Synapsins and the Synaptic Vesicle Reserve Pool: Floats or Anchors?
Abstract
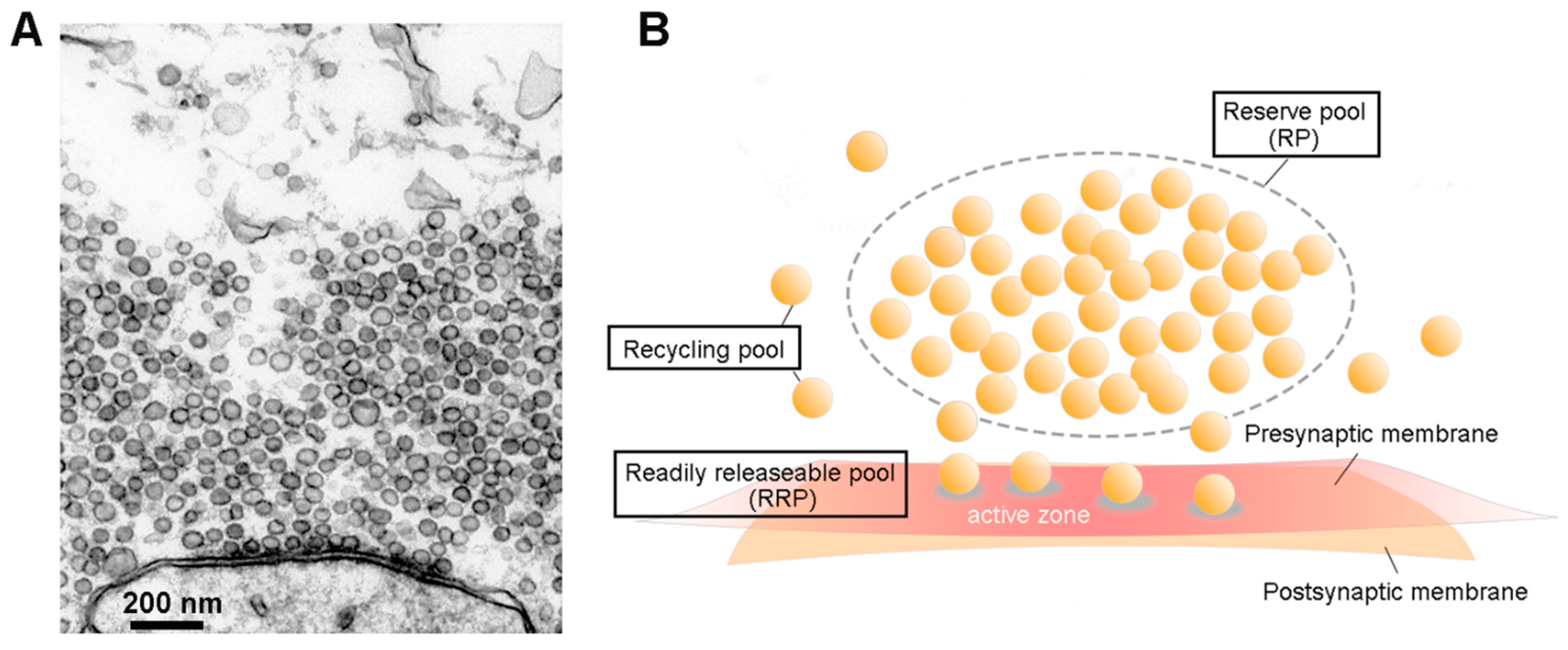
Introduction
Synapsins Bind to SVs

Synapsins Maintain the SV Reserve Pool
Models for Synapsin-Dependent Clustering of SVs in the RP
Actin Scaffold Model
Synaptic Vesicle Crosslinking
Liquid-Liquid Phase Separation
Limitations of the Two Models
Perspectives
- For the SV crosslinking hypothesis, which proteins constitute the inter-SV links? If they include synapsins, why do some links persist in TKO neurons? What is the relationship of the tethers to synapsin oligomers? Given the differences in RP formation by different synapsin isoforms in glutamatergic synapses, what differentiates the ability of these isoforms to form the RP?
