630–638. [Google Scholar] [CrossRef] [Green Version]
- Ejaz, S.; Chekarova, I.; Ejaz, A.; Sohail, A.; Lim, C.W. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. DiabetesObes. Metab. 2008, 10, 53–63. [Google Scholar] [CrossRef]
- Hammes, H.P.; Lin, J.; Renner, O.; Shani, M.; Lundqvist, A.; Betsholtz, C.; Brownlee, M.; Deutsch, U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 2002, 51, 3107–3112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfister, F.; Feng, Y.; vom Hagen, F.; Hoffmann, S.; Molema, G.; Hillebrands, J.L.; Shani, M.; Deutsch, U.; Hammes, H.P. Pericyte migration: A novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes 2008, 57, 2495–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamagishi, S.; Imaizumi, T. Pericyte biology and diseases. Int. J. Tissue React. 2005, 27, 125–135. [Google Scholar]
- Chintalgattu, V.; Rees, M.L.; Culver, J.C.; Goel, A.; Jiffar, T.; Zhang, J.; Dunner, K., Jr.; Pati, S.; Bankson, J.A.; Pasqualini, R.; et al. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci. Transl. Med. 2013, 5, 187ra169. [Google Scholar] [CrossRef] [Green Version]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Klett, F.; Priller, J. Diverse functions of pericytes in cerebral blood flow regulation and ischemia. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2015, 35, 883–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuuminen, R.; Syrjälä, S.; Krebs, R.; Keränen, M.A.; Koli, K.; Abo-Ramadan, U.; Neuvonen, P.J.; Tikkanen, J.M.; Nykänen, A.I.; Lemström, K.B. Donor simvastatin treatment abolishes rat cardiac allograft ischemia/reperfusion injury and chronic rejection through microvascular protection. Circulation 2011, 124, 1138–1150. [Google Scholar] [CrossRef] [Green Version]
- Nykänen, A.I.; Tuuminen, R.; Lemström, K.B. Donor simvastatin treatment and cardiac allograft ischemia/reperfusion injury. Trends Cardiovasc. Med. 2013, 23, 85–90. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, F.M.; Attwell, D. A role for pericytes in coronary no-reflow. Nat. Rev. Cardiol. 2014, 11, 427–432. [Google Scholar] [CrossRef]
- Jin, S.; Hansson, E.M.; Tikka, S.; Lanner, F.; Sahlgren, C.; Farnebo, F.; Baumann, M.; Kalimo, H.; Lendahl, U. Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ. Res. 2008, 102, 1483–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, D.S. A novel mechanism of vascular smooth muscle cell regulation by Notch: Platelet-derived growth factor receptor-beta expression? Circ. Res. 2008, 102, 1448–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Liu, X.Y.; Fagan, A.; Gonzalez-Toledo, M.E.; Zhao, L.R. Ultrastructural changes in cerebral capillary pericytes in aged Notch3 mutant transgenic mice. Ultrastruct. Pathol. 2012, 36, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, L.; Moens, C.B.; Appel, B. Notch3 establishes brain vascular integrity by regulating pericyte number. Development 2014, 141, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Boulos, N.; Helle, F.; Dussaule, J.C.; Placier, S.; Milliez, P.; Djudjaj, S.; Guerrot, D.; Joutel, A.; Ronco, P.; Boffa, J.J.; et al. Notch3 is essential for regulation of the renal vascular tone. Hypertension 2011, 57, 1176–1182. [Google Scholar] [CrossRef] [Green Version]
