Acanthocyte Sedimentation Rate as a Diagnostic Biomarker for Neuroacanthocytosis Syndromes: Experimental Evidence and Physical Justification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Blood Sampling
2.2. Measurements of the ESR
2.3. Blood Smear Preparation and Evaluation
2.4. Laboratory Parameters and Erythrocyte Preparation
2.5. Widefield Microscopy
2.6. Two-Dimensional Simulations
2.7. Measurement of Aggregation Forces
2.8. Statistical Analysis
2.9. Confocal Microscopy and 3D Rendering
3. Results
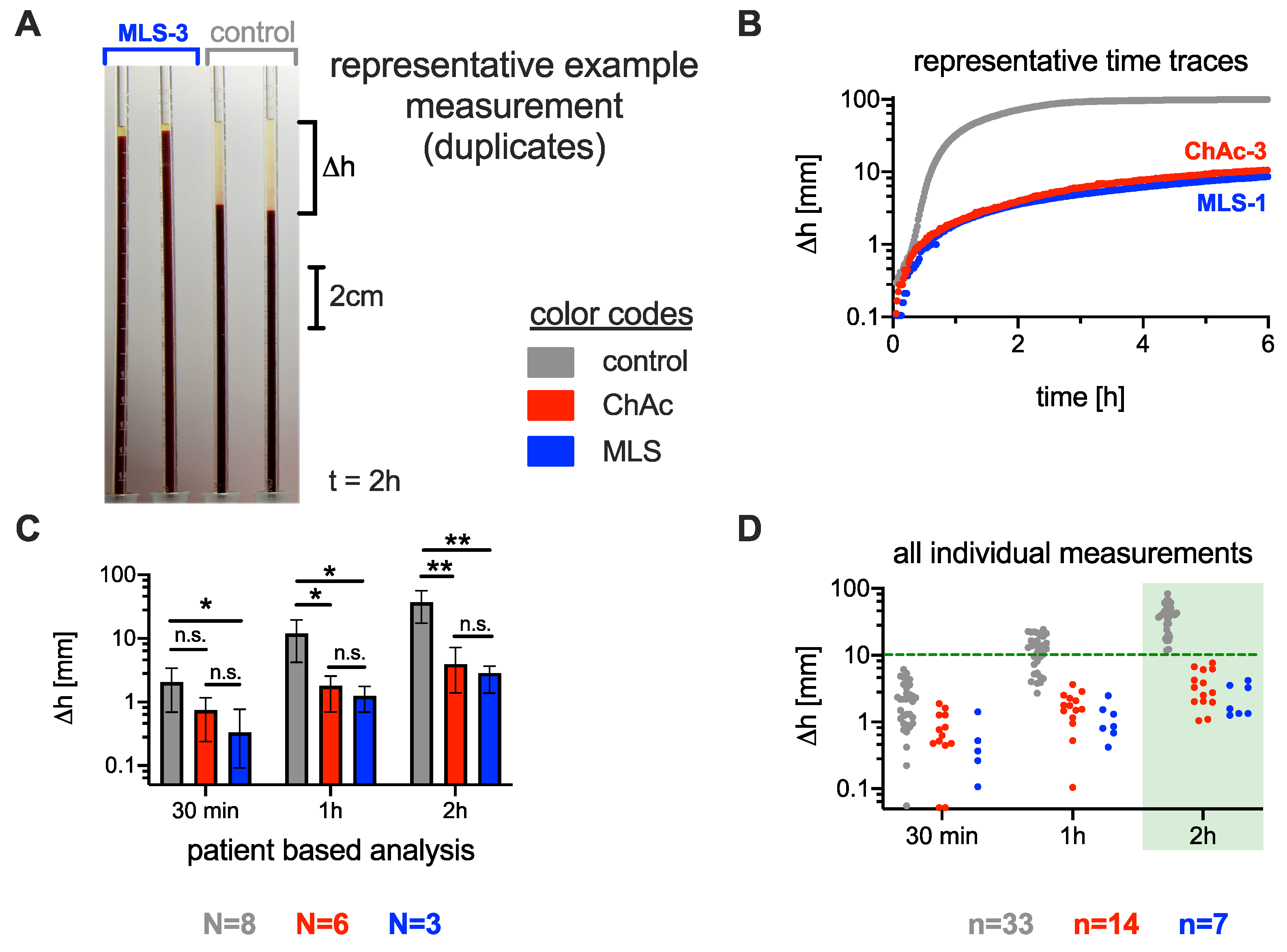
3.1. Significance of the ESR in Neuroacanthocytosis Syndrome Patients
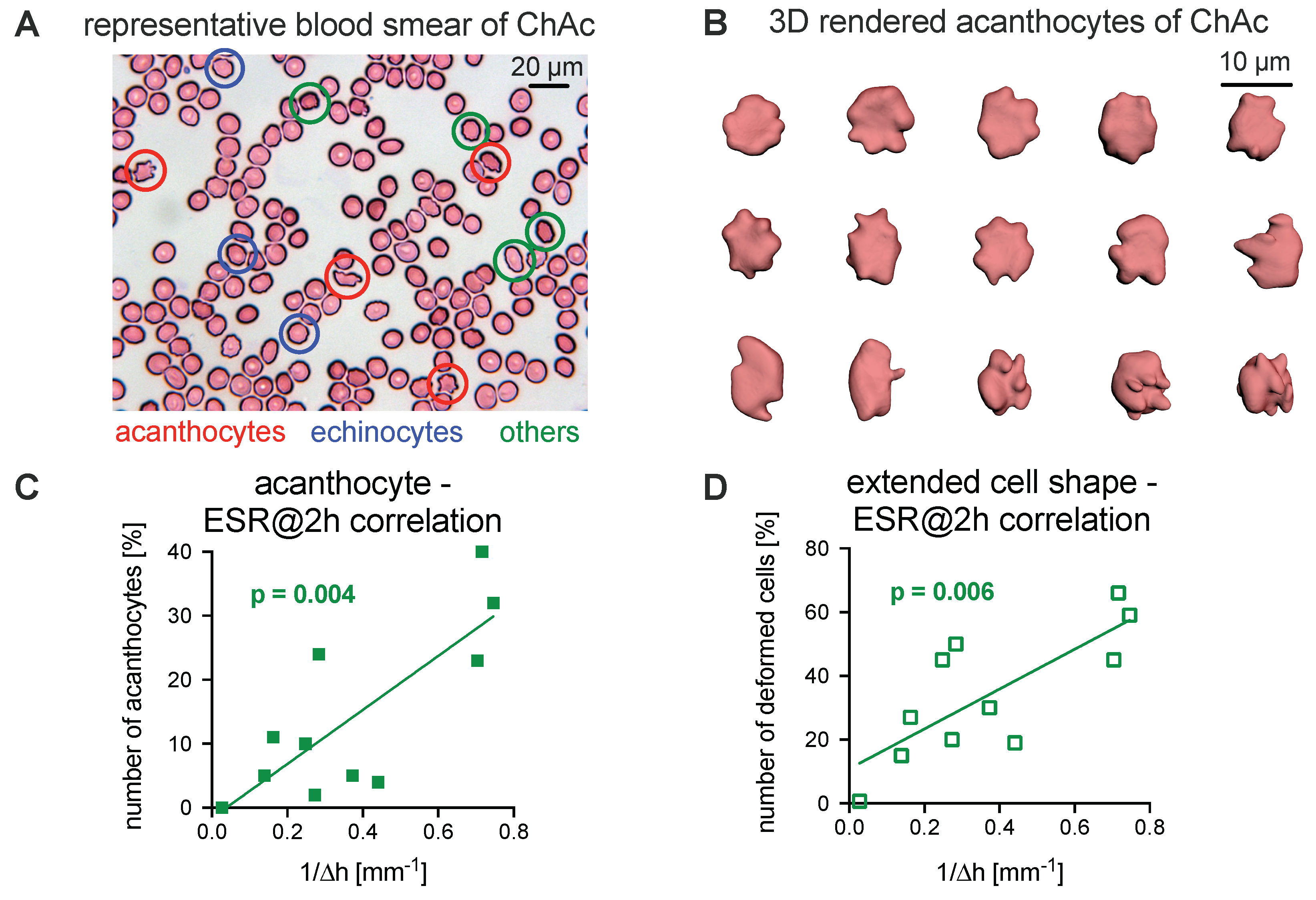
3.2. Comparison of the Acanthocyte Percentage with the ESR
3.3. Explanations for Differences in the ESR
3.3.1. Role of Erythrocytes in Sedimentation
3.3.2. Role of Plasma in the Sedimentation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESR | Erythrocyte sedimentation rate |
| ASR | Acanthocyte sedimentation rate |
| NAS | Neuroacanthocytosis syndrome |
| MLS | McLeod syndrome |
| ChAc | Chorea-acanthocytosis |
| PRN | Pro Re Nata: administration timing of prescribed medication is left to the patient/caregiver |
| PBS | Phosphate buffered saline |
| EDTA | EthyleneDiamine Tetraacetic Acid |
References
