Antidepressant Screening Demonstrated Non-Monotonic Responses to Amitriptyline, Amoxapine and Sertraline in Locomotor Activity Assay in Larval Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Antidepressant Drugs
2.2. Animal Housing and Ethics
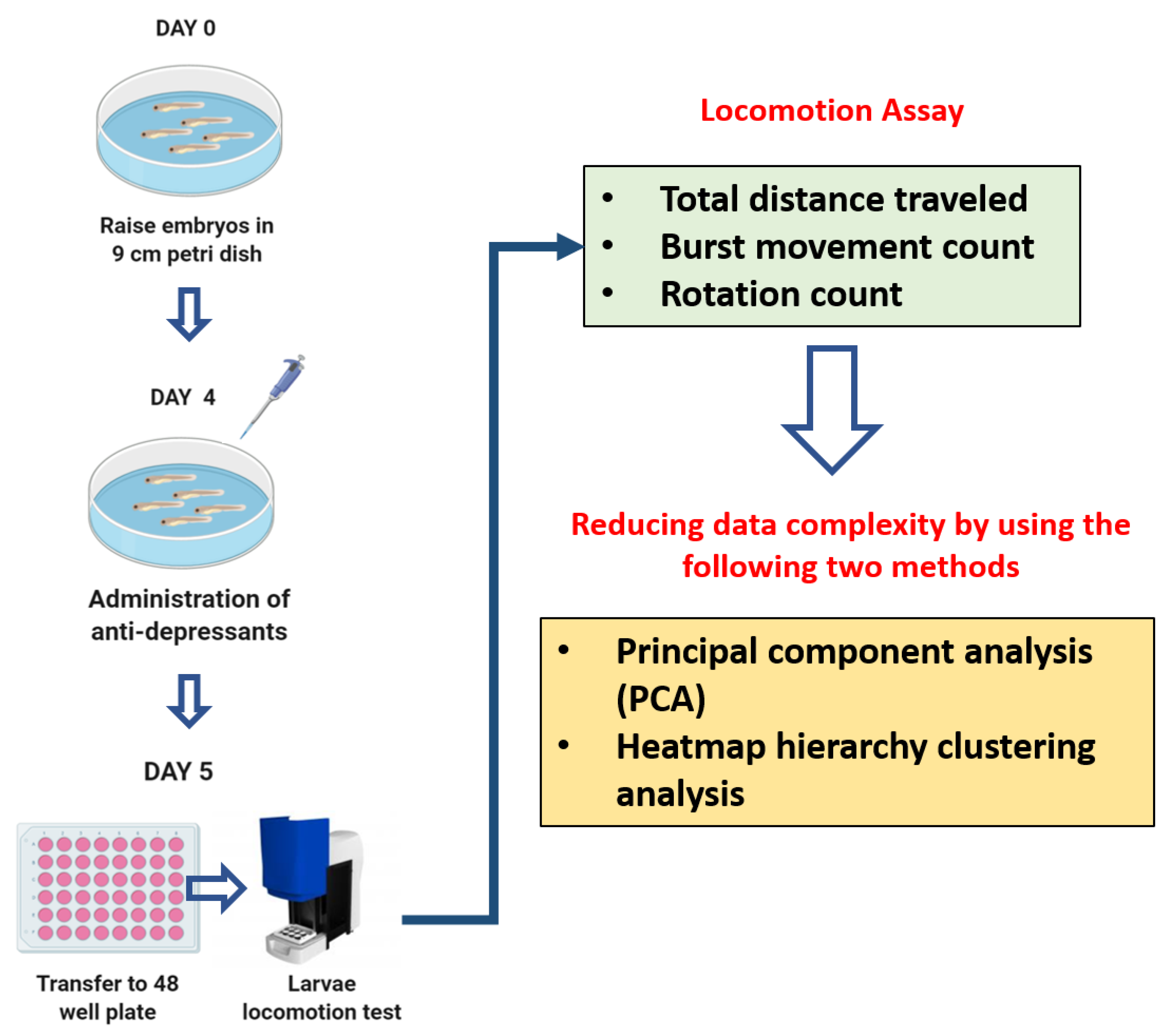
2.3. Antidepressants Exposure on Zebrafish Larvae
2.4. Zebrafish Larvae Locomotion Test
2.5. PCA, Heatmap, and Clustering Analysis
2.6. Statistical Analysis
3. Results
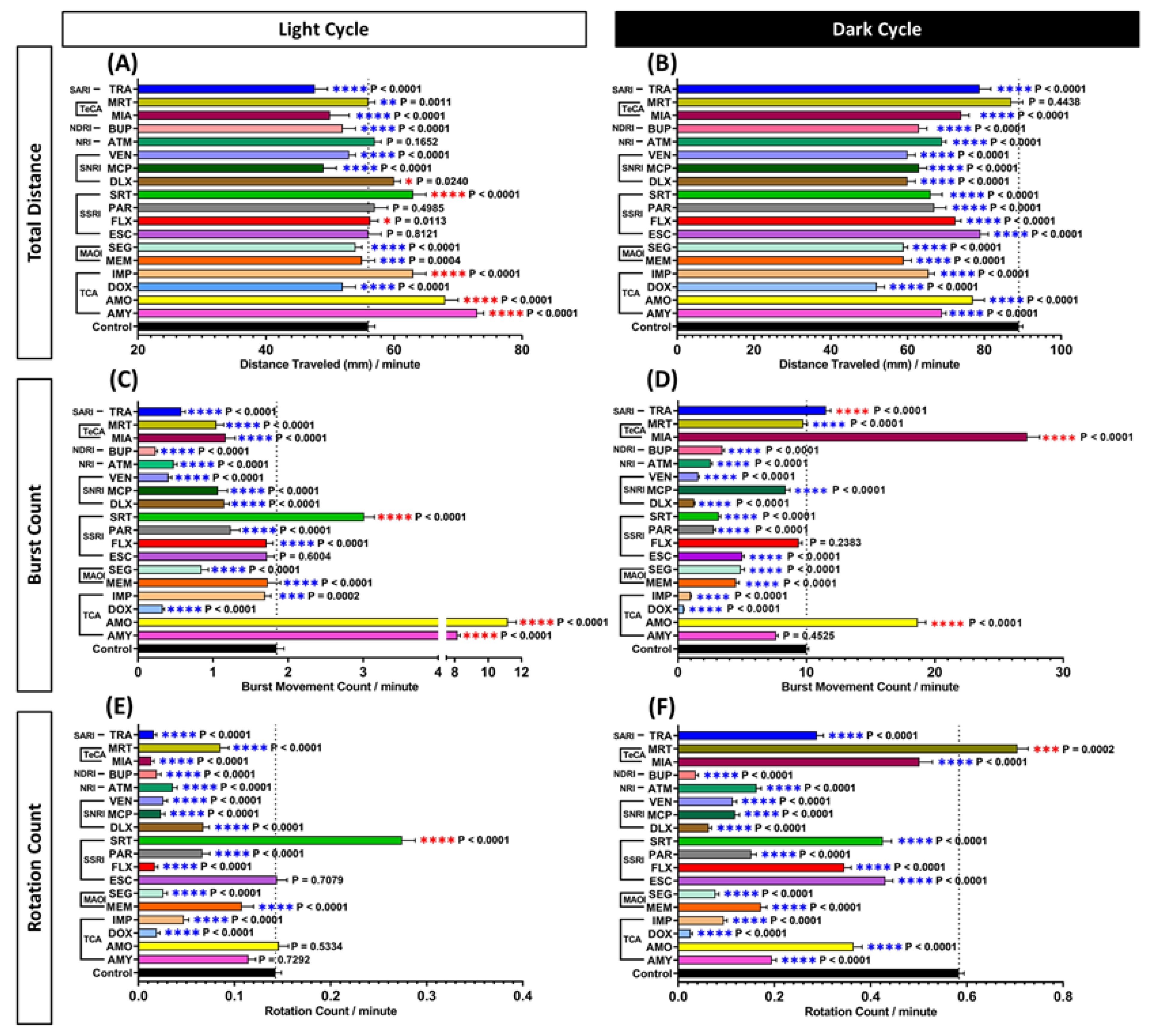
3.1. Locomotor Activity Evaluation of Antidepressants Exposure in Zebrafish Larvae
3.2. Analysis of Locomotion Alteration in Zebrafish after Exposure to Antidepressants by a Phenomic Approach
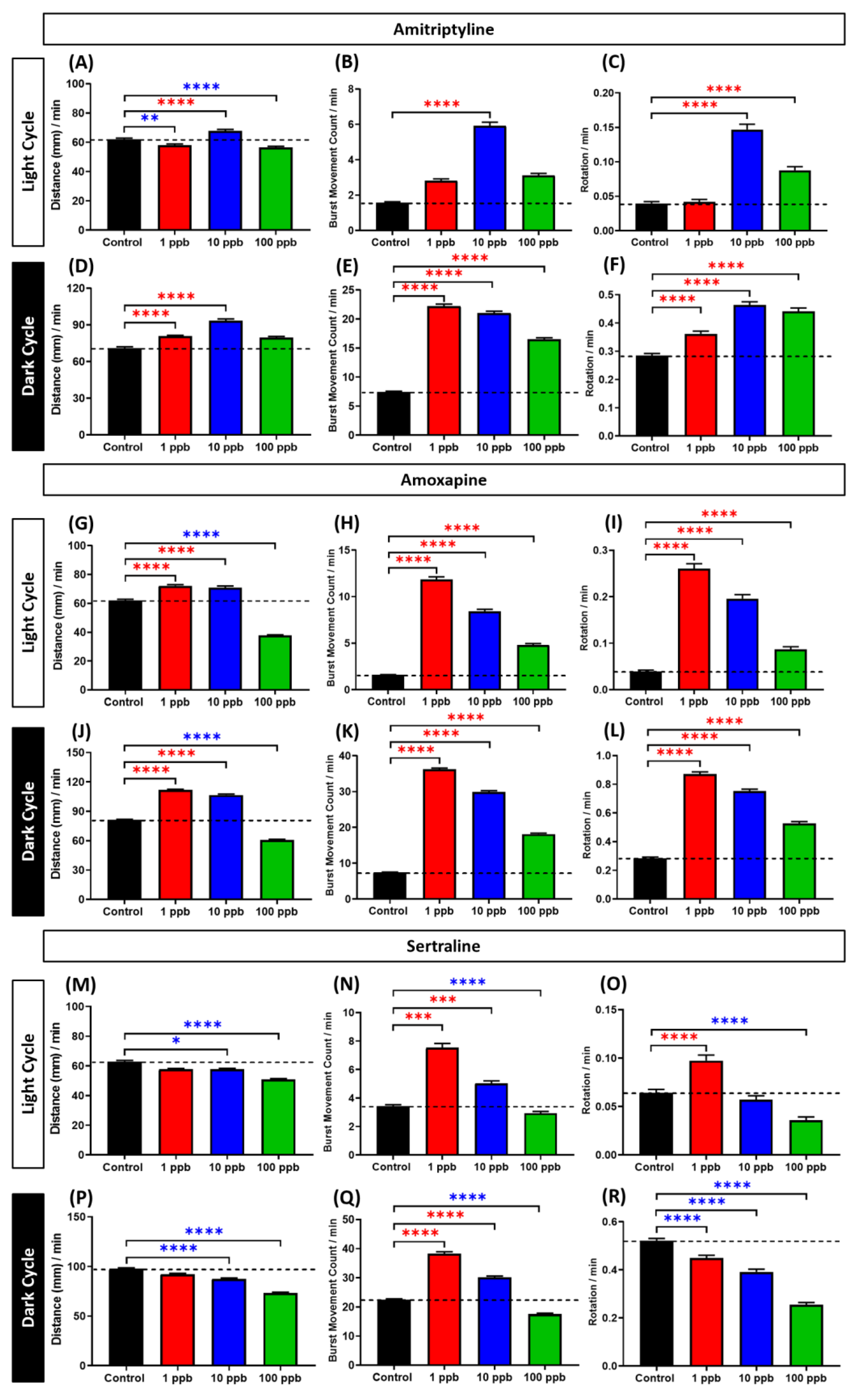
3.3. Locomotor Activity Evaluation of Amitriptyline, Amoxapine, and Sertraline in Three Different Concentrations
4. Discussion
4.1. Acute Exposure of Antidepressants Altered Zebrafish Larvae Locomotor Activity
4.2. Several Antidepressants Induced a Hyperactive Response in Zebrafish Larval Locomotor Activity
4.3. Biphasic Pattern of Amitriptyline, Amoxapine, and Sertraline in Zebrafish Larvae Locomotor Activity
4.4. Possible Mechanisms of Antidepressant Effects and Limitations in This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khushboo, S.B.; Sharma, B. Antidepressants: Mechanism of action, toxicity and possible amelioration. J. Appl. Biotechnol. Bioeng 2017, 3, 1–13. [Google Scholar]
- Aan het Rot, M.; Mathew, S.J.; Charney, D.S. Neurobiological mechanisms in major depressive disorder. CMAJ 2009, 180, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.L. Depression: The case for a monoamine deficiency. J. Clin. Psychiatry 2000, 61, 7–11. [Google Scholar] [PubMed]
- Nguyen, M.; Stewart, A.M.; Kalueff, A.V. Aquatic blues: Modeling depression and antidepressant action in zebrafish. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2014, 55, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Shenouda, R.; Desan, P.H. Abuse of tricyclic antidepressant drugs: A case series. J. Clin. Psychopharmacol. 2013, 33, 440–442. [Google Scholar] [CrossRef]
- Stall, N.; Godwin, J.; Juurlink, D. Bupropion abuse and overdose. CMAJ 2014, 186, 1015-1015. [Google Scholar] [CrossRef]
- Ramesh, S.; Khandelwal, S.K. Antidepressant induced mania: Is it a risk factor for antidepressant abuse? Ind. J. Psychiatry 2003, 45, 194. [Google Scholar]
- Evans, E.A.; Sullivan, M.A. Abuse and misuse of antidepressants. Subst. Abus. Rehab. 2014, 5, 107. [Google Scholar]
- Baribeau, D.; Araki, K.F. Intravenous bupropion: A previously undocumented method of abuse of a commonly prescribed antidepressant agent. J. Addict. Med. 2013, 7, 216–217. [Google Scholar] [CrossRef]
- Moncrieff, J. Antidepressants: Misnamed and misrepresented. World Psychiatry 2015, 14, 302. [Google Scholar] [CrossRef]
- Hawton, K.; Bergen, H.; Simkin, S.; Cooper, J.; Waters, K.; Gunnell, D.; Kapur, N. Toxicity of antidepressants: Rates of suicide relative to prescribing and non-fatal overdose. Br. J. Psychiatry 2010, 196, 354–358. [Google Scholar] [CrossRef]