- Ragot, H.; Monfort, A.; Baudet, M.; Azibani, F.; Fazal, L.; Merval, R.; Polidano, E.; Cohen-Solal, A.; Delcayre, C.; Vodovar, N.; et al. Loss of Notch3 Signaling in Vascular Smooth Muscle Cells Promotes Severe Heart Failure Upon Hypertension. Hypertension 2016, 68, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.K.; Zeng, H.; Zhang, G.Q.; Chen, S.T.; Xie, X.J.; He, X.; Wang, S.; Wen, H.; Chen, J.X. Notch3 deficiency impairs coronary microvascular maturation and reduces cardiac recovery after myocardial ischemia. Int. J. Cardiol. 2017, 236, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.X.; Chen, S.T.; Tao, Y.K. Cardiac pericyte is promising target for ischemic heart diseases: Role of Notch3. Int. J. Cardiol. 2017, 246, 57. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Vaka, V.R.; He, X.; Booz, G.W.; Chen, J.X. High-fat diet induces cardiac remodelling and dysfunction: Assessment of the role played by SIRT3 loss. J. Cell Mol. Med. 2015, 19, 1847–1856. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Zeng, H.; Chen, J.X. Ablation of SIRT3 causes coronary microvascular dysfunction and impairs cardiac recovery post myocardial ischemia. Int. J. Cardiol. 2016, 215, 349–357. [Google Scholar] [CrossRef]
- Zeng, H.; He, X.; Tuo, Q.H.; Liao, D.F.; Zhang, G.Q.; Chen, J.X. LPS causes pericyte loss and microvascular dysfunction via disruption of Sirt3/angiopoietins/Tie-2 and HIF-2α/Notch3 pathways. Sci. Rep. 2016, 6, 20931. [Google Scholar] [CrossRef] [Green Version]
- Souders, C.A.; Bowers, S.L.; Baudino, T.A. Cardiac fibroblast: The renaissance cell. Circ. Res. 2009, 105, 1164–1176. [Google Scholar] [CrossRef]
- Lin, S.L.; Kisseleva, T.; Brenner, D.A.; Duffield, J.S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 2008, 173, 1617–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, H.; Chen, J.X. Sirtuin 3, Endothelial Metabolic Reprogramming, and Heart Failure With Preserved Ejection Fraction. J. Cardiovasc. Pharmacol. 2019, 74, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Avolio, E.; Alvino, V.V.; Ghorbel, M.T.; Campagnolo, P. Perivascular cells and tissue engineering: Current applications and untapped potential. Pharmacol. Ther. 2017, 171, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Koike, N.; Fukumura, D.; Gralla, O.; Au, P.; Schechner, J.S.; Jain, R.K. Tissue engineering: Creation of long-lasting blood vessels. Nature 2004, 428, 138–139. [Google Scholar] [CrossRef]
- Weinberg, C.B.; Bell, E. A blood vessel model constructed from collagen and cultured vascular cells. Science 1986, 231, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Peppiatt, C.M.; Howarth, C.; Mobbs, P.; Attwell, D. Bidirectional control of CNS capillary diameter by pericytes. Nature 2006, 443, 700–704. [Google Scholar] [CrossRef] [Green Version]
- O’Farrell, F.M.; Mastitskaya, S.; Hammond-Haley, M.; Freitas, F.; Wah, W.R.; Attwell, D. Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Yemisci, M.; Gursoy-Ozdemir, Y.; Vural, A.; Can, A.; Topalkara, K.; Dalkara, T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 2009, 15, 1031–1037. [Google Scholar] [CrossRef]
- Almaça, J.; Weitz, J.; Rodriguez-Diaz, R.; Pereira, E.; Caicedo, A. The Pericyte of the Pancreatic Islet Regulates Capillary Diameter and Local Blood Flow. Cell Metab. 2018, 27, 630–644.e4. [Google Scholar] [CrossRef] [Green Version]
- Kirton, J.P.; Wilkinson, F.L.; Canfield, A.E.; Alexander, M.Y. Dexamethasone downregulates calcification-inhibitor molecules and accelerates osteogenic differentiation of vascular pericytes: Implications for vascular calcification. Circ. Res. 2006, 98, 1264–1272. [Google Scholar] [CrossRef] [Green Version]
- Nees, S.; Weiss, D.R.; Juchem, G. Focus on cardiac pericytes. Pflug. Arch. Eur. J. Physiol. 2013, 465, 779–787. [Google Scholar] [CrossRef]
- Birbrair, A.; Zhang, T.; Wang, Z.M.; Messi, M.L.; Mintz, A.; Delbono, O. Pericytes at the intersection between tissue regeneration and pathology. Clin. Sci. 2015, 128, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Acharya, A.; Baek, S.T.; Huang, G.; Eskiocak, B.; Goetsch, S.; Sung, C.Y.; Banfi, S.; Sauer, M.F.; Olsen, G.S.; Duffield, J.S.; et al. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 2012, 139, 2139–2149. [Google Scholar] [CrossRef] [Green Version]