- Adam, M.P.; Ardinger, H.H.; Pagon, R.A.; Wallace, S.E.; Bean, L.J.; Stephens, K.; Amemiya, A. McLeod neurocanthocytosis Syndrom. In GeneReviews®; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Danek, A.; Rubio, J.P.; Rampoldi, L.; Ho, M.; Dobson-Stone, C.; Tison, F.; Symmans, W.A.; Oechsner, M.; Kalckreuth, W.; Watt, J.M.; et al. McLeod neuroacanthocytosis: Genotype and phenotype. Ann. Neurol. 2001, 50, 755–764. [Google Scholar] [CrossRef]
- Baeza, A.V.; Dobson-Stone, C.; Rampoldi, L.; Bader, B.; Walker, R.H.; Danek, A.; Monaco, A.P. Chorea-acanthocytosis. In GeneReviews®; University of Washington: Seattle, WA, USA, 2019. [Google Scholar]
- Siegl, C.; Hamminger, P.; Jank, H.; Ahting, U.; Bader, B.; Danek, A.; Gregory, A.; Hartig, M.; Hayflick, S.; Hermann, A.; et al. Alterations of red cell membrane properties in nneuroacanthocytosis. PLoS ONE 2013, 8, e76715. [Google Scholar]
- Peikert, K.; Danek, A.; Hermann, A. Current state of knowledge in Chorea-Acanthocytosis as core Neuroacanthocytosis syndrome. Eur. J. Med. Genet. 2018, 61, 699–705. [Google Scholar] [CrossRef]
- Adjobo-Hermans, M.J.; Cluitmans, J.C.; Bosman, G.J. Neuroacanthocytosis: Observations, theories and perspectives on the origin and significance of acanthocytes. Tremor Other Hyperkinetic Mov. 2015, 5, 328. [Google Scholar] [CrossRef]
- Rubio, J.P.; Danek, A.; Stone, C.; Chalmers, R.; Wood, N.; Verellen, C.; Ferrer, X.; Malandrini, A.; Fabrizi, G.M.; Manfredi, M.; et al. Chorea-acanthocytosis: Genetic linkage to chromosome 9q21. Am. J. Hum. Genet. 1997, 61, 899–908. [Google Scholar] [CrossRef] [Green Version]