- White, N.C.; Litovitz, T.; Clancy, C. Suicidal antidepressant overdoses: A comparative analysis by antidepressant type. J. Med. Toxicol. 2008, 4, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Judge, B.S.; Rentmeester, L.L. Antidepressant overdose–induced seizures. Neurol. Clin. 2011, 29, 565–580. [Google Scholar] [CrossRef]
- Goldsmith, L.; Moncrieff, J. The psychoactive effects of antidepressants and their association with suicidality. Curr. Drug Saf. 2011, 6, 115–121. [Google Scholar] [CrossRef]
- Veldman, M.B.; Lin, S. Zebrafish as a developmental model organism for pediatric research. Pediatr. Res. 2008, 64, 470–476. [Google Scholar] [CrossRef]
- Raldua, D.; Campos, B.; Barata, C.; Piña, B.; García-Reyero, N.; Babin, P.J. Deciphering emerging toxicological effects of pharmaceuticals on aquatic organisms by using daphnia magna and danio rerio as model organisms. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; Volume 62, pp. 611–647. [Google Scholar]
- Kari, G.; Rodeck, U.; Dicker, A.P. Zebrafish: An emerging model system for human disease and drug discovery. Clin. Pharmacol. Ther. 2007, 82, 70–80. [Google Scholar] [CrossRef]
- Tran, S.; Gerlai, R. Zebrafish models of alcohol addiction. In Addictive Substances and Neurological Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 59–66. [Google Scholar]
- Legradi, J.; El Abdellaoui, N.; Van Pomeren, M.; Legler, J. Comparability of behavioural assays using zebrafish larvae to assess neurotoxicity. Environ. Sci. Pollut. Res. 2015, 22, 16277–16289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Willett, C.; Fremgen, T. Zebrafish: An animal model for toxicological studies. Curr. Prot. Toxicol. 2003, 17, 1–17. [Google Scholar] [CrossRef]
- Sarvaiya, V.; Sadariya, K.; Rana, M.; Thaker, A. Zebrafish as model organism for drug discovery and toxicity testing: A review. Vet. Clin. Sci. 2014, 2, 31–38. [Google Scholar]
- Lockwood, B.; Bjerke, S.; Kobayashi, K.; Guo, S. Acute effects of alcohol on larval zebrafish: A genetic system for large-scale screening. Pharmacol. Biochem. Behav. 2004, 77, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Neelkantan, N.; Mikhaylova, A.; Stewart, A.M.; Arnold, R.; Gjeloshi, V.; Kondaveeti, D.; Poudel, M.K.; Kalueff, A.V. Perspectives on zebrafish models of hallucinogenic drugs and related psychotropic compounds. ACS Chem. Neurosci. 2013, 4, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Costa de Melo, N.; Sánchez-Ortiz, B.L.; dos Santos Sampaio, T.I.; Matias Pereira, A.C.; Pinheiro da Silva Neto, F.L.; Ribeiro da Silva, H.; Alves Soares Cruz, R.; Keita, H.; Soares Pereira, A.M.; Tavares Carvalho, J.C. Anxiolytic and antidepressant effects of the hydroethanolic extract from the leaves of Aloysia polystachya (Griseb.) Moldenke: A study on zebrafish (Danio rerio). Pharmaceuticals 2019, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Tufi, S.; Leonards, P.; Lamoree, M.; de Boer, J.; Legler, J.; Legradi, J. Changes in neurotransmitter profiles during early zebrafish (Danio rerio) development and after pesticide exposure. Environ. Sci. Technol. 