- For the liquid phase separation hypothesis, how quickly can synapsin droplets form in neurons? How do regulatory protein kinases and phosphatases move in and out of the proposed synapsin condensate? How do the other synaptic proteins contribute to the liquid-liquid phase separation of synapsin?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schikorski, T.; Stevens, C.F. Quantitative Ultrastructural Analysis of Hippocampal Excitatory Synapses. J. Neurosci. 1997, 17, 5858–5867. [Google Scholar] [CrossRef]
- Alabi, A.A.; Tsien, R.W. Synaptic Vesicle Pools and Dynamics. Cold Spring Harb. Perspect. Biol. 2012, 4, a013680. [Google Scholar] [CrossRef]
- Denker, A.; Rizzoli, S. Synaptic Vesicle Pools: An Update. Front. Synaptic Neurosci. 2010, 2, 135. [Google Scholar] [CrossRef]
- Rizzoli, S.O.; Betz, W.J. Synaptic vesicle pools. Nat. Rev. Neurosci. 2005, 6, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Rosenmund, C.; Stevens, C.F. Definition of the Readily Releasable Pool of Vesicles at Hippocampal Synapses. Neuron 1996, 16, 1197–1207. [Google Scholar] [CrossRef]
- Pieribone, V.A.; Shupliakov, O.; Brodin, L.; Hilfiker-Rothenfluh, S.; Czernik, A.J.; Greengard, P. Distinct pools of synaptic vesicles in neurotransmitter release. Nat. Cell Biol. 1995, 375, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Shupliakov, O.; Haucke, V.; Pechstein, A. How synapsin I may cluster synaptic vesicles. Semin. Cell Dev. Biol. 2011, 22, 393–399. [Google Scholar] [CrossRef]
- Rose, T.; Schoenenberger, P.; Jezek, K.; Oertner, T.G. Developmental Refinement of Vesicle Cycling at Schaffer Collateral Synapses. Neuron 2013, 77, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Rey, S.; Marra, V.; Smith, C.; Staras, K. Nanoscale Remodeling of Functional Synaptic Vesicle Pools in Hebbian Plasticity. Cell Rep. 2020, 30, 2006–2017.e3. [Google Scholar] [CrossRef]
- Vandael, D.; Borges-Merjane, C.; Zhang, X.; Jonas, P. Short-Term Plasticity at Hippocampal Mossy Fiber Synapses Is Induced by Natural Activity Patterns and Associated with Vesicle Pool Engram Formation. Neuron 2020, 107, 509–521.e7. [Google Scholar] [CrossRef]
- Burrone, J.; Li, Z.; Murthy, V.N. Studying vesicle cycling in presynaptic terminals using the genetically encoded probe synaptopHluorin. Nat. Protoc. 2006, 1, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Lavian, H.; Korngreen, A. Short-term depression shapes information transmission in a constitutively active GABAergic synapse. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Rosenbaum, R.; Rubin, J.; Doiron, B. Short Term Synaptic Depression Imposes a Frequency Dependent Filter on Synaptic Information Transfer. PLoS Comput. Biol. 2012, 8, e1002557. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, E.; Lo, D.C.; Wesseling, J.F. Kinetic Isolation of a Slowly Recovering Component of Short-Term Depression During Exhaustive Use at Excitatory Hippocampal Synapses. J. Neurophysiol. 2008, 100, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Gitler, D.; Takagishi, Y.; Feng, J.; Ren, Y.; Rodriguiz, R.M.; Wetsel, W.C.; Greengard, P.; Augustine, G.J. Different Presynaptic Roles of Synapsins at Excitatory and Inhibitory Synapses. J. Neurosci. 2004, 24, 11368–11380. [Google Scholar] [CrossRef] [PubMed]