- Walker, R.H.; Miranda, M.; Jung, H.H.; Danek, A. Life expectancy and mortality in chorea-acanthocytosis and McLeod syndrome. Parkinsonism Relat. Disord. 2019, 60, 158–161. [Google Scholar] [CrossRef]
- Walker, R.H. Management of neuroacanthocytosis syndromes. Tremor Other Hyperkinetic Mov. 2015, 5, 346. [Google Scholar] [CrossRef]
- Storch, A.; Kornhass, M.; Schwarz, J. Testing for acanthocytosis. J. Neurol. 2005, 252, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Dobson-Stone, C.; Velayos-Baeza, A.; Filippone, L.A.; Westbury, S.; Storch, A.; Erdmann, T.; Wroe, S.J.; Leenders, K.L.; Lang, A.E.; Dotti, M.T.; et al. Chorein detection for the diagnosis of chorea-acanthocytosis. Ann. Neurol. 2004, 56, 299–302. [Google Scholar] [CrossRef]
- Makhro, A.; Huisjes, R.; Verhagen, L.P.; Manu-Pereira, M.d.M.; Llaudet-Planas, E.; Petkova-Kirova, P.; Wang, J.; Eichler, H.; Bogdanova, A.; van Wijk, R.; et al. Red cell properties after different modes of blood transportation. Front. Physiol. 2016, 7, 288. [Google Scholar] [CrossRef] [Green Version]
- D’Errico, J. SLM-Shape Language Modeling. Mathworks. 2009. Available online: http://www.mathworks.com/matlabcentral/fileexchange/24443-slm-shape-language-modeling (accessed on 31 March 2020).