2016, 50, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Rivetti, C.; Campos, B.; Barata, C. Low environmental levels of neuro-active pharmaceuticals alter phototactic behaviour and reproduction in Daphnia magna. Aquat. Toxicol. 2016, 170, 289–296. [Google Scholar] [CrossRef]
- Nieoczym, D.; Socała, K.; Gawel, K.; Esguerra, C.V.; Wyska, E.; Wlaź, P. Anticonvulsant activity of pterostilbene in zebrafish and mouse acute seizure tests. Neurochem. Res. 2019, 44, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, Z.; Chen, Y.; Zhu, T.; Wang, R. A zebrafish behavior assay for assessing anti-epileptic drug efficacy. NeuroQuantology 2019, 17, 1–9. [Google Scholar] [CrossRef]
- Giacomini, N.J.; Rose, B.; Kobayashi, K.; Guo, S. Antipsychotics produce locomotor impairment in larval zebrafish. Neurotoxicol. Teratol. 2006, 28, 245–250. [Google Scholar] [CrossRef]
- Muniandy, Y. The use of larval zebrafish (Danio rerio) model for identifying new anxiolytic drugs from herbal medicine. Zebrafish 2018, 15, 321–339. [Google Scholar] [CrossRef]
- Gawel, K.; Kukula-Koch, W.; Nieoczym, D.; Stepnik, K.; Van Der Ent, W.; Banono, N.S.; Tarabasz, D.; Turski, W.A.; Esguerra, C.V. The influence of palmatine isolated from Berberis sibirica radix on pentylenetetrazole-induced seizures in zebrafish. Cells 2020, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of zebrafish in drug discovery toxicology. Chem. Res. Toxicol. 2019, 33, 95–118. [Google Scholar] [CrossRef]
- Reif, D.M.; Truong, L.; Mandrell, D.; Marvel, S.; Zhang, G.; Tanguay, R.L. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch. Toxicol. 2016, 90, 1459–1470. [Google Scholar] [CrossRef]
- Rihel, J.; Schier, A.F. Behavioral screening for neuroactive drugs in zebrafish. Dev. Neurobiol. 2012, 72, 373–385. [Google Scholar] [CrossRef]
- Poopal, R.-K.; He, Y.; Zhao, R.; Li, B.; Ramesh, M.; Ren, Z. Organophosphorus-based chemical additives induced behavioral changes in zebrafish (Danio rerio): Swimming activity is a sensitive stress indicator. Neurotoxicol. Teratol. 2021, 83, 106945. [Google Scholar] [CrossRef]
- Soni, R.; Verma, S.K. Acute toxicity and behavioural responses in Clarias batrachus (Linnaeus) exposed to herbicide pretilachlor. Heliyon 2018, 4, e01090. [Google Scholar] [CrossRef]
- Pereira, A.; Silva, L.; Laranjeiro, C.; Lino, C.; Pena, A. Selected pharmaceuticals in different aquatic compartments: Part II—Toxicity and environmental risk assessment. Molecules 2020, 25, 1796. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qiu, W.; Chen, J.; Zhan, J.; Pan, C.; Lei, X.; Wu, M. Growth inhibition and coordinated physiological regulation of zebrafish (Danio rerio) embryos upon sublethal exposure to antidepressant amitriptyline. Aquat. Toxicol. 2014, 151, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Brunello, N.; Mendlewicz, J.; Kasper, S.; Leonard, B.; Montgomery, S.; Nelson, J.C.; Paykel, E.; Versiani, M.; Racagni, G. The role of noradrenaline and selective noradrenaline reuptake inhibition in depression. Eur. Neuropsychopharmacol. 2002, 12, 461–475. [Google Scholar] [CrossRef]