- Rosahl, T.W.; Spillane, D.; Missler, M.; Herz, J.; Selig, D.K.; Wolff, J.R.; Hammer, R.E.; Malenka, R.C.; Südhof, T.C. Essential functions of synapsins I and II in synaptic vesicle regulation. Nat. Cell Biol. 1995, 375, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Lignani, G.; Raimondi, A.; Ferrea, E.; Rocchi, A.; Paonessa, F.; Cesca, F.; Orlando, M.; Tkatch, T.; Valtorta, F.; Cossette, P.; et al. Epileptogenic Q555X SYN1 mutant triggers imbalances in release dynamics and short-term plasticity. Hum. Mol. Genet. 2013, 22, 2186–2199. [Google Scholar] [CrossRef]
- Farisello, P.; Boido, D.; Nieus, T.; Medrihan, L.; Cesca, F.; Valtorta, F.; Baldelli, P.; Benfenati, F. Synaptic and Extrasynaptic Origin of the Excitation/Inhibition Imbalance in the Hippocampus of Synapsin I/II/III Knockout Mice. Cereb. Cortex 2012, 23, 581–593. [Google Scholar] [CrossRef]
- Greengard, P.; Valtorta, F.; Czernik, A.; Benfenati, F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 1993, 259, 780–785. [Google Scholar] [CrossRef]
- Südhof, T.C. The synaptic vesicle cycle: A cascade of protein–protein interactions. Nat. Cell Biol. 1995, 375, 645–653. [Google Scholar] [CrossRef]
- Benfenati, F.; Bähler, M.; Jahn, R.; Greengard, P. Interactions of synapsin I with small synaptic vesicles: Distinct sites in synapsin I bind to vesicle phospholipids and vesicle proteins. J. Cell Biol. 1989, 108, 1863–1872. [Google Scholar] [CrossRef]
- Cheetham, J.J.; Hilfiker, S.; Benfenati, F.; Weber, T.; Greengard, P.; Czernik, A.J. Identification of Synapsin I Peptides that Insert into Lipid Membranes. Biochem. J. 2001, 354 Pt 1, 57–66. [Google Scholar] [CrossRef]
- Takamori, S.; Holt, M.; Stenius, K.; Lemke, E.A.; Grønborg, M.; Riedel, D.; Urlaub, H.; Schenck, S.; Brügger, B.; Ringler, P.; et al. Molecular Anatomy of a Trafficking Organelle. Cell 2006, 127, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, B.G.; Mandad, S.; Truckenbrodt, S.; Kröhnert, K.; Schäfer, C.; Rammner, B.; Koo, S.J.; Classen, G.A.; Krauss, M.; Haucke, V.; et al. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 2014, 344, 1023–1028. [Google Scholar] [CrossRef]
- Orlando, M.; Lignani, G.; Maragliano, L.; Fassio, A.; Onofri, F.; Baldelli, P.; Giovedi, S.; Benfenati, F. Functional Role of ATP Binding to Synapsin I In Synaptic Vesicle Trafficking and Release Dynamics. J. Neurosci. 2014, 34, 14752–14768. [Google Scholar] [CrossRef]
- Tao-Cheng, J.-H. Activity-related redistribution of presynaptic proteins at the active zone. Neuroscience 2006, 141, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Pechstein, A.; Shupliakov, O. Taking a Back Seat: Synaptic Vesicle Clustering in Presynaptic Terminals. Front. Synaptic Neurosci. 2010, 2, 143. [Google Scholar] [CrossRef] [PubMed]
- Evergren, E.; Zotova, E.; Brodin, L.; Shupliakov, O. Differential efficiency of the endocytic machinery in tonic and phasic synapses. Neuroscience 2006, 141, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Bloom, O.; Evergren, E.; Tomilin, N.; Kjaerulff, O.; Lö, W.P.; Brodin, L.; Pieribone, V.A.; Greengard, P.; Shupliakov, O. Colocalization of synapsin and actin during synaptic vesicle recycling. J. Cell Biol. 2003, 161, 737–747. [Google Scholar] [CrossRef]