- Zhang, Z.; Henry, E.; Gompper, G.; Fedosov, D.A. Behavior of rigid and deformable particles in deterministic lateral displacement devices with different post shapes. J. Chem. Phys. 2015, 143, 243145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cluitmans, J.C.; Tomelleri, C.; Yapici, Z.; Dinkla, S.; Bovee-Geurts, P.; Chokkalingam, V.; De Franceschi, L.; Brock, R.; Bosman, G.J. Abnormal red cell structure and function in neuroacanthocytosis. PLoS ONE 2015, 10, e0125580. [Google Scholar] [CrossRef]
- Steffen, P.; Jung, A.; Nguyen, D.B.; Müller, T.; Bernhardt, I.; Kaestner, L.; Wagner, C. Stimulation of human red blood cells leads to Ca2+-mediated intercellular adhesion. Cell Calcium 2011, 50, 54–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svoboda, K.; Block, S.M. Biological applications of optical forces. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 247–285. [Google Scholar] [CrossRef] [PubMed]
- Abay, A.; Simionato, G.; Chachanidze, R.; Bogdanova, A.; Hertz, L.; Bianchi, P.; Van Den Akker, E.; Von Lindern, M.; Leonetti, M.; Minetti, G.; et al. Glutaraldehyde—A subtle tool in the investigation of healthy and pathologic red blood cells. Front. Physiol. 2019, 10, 514. [Google Scholar] [CrossRef]
- Simionato, G.; Hinkelmann, K.; Chachanidze, R.; Bianchi, P.; Fermo, E.; van Wijk, R.; Leonetti, M.; Wagner, C.; Kaestner, L. Red blood cell phenotyping from 3D confocal images using artificial neural networks. PLoS Comput. Biol. 2021, accepted. [Google Scholar]
- Brigden, M.L.; Page, N.E. Three closed-tube methods for determining erythrocyte sedimentation rate. Lab. Med. 1993, 24, 97–102. [Google Scholar] [CrossRef]
- Pribush, A.; Meyerstein, D.; Meyerstein, N. The mechanism of erythrocyte sedimentation. Part 1: Channeling in sedimenting blood. Colloids Surf. B Biointerfaces 2010, 75, 214–223. [Google Scholar] [CrossRef]
- Pribush, A.; Meyerstein, D.; Meyerstein, N. The mechanism of erythrocyte sedimentation. Part 2: The global collapse of settling erythrocyte network. Colloids Surf. B Biointerfaces 2010, 75, 224–229. [Google Scholar] [CrossRef]
- Allain, C.; Cloitre, M.; Wafra, M. Aggregation and sedimentation in colloidal suspensions. Phys. Rev. Lett. 1995, 74, 1478. [Google Scholar] [CrossRef]
- Manley, S.; Skotheim, J.; Mahadevan, L.; Weitz, D.A. Gravitational collapse of colloidal gels. Phys. Rev. Lett. 2005, 94, 218302. [Google Scholar] [CrossRef]
- Derec, C.; Senis, D.; Talini, L.; Allain, C. Rapid settling of a colloidal gel. Phys. Rev. E 2003, 67, 062401. [Google Scholar] [CrossRef] [PubMed]
- Senis, D.; Gorre-Talini, L.; Allain, C. Systematic study of the settling kinetics in an aggregating colloidal suspension. Eur. Phys. J. E Soft Matter 2001, 4, 59–68. [Google Scholar] [CrossRef]
- Buscall, R.; Choudhury, T.H.; Faers, M.A.; Goodwin, J.W.; Luckham, P.A.; Partridge, S.J. Towards rationalising collapse times for the delayed sedimentation of weakly-aggregated colloidal gels. Soft Matter 2009, 5, 1345–1349. [Google Scholar] [CrossRef]
- Lindström, S.B.; Kodger, T.E.; Sprakel, J.; Weitz, D.A. Structures, stresses, and fluctuations in the delayed failure of colloidal gels. Soft Matter 2012, 8, 3657–3664. [Google Scholar] [CrossRef] [Green Version]
- Bedell, S.E.; Bush, B.T. Erythrocyte sedimentation rate. From folklore to facts. Am. J. Med. 1985, 78, 1001–1009. [Google Scholar] [CrossRef]