- Riediger, C.; Schuster, T.; Barlinn, K.; Maier, S.; Weitz, J.; Siepmann, T. Adverse effects of antidepressants for chronic pain: A systematic review and meta-analysis. Front. Neurol. 2017, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Apiquian, R.; Fresan, A.; Ulloa, R.-E.; De la Fuente-Sandoval, C.; Herrera-Estrella, M.; Vazquez, A.; Nicolini, H.; Kapur, S. Amoxapine as an atypical antipsychotic: A comparative study vs risperidone. Neuropsychopharmacology 2005, 30, 2236–2244. [Google Scholar] [CrossRef] [PubMed]
- Almasi, A.; Meza, C.E. Doxepin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Schliessbach, J.; Siegenthaler, A.; Bütikofer, L.; Limacher, A.; Juni, P.; Vuilleumier, P.H.; Stamer, U.; Arendt-Nielsen, L.; Curatolo, M. Effect of single-dose imipramine on chronic low-back and experimental pain. A randomized controlled trial. PLoS ONE 2018, 13, e0195776. [Google Scholar]
- Ramachandraih, C.T.; Subramanyam, N.; Bar, K.J.; Baker, G.; Yeragani, V.K. Antidepressants: From MAOIs to SSRIs and more. Ind. J. Psychiatry 2011, 53, 180. [Google Scholar]
- Avasthi, A.; Kulhara, P.; Singh, G.; Sharma, R.; Kaur, R.P. Comparison of the efficacy and safety of moclobemide and imipramine in the treatment of depression in Indian patients. Ind. J. Psychiatry 2005, 47, 84. [Google Scholar] [CrossRef] [PubMed]
- Löhle, M.; Reichmann, H. Controversies in neurology: Why monoamine oxidase B inhibitors could be a good choice for the initial treatment of Parkinson’s disease. BMC Neurol. 2011, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.T.; Bronstein, A.C. Selective serotonin reuptake inhibitor exposure. Top. Compan. Anim. Med. 2013, 28, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Polychroniou, P.E.; Mayberg, H.S.; Craighead, W.E.; Rakofsky, J.J.; Aponte Rivera, V.; Haroon, E.; Dunlop, B.W. Temporal profiles and dose-responsiveness of side effects with escitalopram and duloxetine in treatment-naïve depressed adults. Behav. Sci. 2018, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Barraco, A.; Donda, P. Fluoxetine: A review on evidence based medicine. Ann. Gen. Hosp. Psychiatry 2004, 3, 2. [Google Scholar] [CrossRef]
- Nevels, R.M.; Gontkovsky, S.T.; Williams, B.E. Paroxetine—The antidepressant from hell? Probably not, but caution required. Psychopharmacol. Bull. 2016, 46, 77. [Google Scholar]
- Wang, S.-M.; Han, C.; Bahk, W.-M.; Lee, S.-J.; Patkar, A.A.; Masand, P.S.; Pae, C.-U. Addressing the side effects of contemporary antidepressant drugs: A comprehensive review. Chonnam. Med. J. 2018, 54, 101–112. [Google Scholar] [CrossRef]