- Pieribone, V.A.; Porton, B.; Rendon, B.; Feng, J.; Greengard, P.; Kao, H.-T. Expression of synapsin III in nerve terminals and neurogenic regions of the adult brain. J. Comp. Neurol. 2002, 454, 105–114. [Google Scholar] [CrossRef]
- Song, S.-H.; Augustine, G.J. Synapsin Isoforms Regulating GABA Release from Hippocampal Interneurons. J. Neurosci. 2016, 36, 6742–6757. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Maeno, H.; Greengard, P. Regulation of Endogenous Phosphorylation of Specific Proteins in Synaptic Membrane Fractions from Rat Brain by Adenosine 3′:5′-Monophosphate. J. Biol. Chem. 1973, 248, 8295–8305. [Google Scholar] [CrossRef]
- Huttner, W.B.; Schiebler, W.; Greengard, P.; De Camilli, P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J. Cell Biol. 1983, 96, 1374–1388. [Google Scholar] [CrossRef] [PubMed]
- Schiebler, W.; Jahn, R.; Doucet, J.P.; Rothlein, J.; Greengard, P. Characterization of synapsin I binding to small synaptic vesicles. J. Biol. Chem. 1986, 261, 8383–8390. [Google Scholar] [CrossRef]
- Benfenati, F.; Valtorta, F.; Bähler, M.; Greengard, P. Synapsin I, a Neuron-Specific Phosphoprotein Interacting with Small Synaptic Vesicles and F-Actin. Cell Biol. Int. Rep. 1989, 13, 1007–1021. [Google Scholar] [CrossRef]
- Thiel, G.; Südhof, T.C.; Greengard, P. Synapsin II. Mapping of a Domain in the NH2-Terminal Region Which Binds to Small Synaptic Vesicles. J. Biol. Chem. 1990, 265, 16527–16533. [Google Scholar] [CrossRef]
- Hosaka, M.; Hammer, R.E.; Südhof, T.C. A Phospho-Switch Controls the Dynamic Association of Synapsins with Synaptic Vesicles. Neuron 1999, 24, 377–387. [Google Scholar] [CrossRef]
- Llinás, R.; McGuinness, T.L.; Leonard, C.S.; Sugimori, M.; Greengard, P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc. Natl. Acad. Sci. USA 1985, 82, 3035–3039. [Google Scholar] [CrossRef]
- Chi, P.; Greengard, P.; Ryan, T.A. Synapsin dispersion and reclustering during synaptic activity. Nat. Neurosci. 2001, 4, 1187–1193. [Google Scholar] [CrossRef]
- Chi, P.; Greengard, P.; Ryan, T.A. Synaptic Vesicle Mobilization Is Regulated by Distinct Synapsin I Phosphorylation Pathways at Different Frequencies. Neuron 2003, 38, 69–78. [Google Scholar] [CrossRef]
- Song, S.-H.; Augustine, G.J. Synapsin Isoforms and Synaptic Vesicle Trafficking. Mol. Cells 2015, 38, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, Y.; Deisenhofer, J.; Brautigam, C.A. Tetramerization and ATP Binding by a Protein Comprising the A, B, and C Domains of Rat Synapsin I. J. Biol. Chem. 2004, 279, 11948–11956. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.S.; Bradley, A.R.; Valasatava, Y.; Duarte, J.M.; Prlić, A.; Rose, P.W. NGL viewer: Web-based molecular graphics for large complexes. Bioinformatics 2018, 34, 3755–3758. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker, S.; Schweizer, F.E.; Kao, H.-T.; Czernik, A.J.; Greengard, P.; Augustine, G.J. Two sites of action for synapsin domain E in regulating neurotransmitter release. Nat. Neurosci. 1998, 1, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker, S.; Benfenati, F.; Doussau, F.; Nairn, A.C.; Czernik, A.J.; Augustine, G.J.; Greengard, P. Structural Domains Involved in the Regulation of Transmitter Release by Synapsins. J. Neurosci. 2005, 25, 2658–2669. [Google Scholar] [CrossRef]