- Braitsch, C.M.; Combs, M.D.; Quaggin, S.E.; Yutzey, K.E. Pod1/Tcf21 is regulated by retinoic acid signaling and inhibits differentiation of epicardium-derived cells into smooth muscle in the developing heart. Dev. Biol. 2012, 368, 345–357. [Google Scholar] [CrossRef] [Green Version]
- Sacchetti, B.; Funari, A.; Remoli, C.; Giannicola, G.; Kogler, G.; Liedtke, S.; Cossu, G.; Serafini, M.; Sampaolesi, M.; Tagliafico, E.; et al. No Identical “Mesenchymal Stem Cells” at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Rep. 2016, 6, 897–913. [Google Scholar] [CrossRef] [Green Version]
- Katare, R.G.; Madeddu, P. Pericytes from human veins for treatment of myocardial ischemia. Trends Cardiovasc. Med. 2013, 23, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Katare, R.; Riu, F.; Mitchell, K.; Gubernator, M.; Campagnolo, P.; Cui, Y.; Fortunato, O.; Avolio, E.; Cesselli, D.; Beltrami, A.P.; et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ. Res. 2011, 109, 894–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohnishi, S.; Yanagawa, B.; Tanaka, K.; Miyahara, Y.; Obata, H.; Kataoka, M.; Kodama, M.; Ishibashi-Ueda, H.; Kangawa, K.; Kitamura, S.; et al. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J. Mol. Cell Cardiol. 2007, 42, 88–97. [Google Scholar] [CrossRef]
- Graumann, U.; Ritz, M.F.; Rivero, B.G.; Hausmann, O. CD133 expressing pericytes and relationship to SDF-1 and CXCR4 in spinal cord injury. Curr. Neurovascular Res. 2010, 7, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Ellison-Hughes, G.M.; Madeddu, P. Exploring pericyte and cardiac stem cell secretome unveils new tactics for drug discovery. Pharmacol. Ther. 2017, 171, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.G.; Andrejecsk, J.W.; Kluger, M.S.; Saltzman, W.M.; Pober, J.S. Pericytes modulate endothelial sprouting. Cardiovasc. Res. 2013, 100, 492–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendel, J.S.; Ye, L.; Zhang, P.; Tranquillo, R.T.; Zhang, J.J. Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng. Part A 2014, 20, 1325–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, K.T.; Dries-Devlin, J.L.; Tranquillo, R.T. Engineered microvessels with strong alignment and high lumen density via cell-induced fibrin gel compaction and interstitial flow. Tissue Eng. Part A 2014, 20, 553–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltrami, A.P.; Cesselli, D.; Bergamin, N.; Marcon, P.; Rigo, S.; Puppato, E.; D’Aurizio, F.; Verardo, R.; Piazza, S.; Pignatelli, A.; et al. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow). Blood 2007, 110, 3438–3446. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, A.P.; Madeddu, P. Pericytes and cardiac stem cells: Common features and peculiarities. Pharmacol. Res. 2018, 127, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.C.; Baily, J.E.; Corselli, M.; Díaz, M.E.; Sun, B.; Xiang, G.; Gray, G.A.; Huard, J.; Péault, B. Human myocardial pericytes: Multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells 2015, 33, 557–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. CMLS 2014, 71, 549–574. [Google Scholar] [CrossRef] [Green Version]
- Berk, B.C.; Fujiwara, K.; Lehoux, S. ECM remodeling in hypertensive heart disease. J. Clin. Investig. 2007, 117, 568–575. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol. Asp. Med. 2019, 65, 70–99. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Regulation of the inflammatory response in cardiac repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Asp. Med. 2019, 65, 2–15. [Google Scholar] [CrossRef]