- Sansone, R.A.; Sansone, L.A. Serotonin norepinephrine reuptake inhibitors: A pharmacological comparison. Innov. Clin. Neurosci. 2014, 11, 37. [Google Scholar]
- Knadler, M.P.; Lobo, E.; Chappell, J.; Bergstrom, R. Duloxetine. Clin. Pharmacokinet. 2011, 50, 281–294. [Google Scholar] [CrossRef]
- Kasper, S.; Pail, G. Milnacipran: A unique antidepressant? Neuropsychiatr. Dis. Treat. 2010, 6, 23. [Google Scholar]
- Örüm, M.H.; Kapıcı, Y. Venlafaxine-induced acute dystonia. Demiroglu Sci. Univ. Florence Nightingale J. Med. 2019, 5, 146–148. [Google Scholar] [CrossRef]
- Shams, Y. Serotonin norepinephrine re-uptake inhibitor (SNRI)-, selective norepinephrine reuptake inhibitor (S-NRI)-, and exogenously administered norepinephrine-induced takotsubo syndrome: Analysis of published cases. Int. J. Cardiol. 2017, 231, 228–233. [Google Scholar]
- Guldiken, G.; Karayagmurlu, A. A severe adverse effect of atomoxetine: Hypertensive crisis. Clin. Neuropharmacol. 2020, 43, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M.; Pradko, J.F.; Haight, B.R.; Modell, J.G.; Rockett, C.B.; Learned-Coughlin, S. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim. Care Compan. J. Clin. Psychiatry 2004, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.M.; Phillips, D.J. Nonepileptic myoclonus following bupropion overdose. Clin. Pediatr. 2018, 57, 1100–1102. [Google Scholar] [CrossRef] [PubMed]
- Fasipe, O.J. Neuropharmacological classification of antidepressant agents based on their mechanisms of action. Arch. Med. Health Sci. 2018, 6, 81. [Google Scholar] [CrossRef]
- Gundogmus, I.; Ispir, M.; Algul, A. Mianserin induced periorbital edema: A case report. Psychiatry Clin. Psychopharmacol. 2017, 27, 96–98. [Google Scholar] [CrossRef]
- Menon, V.; Madhavapuri, P. Low-dose mirtazapine-induced nightmares necessitating its discontinuation in a young adult female. J. Pharmacol. Pharmacother. 2017, 8, 182. [Google Scholar] [CrossRef]
- Jaffer, K.Y.; Chang, T.; Vanle, B.; Dang, J.; Steiner, A.J.; Loera, N.; Abdelmesseh, M.; Danovitch, I.; Ishak, W.W. Trazodone for insomnia: A systematic review. Innov. Clin. Neurosci. 2017, 14, 24. [Google Scholar]
- Hussain, A.; Audira, G.; Siregar, P.; Lin, Y.-C.; Villalobos, O.; Villaflores, O.; Wang, W.-D.; Hsiao, C.-D. Waterborne exposure of paclobutrazol at environmental relevant concentration induce locomotion hyperactivity in larvae and anxiolytic exploratory behavior in adult zebrafish. Int. J. Environ. Res. Public Health 2020, 17, 4632. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Audira, G.; Malhotra, N.; Uapipatanakul, B.; Chen, J.-R.; Lai, Y.-H.; Huang, J.-C.; Chen, K.H.-C.; Lai, H.-T.; Hsiao, C.-D. Multiple screening of pesticides toxicity in zebrafish and daphnia based on locomotor activity alterations. Biomolecules 2020, 10, 1224. [Google Scholar] [CrossRef] [PubMed]
- ZebraBox for Embryos or Larvae. Available online: http://www.viewpoint.fr/en/p/equipment/zebrabox-for-embryos-or-larvae (accessed on 25 March 2021).