- Tao, C.-L.; Liu, Y.-T.; Sun, R.; Zhang, B.; Qi, L.; Shivakoti, S.; Tian, C.-L.; Zhang, P.; Lau, P.-M.; Zhou, Z.H.; et al. Differentiation and Characterization of Excitatory and Inhibitory Synapses by Cryo-electron Tomography and Correlative Microscopy. J. Neurosci. 2018, 38, 1493–1510. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.M.; Boudin, H. Molecular heterogeneity of central synapses: Afferent and target regulation. Nat. Neurosci. 2001, 4, 569–578. [Google Scholar] [CrossRef]
- Gitler, D.; Cheng, Q.; Greengard, P.; Augustine, G.J. Synapsin IIa Controls the Reserve Pool of Glutamatergic Synaptic Vesicles. J. Neurosci. 2008, 28, 10835–10843. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Song, S.-H.; Augustine, G.J. Molecular Mechanisms of Short-Term Plasticity: Role of Synapsin Phosphorylation in Augmentation and Potentiation of Spontaneous Glutamate Release. Front. Synaptic Neurosci. 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Matos, H.; Quiles, R.; Andrade, R.; Bykhovskaia, M. Growth and excitability at synapsin II deficient hippocampal neurons. Mol. Cell. Neurosci. 2019, 96, 25–34. [Google Scholar] [CrossRef]
- Hirokawa, N.; Sobue, K.; Kanda, K.; Harada, A.; Yorifuji, H. The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1. J. Cell Biol. 1989, 108, 111–126. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Atluri, P.P.; Ryan, T.A. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat. Neurosci. 2003, 6, 127–135. [Google Scholar] [CrossRef]
- Fernández-Busnadiego, R.; Zuber, B.; Maurer, U.E.; Cyrklaff, M.; Baumeister, W.; Lučić, V. Quantitative analysis of the native presynaptic cytomatrix by cryoelectron tomography. J. Cell Biol. 2010, 188, 145–156. [Google Scholar] [CrossRef]
- Shupliakov, O.; Bloom, O.; Gustafsson, J.S.; Kjaerulff, O.; Löw, P.; Tomilin, N.; Pieribone, V.A.; Greengard, P.; Brodin, L. Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc. Natl. Acad. Sci. USA 2002, 99, 14476–14481. [Google Scholar] [CrossRef] [PubMed]
- Siksou, L.; Rostaing, P.; Lechaire, J.-P.; Boudier, T.; Ohtsuka, T.; Fejtová, A.; Kao, H.-T.; Greengard, P.; Gundelfinger, E.D.; Triller, A.; et al. Three-Dimensional Architecture of Presynaptic Terminal Cytomatrix. J. Neurosci. 2007, 27, 6868–6877. [Google Scholar] [CrossRef]
- Landis, D.M.; Hall, A.K.; Weinstein, L.A.; Reese, T.S. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron 1988, 1, 201–209. [Google Scholar] [CrossRef]
- Cole, A.A.; Chen, X.; Reese, T.S. A Network of Three Types of Filaments Organizes Synaptic Vesicles for Storage, Mobilization, and Docking. J. Neurosci. 2016, 36, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Takei, Y.; Harada, A.; Takeda, S.; Kobayashi, K.; Terada, S.; Noda, T.; Takahashi, T.; Hirokawa, N. Synapsin I deficiency results in the structural change in the presynaptic terminals in the murine nervous system. J. Cell Biol. 1995, 131, 1789–1800. [Google Scholar] [CrossRef]