- Leask, A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ. Res. 2010, 106, 1675–1680. [Google Scholar] [CrossRef] [Green Version]
- Frangogiannis, N.G. Galectin-3 in the fibrotic response: Cellular targets and molecular mechanisms. Int. J. Cardiol. 2018, 258, 226–227. [Google Scholar] [CrossRef]
- Birbrair, A.; Zhang, T.; Wang, Z.M.; Messi, M.L.; Enikolopov, G.N.; Mintz, A.; Delbono, O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013, 22, 2298–2314. [Google Scholar] [CrossRef] [Green Version]
- Sottile, J.; Hocking, D.C. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol. Biol. Cell 2002, 13, 3546–3559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.W.; Okada, M.; Proto, J.D.; Gao, X.; Sekiya, N.; Beckman, S.A.; Corselli, M.; Crisan, M.; Saparov, A.; Tobita, K.; et al. Human pericytes for ischemic heart repair. Stem Cells 2013, 31, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Alvino, V.V.; Fernández-Jiménez, R.; Rodriguez-Arabaolaza, I.; Slater, S.; Mangialardi, G.; Avolio, E.; Spencer, H.; Culliford, L.; Hassan, S.; Sueiro Ballesteros, L.; et al. Transplantation of Allogeneic Pericytes Improves Myocardial Vascularization and Reduces Interstitial Fibrosis in a Swine Model of Reperfused Acute Myocardial Infarction. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Maadawi, Z.M. A Tale of Two Cells: Telocyte and Stem Cell Unique Relationship. Adv. Exp. Med. Biol. 2016, 913, 359–376. [Google Scholar] [CrossRef]
- Iancu, C.B.; Rusu, M.C.; Mogoantă, L.; Hostiuc, S.; Grigoriu, M. Myocardial Telocyte-Like Cells: A Review Including New Evidence. CellsTissuesOrgans 2018, 206, 16–25. [Google Scholar] [CrossRef]
- Gherghiceanu, M.; Popescu, L.M. Cardiac telocytes—Their junctions and functional implications. Cell Tissue Res. 2012, 348, 265–279. [Google Scholar] [CrossRef] [Green Version]
- Suciu, L.C.; Popescu, B.O.; Kostin, S.; Popescu, L.M. Platelet-derived growth factor receptor-β-positive telocytes in skeletal muscle interstitium. J. Cell. Mol. Med. 2012, 16, 701–707. [Google Scholar] [CrossRef]
- Grigoriu, F.; Hostiuc, S.; Vrapciu, A.D.; Rusu, M.C. Subsets of telocytes: The progenitor cells in the human endocardial niche. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2016, 57, 767–774. [Google Scholar]
- Ziegler, T.; Horstkotte, J.; Schwab, C.; Pfetsch, V.; Weinmann, K.; Dietzel, S.; Rohwedder, I.; Hinkel, R.; Gross, L.; Lee, S.; et al. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J. Clin. Investig. 2013, 123, 3436–3445. [Google Scholar] [CrossRef] [Green Version]
- Summerhill, V.; Orekhov, A. Pericytes in Atherosclerosis. Adv. Exp. Med. Biol. 2019, 1147, 279–297. [Google Scholar] [CrossRef]
- Hamilton, N.B.; Attwell, D.; Hall, C.N. Pericyte-mediated regulation of capillary diameter: A component of neurovascular coupling in health and disease. Front. Neuroenergetics 2010, 2. [Google Scholar] [CrossRef] [Green Version]
- Matsugi, T.; Chen, Q.; Anderson, D.R. Adenosine-induced relaxation of cultured bovine retinal pericytes. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2695–2701. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Korn, J.; Christ, B.; Kurz, H. Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J. Comp. Neurol. 2002, 442, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [Green Version]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Orekhov, A.N.; Andreeva, E.R.; Bobryshev, Y.V. Cellular mechanisms of human atherosclerosis: Role of cell-to-cell communications in subendothelial cell functions. Tissue Cell 2016, 48, 25–34. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Bobryshev, Y.V.; Chistiakov, D.A. The complexity of cell composition of the intima of large arteries: Focus on pericyte-like cells. Cardiovasc. Res. 2014, 103, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Davaine, J.M.; Quillard, T.; Chatelais, M.; Guilbaud, F.; Brion, R.; Guyomarch, B.; Brennan, M.; Heymann, D.; Heymann, M.F.; Gouëffic, Y. Bone Like Arterial Calcification in Femoral Atherosclerotic Lesions: Prevalence and Role of Osteoprotegerin and Pericytes. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2016, 51, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Shashkin, P.; Dragulev, B.; Ley, K. Macrophage differentiation to foam cells. Curr. Pharm. Des. 2005, 11, 3061–3072. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.S.; Cai, J.; Towler, D.A. Molecular mechanisms of vascular calcification: Lessons learned from the aorta. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1423–1430. [Google Scholar] [CrossRef]
- Liu, R.; Lauridsen, H.M.; Amezquita, R.A.; Pierce, R.W.; Jane-Wit, D.; Fang, C.; Pellowe, A.S.; Kirkiles-Smith, N.C.; Gonzalez, A.L.; Pober, J.S. IL-17 Promotes Neutrophil-Mediated Immunity by Activating Microvascular Pericytes and Not Endothelium. J. Immunol. 2016, 197, 2400–2408. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Andreeva, E.R.; Andrianova, I.V.; Bobryshev, Y.V. Peculiarities of cell composition and cell proliferation in different type atherosclerotic lesions in carotid and coronary arteries. Atherosclerosis 2010, 212, 436–443. [Google Scholar] [CrossRef]
- Mohler, E.R., 3rd; Gannon, F.; Reynolds, C.; Zimmerman, R.; Keane, M.G.; Kaplan, F.S. Bone formation and inflammation in cardiac valves. Circulation 2001, 103, 1522–1528. [Google Scholar] [CrossRef]
- Collett, G.D.; Canfield, A.E. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ. Res. 2005, 96, 930–938. [Google Scholar] [CrossRef]
- Mohler, E.R., 3rd. Mechanisms of aortic valve calcification. Am. J. Cardiol. 2004, 94, 1396–1402. [Google Scholar] [CrossRef]
- Rajamannan, N.M.; Gersh, B.; Bonow, R.O. Calcific aortic stenosis: From bench to the bedside--emerging clinical and cellular concepts. Heart 2003, 89, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.M.; Miller, J.D.; Heistad, D.D. Fibrocalcific aortic valve disease: Opportunity to understand disease mechanisms using mouse models. Circ. Res. 2013, 113, 209–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolle, I.G.; Crivellari, I.; Zanello, A.; Mazzega, E.; Dalla, E.; Bulfoni, M.; Avolio, E.; Battistella, A.; Lazzarino, M.; Cellot, A.; et al. Heart failure impairs the mechanotransduction propeties of human cardiac pericytes. J. Mol. Cell. Cardiol. 2020, 151, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Avolio, E.; Meloni, M.; Spencer, H.L.; Riu, F.; Katare, R.; Mangialardi, G.; Oikawa, A.; Rodriguez-Arabaolaza, I.; Dang, Z.; Mitchell, K.; et al. Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circ. Res. 2015, 116, e81–e94. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, S.; Sumiyoshi, H.; Kitamura, S.; Nagaya, N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett. 2007, 581, 3961–3966. [Google Scholar] [CrossRef] [Green Version]
- Mias, C.; Lairez, O.; Trouche, E.; Roncalli, J.; Calise, D.; Seguelas, M.H.; Ordener, C.; Piercecchi-Marti, M.D.; Auge, N.; Salvayre, A.N.; et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells 2009, 27, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- Dobaczewski, M.; Gonzalez-Quesada, C.; Frangogiannis, N.G. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J. Mol. Cell Cardiol. 2010, 48, 504–511. [Google Scholar] [CrossRef] [Green Version]