- Kristofco, L.A.; Cruz, L.C.; Haddad, S.P.; Behra, M.L.; Chambliss, C.K.; Brooks, B.W. Age matters: Developmental stage of Danio rerio larvae influences photomotor response thresholds to diazinion or diphenhydramine. Aquat. Toxicol. 2016, 170, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- ClustVis: A Web Tool for Visualizing Clustering of Multivariate Data (BETA). Available online: https://biit.cs.ut.ee/clustvis/ (accessed on 25 March 2021).
- Prism: Analyze, Graph and Present Your Scientific Work. Available online: https://www.graphpad.com/ (accessed on 25 March 2021).
- Martins, J.; Teles, L.O.; Vasconcelos, V. Assays with Daphnia magna and Danio rerio as alert systems in aquatic toxicology. Environ. Int. 2007, 33, 414–425. [Google Scholar] [CrossRef]
- Cunha, D.L.; Mendes, M.P.; Marques, M. Environmental risk assessment of psychoactive drugs in the aquatic environment. Environ. Sci. Pollut. Res. 2019, 26, 78–90. [Google Scholar] [CrossRef]
- Kedra, M.; Banasiak, K.; Kisielewska, K.; Wolinska-Niziol, L.; Jaworski, J.; Zmorzynska, J. TrkB hyperactivity contributes to brain dysconnectivity, epileptogenesis, and anxiety in zebrafish model of Tuberous Sclerosis Complex. Proc. Natl. Acad. Sci. USA 2020, 117, 2170–2179. [Google Scholar] [CrossRef]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish larvae as a behavioral model in neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef]
- Gawel, K.; Turski, W.A.; van der Ent, W.; Mathai, B.J.; Kirstein-Smardzewska, K.J.; Simonsen, A.; Esguerra, C.V. Phenotypic characterization of larval zebrafish (Danio rerio) with partial knockdown of the cacna1a gene. Mol. Neurobiol. 2020, 57, 1904–1916. [Google Scholar] [CrossRef]
- De Esch, C.; van der Linde, H.; Slieker, R.; Willemsen, R.; Wolterbeek, A.; Woutersen, R.; De Groot, D. Locomotor activity assay in zebrafish larvae: Influence of age, strain and ethanol. Neurotoxicol. Teratol. 2012, 34, 425–433. [Google Scholar] [CrossRef]
- Harmer, C.J.; Duman, R.S.; Cowen, P.J. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 2017, 4, 409–418. [Google Scholar] [CrossRef]
- Andreatta, R.D. Neuroscience Fundamentals for Communication Sciences and Disorders; Plural Publishing: San Diego, CA, USA, 2018. [Google Scholar]
- Kawai, H.; Iwadate, R.; Ishibashi, T.; Kudo, N.; Kawashima, Y.; Mitsumoto, A. Antidepressants with different mechanisms of action show different chronopharmacological profiles in the tail suspension test in mice. Chronobiol. Int. 2019, 36, 1194–1207. [Google Scholar] [CrossRef]
- Höglund, E.; Øverli, Ø.; Åtland, Å. Assaying waterborne psychoactive drugs by the response to naturalistic predator cues in the stickleback (Gasterosteus aculeatus). Sci. Total Environ. 2020, 737, 140257. [Google Scholar] [CrossRef]
- Fong, P.P.; Ford, A.T. The biological effects of antidepressants on the molluscs and crustaceans: A review. Aquat. Toxicol. 2014, 151, 4–13. [Google Scholar] [CrossRef]
- Gannon, R.L.; Millan, M.J. Evaluation of serotonin, noradrenaline and dopamine reuptake inhibitors on light-induced phase advances in hamster circadian activity rhythms. Psychopharmacology 2007, 195, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, M.; Clesse, D.; Pévet, P.; Challet, E. New light on the serotonergic paradox in the rat circadian system. J. Neurochem. 2009, 110, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Challet, E.; Turek, F.W.; Laute, M.-A.; Van Reeth, O. Sleep deprivation decreases phase-shift responses of circadian rhythms to light in the mouse: Role of serotonergic and metabolic signals. Brain Res. 2001, 909, 81–91. [Google Scholar] [CrossRef]
- Cunha, V.; Rodrigues, P.; Santos, M.; Moradas-Ferreira, P.; Ferreira, M. Fluoxetine modulates the transcription of genes involved in serotonin, dopamine and adrenergic signalling in zebrafish embryos. Chemosphere 2018, 191, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Painter, M.M.; Buerkley, M.A.; Julius, M.L.; Vajda, A.M.; Norris, D.O.; Barber, L.B.; Furlong, E.T.; Schultz, M.M.; Schoenfuss, H.L. Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 2009, 28, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Chiffre, A.; Clérandeau, C.; Dwoinikoff, C.; Le Bihanic, F.; Budzinski, H.; Geret, F.; Cachot, J. Psychotropic drugs in mixture alter swimming behaviour of Japanese medaka (Oryzias latipes) larvae above environmental concentrations. Environ. Sci. Pollut. Res. 2016, 23, 4964–4977. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V. Illustrated zebrafish neurobehavioral glossary. In The rights and Wrongs of Zebrafish: Behavioral Phenotyping of Zebrafish; Springer: Berlin/Heidelberg, Germany, 2017; pp. 291–317. [Google Scholar]
- Banono, N.S.; Gawel, K.; De Witte, L.; Esguerra, C.V. Zebrafish Larvae Carrying a Splice Variant Mutation in cacna1d: A New Model for Schizophrenia-Like Behaviours? Mol. Neurobiol. 2021, 58, 877–894. [Google Scholar] [CrossRef]