- Gray, S.J.; Mitchell, E.B.; Dick, G. Effect of purified protein fractions on sedimentation rate of erythrocytes. Proc. Soc. Exp. Biol. Med. 1942, 51, 403–404. [Google Scholar] [CrossRef]
- Flormann, D.; Kuder, E.; Lipp, P.; Wagner, C.; Kaestner, L. Is there a role of C-reactive protein in red blood cell aggregation? Int. J. Lab. Hematol. 2015, 37, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Aouane, O.; Thiébaud, M.; Flormann, D.; Verdier, C.; Kaestner, L.; Laschke, M.; Selmi, H.; Benyoussef, A.; Podgorski, T.; et al. The plasma protein fibrinogen stabilizes clusters of red blood cells in microcapillary flows. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ghazali, M.E.B.; Argo, Y.; Kyotoh, H.; Adachi, Y. Effect of the concentration of NaCl and cylinder height on the sedimentation of flocculated suspension of Na-montmorillonite in the semi-dilute regime. Paddy Water Environ. 2020, 18, 309–316. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Zia, R. Gravitational collapse of colloidal gels: Non-equilibrium phase separation driven by osmotic pressure. Soft Matter 2018, 14, 3265–3287. [Google Scholar] [CrossRef] [PubMed]
- Razali, A.; Fullerton, C.J.; Turci, F.; Hallett, J.E.; Jack, R.L.; Royall, C.P. Effects of vertical confinement on gelation and sedimentation of colloids. Soft Matter 2017, 13, 3230–3239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harich, R.; Blythe, T.; Hermes, M.; Zaccarelli, E.; Sederman, A.; Gladden, L.F.; Poon, W.C. Gravitational collapse of depletion-induced colloidal gels. Soft Matter 2016, 12, 4300–4308. [Google Scholar] [CrossRef] [Green Version]
- Rampoldi, L.; Danek, A.; Monaco, A.P. Clinical features and molecular bases of neuroacanthocytosis. J. Mol. Med. 2002, 80, 475–491. [Google Scholar] [CrossRef]
- Patton, W.; Meyer, P.; Stuart, J. Evaluation of sealed vacuum extraction method (Seditainer) for measurement of erythrocyte sedimentation rate. J. Clin. Pathol. 1989, 42, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Kratz, A.; Plebani, M.; Peng, M.; Lee, Y.; McCafferty, R.; Machin, S.; International Council for Standardization in Haematology (ICSH). ICSH recommendations for modified and alternate methods measuring the erythrocyte sedimentation rate. Int. J. Lab Hematol. 2017, 39, 448–457. [Google Scholar] [CrossRef] [Green Version]
- Brigden, M.L. Clinical utility of the erythrocyte sedimentation rate. Am. Fam. Phys. 1999, 60, 1443–1450. [Google Scholar]
- Salt, H.; Wolff, O.; Lloyd, J.; Fosbrooke, A.; Cameron, A.; Hubble, D. On having no beta-lipoprotein. A syndrome comprising α-beta-lipoproteinaemia, acanthocytosis, andsteatorrhoea. Lancet 1960, 2, 325–329. [Google Scholar] [CrossRef]
- Reinhart, W.H.; Singh, A.; Straub, P.W. Red blood cell aggregation and sedimentation: The role of the cell shape. Br. J. Haematol. 1989, 73, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Brecher, G.; Bessis, M. Present status of spiculed red cells and their relationship to the discocyte-echinocyte transformation: A critical review. Blood 1972, 40, 333–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Subjects | Sex | Age (y) | Hematocrit (%) | Hemoglobin (g/dL) | RBC Number () | Main Clinical Characteristics | Disease Duration | Acanthocyte Count (%) | Medication |
|---|---|---|---|---|---|---|---|---|---|
| ChAc-1 | m | 53 | 43 | 15.1 | 4.67 | parkinsonism, dystonia, dysarthria, peripheral neuropathy, depression | 15 | 40 | scopoderm transdermal therapeutic system/day |