- Hosaka, M.; Südhof, T.C. Homo- and Heterodimerization of Synapsins. J. Biol. Chem. 1999, 274, 16747–16753. [Google Scholar] [CrossRef]
- Monaldi, I.; Vassalli, M.; Bachi, A.; Giovedi, S.; Millo, E.; Valtorta, F.; Raiteri, R.; Benfenati, F.; Fassio, A. The highly conserved synapsin domain E mediates synapsin dimerization and phospholipid vesicle clustering. Biochem. J. 2010, 426, 55–64. [Google Scholar] [CrossRef]
- Wesseling, J.F.; Phan, S.; Bushong, E.A.; Siksou, L.; Marty, S.; Pérez-Otaño, I.; Ellisman, M. Sparse force-bearing bridges between neighboring synaptic vesicles. Brain Struct. Funct. 2019, 224, 3263–3276. [Google Scholar] [CrossRef]
- Banjade, S.; Rosen, M.K. Phase transitions of multivalent proteins can promote clustering of membrane receptors. eLife 2014, 3, e04123. [Google Scholar] [CrossRef]
- Brangwynne, C.P. Phase transitions and size scaling of membrane-less organelles. J. Cell Biol. 2013, 203, 875–881. [Google Scholar] [CrossRef]
- Hyman, A.A.; Weber, C.A.; Jülicher, F. Liquid-Liquid Phase Separation in Biology. Annu. Rev. Cell Dev. Biol. 2014, 30, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Banjade, S.; Cheng, H.-C.; Kim, S.; Chen, B.; Guo, L.; Llaguno, M.C.; Hollingsworth, J.V.; King, D.S.; Banani, S.F.; et al. Phase transitions in the assembly of multivalent signalling proteins. Nat. Cell Biol. 2012, 483, 336–340. [Google Scholar] [CrossRef]
- Milovanovic, D.; Wu, Y.; Bian, X.; De Camilli, P. A liquid phase of synapsin and lipid vesicles. Science 2018, 361, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Denker, A.; Bethani, I.; Kröhnert, K.; Körber, C.; Horstmann, H.; Wilhelm, B.G.; Barysch, S.V.; Kuner, T.; Neher, E.; Rizzoli, S.O. A small pool of vesicles maintains synaptic activity in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 17177–17182. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.H.; Kaeser, P.S. A Presynaptic Liquid Phase Unlocks the Vesicle Cluster. Trends Neurosci. 2018, 41, 772–774. [Google Scholar] [CrossRef]
- Pechstein, A.; Tomilin, N.; Fredrich, K.; Vorontsova, O.; Sopova, E.; Evergren, E.; Haucke, V.; Brodin, L.; Shupliakov, O. Vesicle Clustering in a Living Synapse Depends on a Synapsin Region that Mediates Phase Separation. Cell Rep. 2020, 30, 2594–2602.e3. [Google Scholar] [CrossRef]
- Wu, X.; Cai, Q.; Shen, Z.; Chen, X.; Zeng, M.; Du, S.; Zhang, M. RIM and RIM-BP Form Presynaptic Active-Zone-like Condensates via Phase Separation. Mol. Cell 2019, 73, 971–984. [Google Scholar] [CrossRef]
- McDonald, N.A.; Fetter, R.D.; Shen, K. Assembly of synaptic active zones requires phase separation of scaffold molecules. Nat. Cell Biol. 2020, 588, 454–458. [Google Scholar] [CrossRef]
- Wu, X.; Ganzella, M.; Zhou, J.; Zhu, S.; Jahn, R.; Zhang, M. Vesicle Tethering on the Surface of Phase-Separated Active Zone Condensates. Mol. Cell 2021, 81, 13–24.e7. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Wu, Y.; Lee, S.-E.; Kim, G.; Jeong, S.; Milovanovic, D.; De Camilli, P.; Chang, S. Cooperative function of synaptophysin and synapsin in the generation of synaptic vesicle-like clusters in non-neuronal cells. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Riback, J.A.; Zhu, L.; Ferrolino, M.C.; Tolbert, M.; Mitrea, D.M.; Sanders, D.W.; Wei, M.-T.; Kriwacki, R.W.; Brangwynne, C.P. Composition-dependent thermodynamics of intracellular phase separation. Nat. Cell Biol. 2020, 581, 209–214. [Google Scholar] [CrossRef]