- Colwill, R.M.; Creton, R. Imaging escape and avoidance behavior in zebrafish larvae. Rev. Neurosci. 2011, 22, 63–73. [Google Scholar] [CrossRef]
- Huang, I.J.; Sirotkin, H.I.; McElroy, A.E. Varying the exposure period and duration of neuroactive pharmaceuticals and their metabolites modulates effects on the visual motor response in zebrafish (Danio rerio) larvae. Neurotoxicol. Teratol. 2019, 72, 39–48. [Google Scholar] [CrossRef]
- Nicolas, L.B.; Kolb, Y.; Prinssen, E.P. A combined marble burying–locomotor activity test in mice: A practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur. J. Pharmacol. 2006, 547, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Dulawa, S.C.; Holick, K.A.; Gundersen, B.; Hen, R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 2004, 29, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Mombereau, C.; Gur, T.L.; Onksen, J.; Blendy, J.A. Differential effects of acute and repeated citalopram in mouse models of anxiety and depression. Int. J. Neuropsychopharmacol. 2010, 13, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Glover, M.E.; Clinton, S.M. Of rodents and humans: A comparative review of the neurobehavioral effects of early life SSRI exposure in preclinical and clinical research. Int. J. Dev. Neurosci. 2016, 51, 50–72. [Google Scholar] [CrossRef]
- Li, F.; Lin, J.; Liu, X.; Li, W.; Ding, Y.; Zhang, Y.; Zhou, S.; Guo, N.; Li, Q. Characterization of the locomotor activities of zebrafish larvae under the influence of various neuroactive drugs. Ann. Transl. Med. 2018, 6, 173. [Google Scholar] [CrossRef] [PubMed]
- Valenti, T.W., Jr.; Gould, G.G.; Berninger, J.P.; Connors, K.A.; Keele, N.B.; Prosser, K.N.; Brooks, B.W. Human therapeutic plasma levels of the selective serotonin reuptake inhibitor (SSRI) sertraline decrease serotonin reuptake transporter binding and shelter-seeking behavior in adult male fathead minnows. Environ. Sci. Technol. 2012, 46, 2427–2435. [Google Scholar] [CrossRef]
- Maj, J.; Rogóż, Z.; Skuza, G. Antidepressant drugs increase the locomotor hyperactivity induced by MK-801 in rats. J. Neural Transm. 1991, 85, 169–179. [Google Scholar] [CrossRef]
- León, L.A.; Cardenas, F.P. Contribution of the dopaminergic system to the effect of chronic fluoxetine in the rat forced swim test. Psychol. Neurosci. 2008, 1, 81. [Google Scholar] [CrossRef]
- Detke, M.J.; Rickels, M.; Lucki, I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 1995, 121, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodynam. Ther. 1977, 229, 327–336. [Google Scholar]
- Maudhuit, C.; Jolas, T.; Lainey, E.; Hamon, M.; Adrien, J. Effects of acute and chronic treatment with amoxapine and cericlamine on the sleep-wakefulness cycle in the rat. Neuropharmacology 1994, 33, 1017–1025. [Google Scholar] [CrossRef]
- Ferguson, L.; Cooper, G.; Loiselle, D.; Roberton, A. A possible mechanism of toxicity by the antidepressant amoxapine based on its effects in three in vitro models. Toxicol. In Vitro 1989, 3, 285–291. [Google Scholar] [CrossRef]
- Sa, D.S.; Kapur, S.; Lang, A.E. Amoxapine shows an antipsychotic effect but worsens motor function in patients with Parkinson’s disease and psychosis. Clin. Neuropharmacol. 2001, 24, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Richendrfer, H.; Creton, R. Cluster analysis profiling of behaviors in zebrafish larvae treated with antidepressants and pesticides. Neurotoxicol. Teratol. 2018, 69, 54–62. [Google Scholar] [CrossRef]
- Herculano, A.M.; Maximino, C. Serotonergic modulation of zebrafish behavior: Towards a paradox. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2014, 55, 50–66. [Google Scholar] [CrossRef]
- Pisani, F.; Spina, E.; Oteri, G. Antidepressant drugs and seizure susceptibility: From in vitro data to clinical practice. Epilepsia 1999, 40, s48–s56. [Google Scholar] [CrossRef]
- Cai, B.; Matsumoto, K.; Ohta, H.; Watanabe, H. Biphasic effects of typical antidepressants and mianserin, an atypical antidepressant, on aggressive behavior in socially isolated mice. Pharmacol. Biochem. Behav. 1993, 44, 519–525. [Google Scholar]
- Lange, S.C.; Julien, R.M.; Fowler, G.W.; Portera, A.; Scheffner, D. Biphasic effects of imipramine in experimental models of epilepsy. Epilepsia 1976, 17, 183–195. [Google Scholar] [CrossRef]
- Somogyi, G.; Perel, J. Biphasic effect of tricyclic antidepressants on the release of norepinephrine from the adrenergic nerves of the rabbit heart. Psychopharmacology 1991, 104, 237–243. [Google Scholar] [CrossRef]
- Coppell, A.; Pei, Q.; Zetterström, T. Bi-phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology 2003, 44, 903–910. [Google Scholar] [CrossRef]
- Kapoor, A.; Iqbal, M.; Petropoulos, S.; Ho, H.L.; Gibb, W.; Matthews, S.G. Effects of sertraline and fluoxetine on p-glycoprotein at barrier sites: In vivo and in vitro approaches. PLoS ONE 2013, 8, e56525. [Google Scholar] [CrossRef] [PubMed]