| ChAc-2 | f | 51 | 40 | 13.8 | 4.21 | epilepsy, parkinsonism dystonia, dysarthria, peripheral neuropathy, cognitive impairment | 30 | 5 | levetiracetam 4 g/day, valproate 2 g/day, clobazam 10 mg/day, zonisamide 200 mg/day, vitamin D |
| ChAc-3 | m | 54 | 40 | 14.2 | 4.15 | epilepsy, parkinsonism, dystonia, dysarthria, dysphagia, peripheral neuropathy, cognitive impairment | 21 | 10 | lamotrigine 110 mg/day, oxcarbazepine 1.5 g/day, lacosamide 300 mg/day, levodopa 300 mg/day, esomeprazole 40 mg/day |
| ChAc-4 | m | 34 | 46 | 16.2 | 5.40 | drug-resistant epilepsy, mild chorea, tics, cognitive impairment, peripheral neuropathy, myopathy | 11 | 4 | lacosamide 550 mg/day, zonisamide 300 mg/day, perampanel 4 mg/day, vitamin D, PRN: lorazepam/midazolam |
| ChAc-5 | m | 29 | 43 | 15.7 | 4.91 | drug-resistant epilepsy, mild chorea, tics, cognitive impairment, irritability, anxiety, depression, psychosis | 15 | 11 | lacosamide 600 mg/day, zonisamide 400 mg/day, mirtazapine 15 mg/day, olanzapine 2.5 mg/day, vitamin D, PRN: lorazepam/midazolam |
| ChAc-6 | m | 42 | 41 | 15.2 | 4.96 | epilepsy, feeding dystonia, orofacial dyskinesia, chorea, peripheral neuropathy, myopathy, impulse control disorder | 12 | 5 | levetiracetame 1 g/day, quetiapine 400 mg/day, ramipril 2.5 mg/day, metoprolol 47.5 mg/day, PRN: metamizole, ibuprofen, pantoprazole |
| MLS-1 | m | 58 | 43 | 16.0 | 4.82 | myopathy | 15 | 2 | magnesium, St. John’s wort extract, pumpkin seed preparation, PRN: pantoprazol |
| MLS-2 | m | 51 | 42 | 15.3 | 4.67 | epilepsy, peripheral neuropathy, myopathy | 40 | 32 | levetiracetame 2 g/day, lamotrigine 400 mg/day, candesartan 8 mg/day, vitamin D, PRN: methylprednisolone |
| MLS-3 | m | 52 | 45 | 16.2 | 5.17 | neuropathy, myopathy | 18 | 24 | No medication |
| female controls (average, N = 4) | 64 ± 10 | 38.5 ± 2.6 | 12.6 ± 1.3 | 4.36 ± 0.19 | — | — | 0 | — | |
| male controls (average, N = 4) | 38 ± 11 | 43.4 ± 2.1 | 15.5 ± 0.8 | 5.17 ± 0.1 | — | — | 0 | — | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darras, A.; Peikert, K.; Rabe, A.; Yaya, F.; Simionato, G.; John, T.; Dasanna, A.K.; Buvalyy, S.; Geisel, J.; Hermann, A.; et al. Acanthocyte Sedimentation Rate as a Diagnostic Biomarker for Neuroacanthocytosis Syndromes: Experimental Evidence and Physical Justification. Cells 2021, 10, 788. https://doi.org/10.3390/cells10040788
Darras A, Peikert K, Rabe A, Yaya F, Simionato G, John T, Dasanna AK, Buvalyy S, Geisel J, Hermann A, et al. Acanthocyte Sedimentation Rate as a Diagnostic Biomarker for Neuroacanthocytosis Syndromes: Experimental Evidence and Physical Justification. Cells. 2021; 10(4):788. https://doi.org/10.3390/cells10040788
Chicago/Turabian StyleDarras, Alexis, Kevin Peikert, Antonia Rabe, François Yaya, Greta Simionato, Thomas John, Anil Kumar Dasanna, Semen Buvalyy, Jürgen Geisel, Andreas Hermann, and et al. 2021. "Acanthocyte Sedimentation Rate as a Diagnostic Biomarker for Neuroacanthocytosis Syndromes: Experimental Evidence and Physical Justification" Cells 10, no. 4: 788. https://doi.org/10.3390/cells10040788
APA StyleDarras, A., Peikert, K., Rabe, A., Yaya, F., Simionato, G., John, T., Dasanna, A. K., Buvalyy, S., Geisel, J., Hermann, A., Fedosov, D. A., Danek, A., Wagner, C., & Kaestner, L. (2021). Acanthocyte Sedimentation Rate as a Diagnostic Biomarker for Neuroacanthocytosis Syndromes: Experimental Evidence and Physical Justification. Cells, 10(4), 788. https://doi.org/10.3390/cells10040788