- Teder, P.; Vandivier, R.W.; Jiang, D.; Liang, J.; Cohn, L.; Puré, E.; Henson, P.M.; Noble, P.W. Resolution of lung inflammation by CD44. Science 2002, 296, 155–158. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Ren, G.; Dewald, O.; Zymek, P.; Haudek, S.; Koerting, A.; Winkelmann, K.; Michael, L.H.; Lawler, J.; Entman, M.L. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation 2005, 111, 2935–2942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, G.; Su, J.; Zhang, L.; Zhao, X.; Ling, W.; L’Huillie, A.; Zhang, J.; Lu, Y.; Roberts, A.I.; Ji, W.; et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 2009, 27, 1954–1962. [Google Scholar] [CrossRef]
- Ren, G.; Michael, L.H.; Entman, M.L.; Frangogiannis, N.G. Morphological characteristics of the microvasculature in healing myocardial infarcts. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2002, 50, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Payne, T.R.; Oshima, H.; Okada, M.; Momoi, N.; Tobita, K.; Keller, B.B.; Peng, H.; Huard, J. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J. Am. Coll. Cardiol. 2007, 50, 1677–1684. [Google Scholar] [CrossRef] [Green Version]
- James, A.W.; Zara, J.N.; Zhang, X.; Askarinam, A.; Goyal, R.; Chiang, M.; Yuan, W.; Chang, L.; Corselli, M.; Shen, J.; et al. Perivascular stem cells: A prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl. Med. 2012, 1, 510–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enciso, J.M.; Hirschi, K.K. Understanding abnormalities in vascular specification and remodeling. Pediatrics 2005, 116, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, S.; Yasuda, T.; Kitamura, S.; Nagaya, N. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells 2007, 25, 1166–1177. [Google Scholar] [CrossRef]
- Au, P.; Tam, J.; Fukumura, D.; Jain, R.K. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 2008, 111, 4551–4558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campagnolo, P.; Cesselli, D.; Al Haj Zen, A.; Beltrami, A.P.; Kränkel, N.; Katare, R.; Angelini, G.; Emanueli, C.; Madeddu, P. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation 2010, 121, 1735–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Pericytes Type-I | Pericytes Type-II | |
|---|---|---|
| Marker | PDGFR-α+/Nestin-GFP−/NG2-DsRed+ | PDGFR-α−/Nestin-GFP+/NG2-DsRed+ |
| Distribution | Capillary phenotype | Arteriolar phenotype |
| Function | Adipocyte deposition Fibrogenesis | Regeneration Angiogenesis |
| Shape | PC-EC Ratio | |
|---|---|---|
| Heart | Spindle-shaped | 1:2–1:3 |
| Nervous system | Solitary and stellate-shaped | 1:1 |
| Skeletal muscle | 1:100 | |
| Kidney | Rounded and compact | |
| Function |
| |
| Molecules Secreted by Pericytes | Functions |
|---|---|
| TNF-α, IP-10, IL-6, 8 Eotaxin and Rantes | Induce inflammatory responses |
| LIF, COX-2, and HMOX-1 | Inhibit inflammatory responses |
| Bmp-4, 6, 7 | Preserve stem-cell regenerative capabilities |
| TGF-β | Stimulates angiogenesis and fibrogenesis |
| Ang-1 | Enhances vessel stabilization |
| VEGF | Stimulates angiogenesis |
| SPARC | Stimulates fibrogenesis |
| S1P | Stabilizes intercellular contacts of PCs and ECs |
| OPG | Contributes to calcification |
| MiR-132 | Induces survival response and differentiation of cardiomyocytes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.; Cantrell, A.C.; Zeng, H.; Zhu, S.-H.; Chen, J.-X. Emerging Role of Pericytes and Their Secretome in the Heart. Cells 2021, 10, 548. https://doi.org/10.3390/cells10030548
Su H, Cantrell AC, Zeng H, Zhu S-H, Chen J-X. Emerging Role of Pericytes and Their Secretome in the Heart. Cells. 2021; 10(3):548. https://doi.org/10.3390/cells10030548
Chicago/Turabian StyleSu, Han, Aubrey C. Cantrell, Heng Zeng, Shai-Hong Zhu, and Jian-Xiong Chen. 2021. "Emerging Role of Pericytes and Their Secretome in the Heart" Cells 10, no. 3: 548. https://doi.org/10.3390/cells10030548