- Sills, T.; Greenshaw, A.; Baker, G.; Fletcher, P. The potentiating effect of sertraline and fluoxetine on amphetamine-induced locomotor activity is not mediated by serotonin. Psychopharmacology 1999, 143, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Preskorn, S.H. Factors affecting the biphasic concentration: Effect relationships of tricyclic antidepressants. In Clinical Pharmacology in Psychiatry; Springer: Berlin/Heidelberg, Germany, 1981; pp. 297–306. [Google Scholar]
- Furukawa, T.A.; Cipriani, A.; Cowen, P.J.; Leucht, S.; Egger, M.; Salanti, G. Optimal dose of selective serotonin reuptake inhibitors, venlafaxine, and mirtazapine in major depression: A systematic review and dose-response meta-analysis. Lancet Psychiatry 2019, 6, 601–609. [Google Scholar] [CrossRef]
- Marken, P.A.; Munro, J.S. Selecting a selective serotonin reuptake inhibitor: Clinically important distinguishing features. Prim. Care Compan. J. Clin. Psychiatry 2000, 2, 205. [Google Scholar] [CrossRef]
- Stahl, S.; Grady, M.M.; Munter, N. The Prescriber’s Guide: Antidepressants: Stahl’s Essential Psychopharmacology; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]

| No. | Class | Mechanism | Name | Side Effects |
|---|---|---|---|---|
| 1 | TCA | Inhibit the reuptake of noradrenaline and serotonin [39] | Amitriptyline (AMY) | Dizziness, constipation, headache, and palpitations [40] |
| 2 | Amoxapine (AMO) | Seizures, severe metabolic acidosis, acute renal failure, and coma [41] | ||
| 3 | Doxepin (DOX) | Dry mouth, constipation, dizziness, tachycardia, grand mal seizure, tremor, and hyperthermia [42] | ||
| 4 | Imipramine (IMP) | Nausea, dizziness, and sedation [43] | ||
| 5 | MAOI | Inhibit monoamine oxidase enzymes (MAO-A/B) [44] | Moclobemide (MEM) | Insomnia, headache, nausea, agitation, diarrhea, and dizziness [45] |
| 6 | Selegiline (SEG) | Anorexia, musculoskeletal injuries, hallucinations, dyskinesia, cardiac arrhythmias, and orthostatic hypotension [46] | ||
| 7 | SSRI | Inhibit the reuptake of serotonin [47] | Escitalopram (ESC) | Ejaculation disorder, insomnia, diarrhea, dry mouth, somnolence, dizziness, hyperhidrosis, and fatigue [48] |
| 8 | Fluoxetine (FLX) | Sexual dysfunction, headache, and nausea [49] | ||
| 9 | Paroxetine (PAR) | Sexual dysfunction, weight gain, sleepiness, dry mouth, headache, and nausea [50] | ||
| 10 | Sertraline (SRT) | Agitation, insomnia, seizure, and sexual dysfunction [51] | ||
| 11 | SNRI | Inhibit reuptake of serotonin and noradrenaline [52] | Duloxetine (DLX) | Dry mouth, insomnia, fatigue, headache, nausea, dizziness, constipation, diarrhea, and hyperhidrosis [53] |
| 12 | Milnacipran (MCP) | Dry mouth, sweating, and constipation [54] | ||
| 13 | Venlafaxine (VEN) | Dry mouth, constipation, dizziness, diaphoresis, decreased libido, and induced acute dystonia [55] | ||
| 14 | NRI | Inhibit reuptake of noradrenaline [56] | Atomoxetine (ATM) | Hypertensive crisis, headache, abdominal pain, decreased appetite, vomiting, and nausea [57] |
| 15 | NDRI | Inhibit reuptake of noradrenaline and dopamine [58] | Bupropion (BUP) | Seizures, nonepileptic myoclonus, and confusion [59] |
| 16 | TeCA (NASSA) | Antagonizing α2-adrenergic and serotonin receptor [60] | Mianserin (MIA) | Periorbital edema [61] |
| 17 | Mirtazapine (MRT) | Induced nightmares and high incidence of somnolence [62] | ||
| 18 | SARI | Inhibit the reuptake of serotonin, noradrenaline, dopamine; antagonizing serotonin and α1-adrenergic receptor [60] | Trazodone (TRA) | Daytime sleepiness, headache, orthostatic hypotension, and drowsiness [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suryanto, M.E.; Audira, G.; Uapipatanakul, B.; Hussain, A.; Saputra, F.; Siregar, P.; Chen, K.H.-C.; Hsiao, C.-D. Antidepressant Screening Demonstrated Non-Monotonic Responses to Amitriptyline, Amoxapine and Sertraline in Locomotor Activity Assay in Larval Zebrafish. Cells 2021, 10, 738. https://doi.org/10.3390/cells10040738
Suryanto ME, Audira G, Uapipatanakul B, Hussain A, Saputra F, Siregar P, Chen KH-C, Hsiao C-D. Antidepressant Screening Demonstrated Non-Monotonic Responses to Amitriptyline, Amoxapine and Sertraline in Locomotor Activity Assay in Larval Zebrafish. Cells. 2021; 10(4):738. https://doi.org/10.3390/cells10040738
Chicago/Turabian StyleSuryanto, Michael Edbert, Gilbert Audira, Boontida Uapipatanakul, Akhlaq Hussain, Ferry Saputra, Petrus Siregar, Kelvin H.-C. Chen, and Chung-Der Hsiao. 2021. "Antidepressant Screening Demonstrated Non-Monotonic Responses to Amitriptyline, Amoxapine and Sertraline in Locomotor Activity Assay in Larval Zebrafish" Cells 10, no. 4: 738. https://doi.org/10.3390/cells10040738
APA StyleSuryanto, M. E., Audira, G., Uapipatanakul, B., Hussain, A., Saputra, F., Siregar, P., Chen, K. H.-C., & Hsiao, C.-D. (2021). Antidepressant Screening Demonstrated Non-Monotonic Responses to Amitriptyline, Amoxapine and Sertraline in Locomotor Activity Assay in Larval Zebrafish. Cells, 10(4), 738. https://doi.org/10.3390/cells10040738