- Hosaka, M.; Südhof, T.C. Synapsin III, a Novel Synapsin with an Unusual Regulation by Ca2+. J. Biol. Chem. 1998, 273, 13371–13374. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, M.; Südhof, T.C. Synapsins I and II Are ATP-binding Proteins with Differential Ca2+ Regulation. J. Biol. Chem. 1998, 273, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.; Czernik, A.; Kao, H.; Takei, K.; Johnston, P.; Horiuchi, A.; Kanazir, S.; Wagner, M.; Périn, M.; De Camilli, P.; et al. Synapsins: Mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science 1989, 245, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Gerth, F.; Jäpel, M.; Pechstein, A.; Kochlamazashvili, G.; Lehmann, M.; Puchkov, D.; Onofri, F.; Benfenati, F.; Nikonenko, A.G.; Fredrich, K.; et al. Intersectin associates with synapsin and regulates its nanoscale localization and function. Proc. Natl. Acad. Sci. USA 2017, 114, 12057–12062. [Google Scholar] [CrossRef] [PubMed]
- Durán, E.; Montes, M.Á.; Jemal, I.; Satterfield, R.; Young, S.; de Toledo, G. Álvarez Synaptotagmin-7 controls the size of the reserve and resting pools of synaptic vesicles in hippocampal neurons. Cell Calcium 2018, 74, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Winther, A.M.E.; Vorontsova, O.; Rees, K.A.; Näreoja, T.; Sopova, E.; Jiao, W.; Shupliakov, O. An Endocytic Scaffolding Protein together with Synapsin Regulates Synaptic Vesicle Clustering in the Drosophila Neuromuscular Junction. J. Neurosci. 2015, 35, 14756–14770. [Google Scholar] [CrossRef]
- Efimova, N.; Korobova, F.; Stankewich, M.C.; Moberly, A.H.; Stolz, D.B.; Wang, J.; Kashina, A.; Ma, M.; Svitkina, T. βIII Spectrin Is Necessary for Formation of the Constricted Neck of Dendritic Spines and Regulation of Synaptic Activity in Neurons. J. Neurosci. 2017, 37, 6442–6459. [Google Scholar] [CrossRef] [PubMed]
- Gitler, D.; Xu, Y.; Kao, H.-T.; Lin, D.; Lim, S.; Feng, J.; Greengard, P.; Augustine, G.J. Molecular Determinants of Synapsin Targeting to Presynaptic Terminals. J. Neurosci. 2004, 24, 3711–3720. [Google Scholar] [CrossRef] [PubMed]
- Staras, K.; Branco, T.; Burden, J.J.; Pozo, K.; Darcy, K.; Marra, V.; Ratnayaka, A.; Goda, Y. A Vesicle Superpool Spans Multiple Presynaptic Terminals in Hippocampal Neurons. Neuron 2010, 66, 37–44. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Augustine, G.J. Synapsins and the Synaptic Vesicle Reserve Pool: Floats or Anchors? Cells 2021, 10, 658. https://doi.org/10.3390/cells10030658
Zhang M, Augustine GJ. Synapsins and the Synaptic Vesicle Reserve Pool: Floats or Anchors? Cells. 2021; 10(3):658. https://doi.org/10.3390/cells10030658
Chicago/Turabian StyleZhang, Minchuan, and George J. Augustine. 2021. "Synapsins and the Synaptic Vesicle Reserve Pool: Floats or Anchors?" Cells 10, no. 3: 658. https://doi.org/10.3390/cells10030658
APA StyleZhang, M., & Augustine, G. J. (2021). Synapsins and the Synaptic Vesicle Reserve Pool: Floats or Anchors? Cells, 10(3), 658. https://doi.org/10.3390/cells10030658





