Human-Induced Pluripotent Stem-Cell-Derived Smooth Muscle Cells Increase Angiogenesis to Treat Hindlimb Ischemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Derivation of hiPSC-SMC
2.2. Human Vascular Smooth Muscle Cells
2.3. Human ADSC Cell Culture
2.4. Quantitative PCR
2.5. Animal Model
2.6. Functional Scoring
2.7. Laser Doppler Perfusion Imaging
2.8. MicroCT Imaging
2.9. Immunofluorescence
2.10. Capillary Density Analysis
2.11. Western Blotting
2.12. Statistics
3. Results
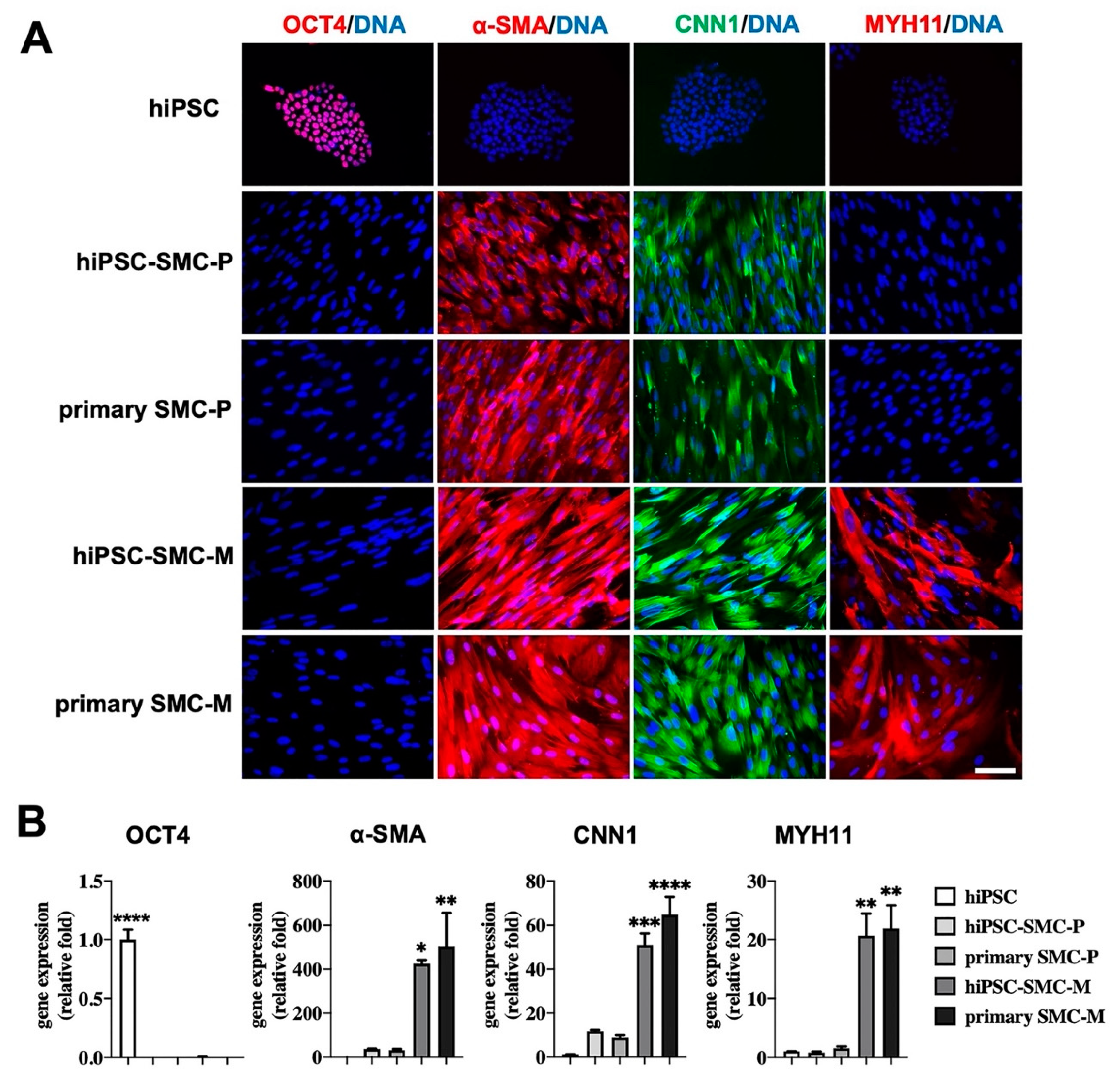
3.1. Characterization of hiPSC-SMC
3.2. hiPSC-SMC Ameliorate Ischemia In Vivo
3.3. hiPSC-SMC Stimulate Angiogenesis
3.4. hiPSC-SMC Treatment is Associated with Fewer Macrophages
3.5. hiPSC-SMC Increase Angiogenesis by Promoting VEGF-A Secretion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



| Gene | Primer Sequence |
|---|---|
| GAPDH-F | TGTTGCCATCAATGACCCCTT |
| GAPDH-R | CTCCACGACGTACTCAGCG |
| α-SMA-F | CTGGGACGACATGGAAAA |
| α-SMA-R | ACATGGCTGGGACATTGA |
| CNN1-F | AGCATGGCGAAGACGAAAGGAA |
| CNN1-R | CCCATCTGCAGGCTGACATTGA |
| MYH11-F | AGAGACAGCTTCACGAGTATGAG |
| MYH11-R | CTTCCAGCTCTCTTTGAAAGTC |
| OCT-4-F | GCAGCTCGGAAGGCAGAT |
| OCT-4-R | TGGATTTTAAAAGGCAGAAGACTTG |
| Antigen | Catalog # | Company | Dilution Used |
|---|---|---|---|
| MYH11 | ab53219 | Abcam | 1:100 |
| α-SMA | A5228 | Sigma | 1:100 |
| CNN1 | C2687 | Sigma | 1:100 |
| OCT4 | ab18976 | Abcam | 1:100 |
| isolectin | DL-1208 | Vector | 1:200 |
| CD68 | MCA1957 | BioRad | 1:200 |
| iNOS | ab15323 | Abcam | 1:200 |
| CD206 | ab64693 | Abcam | 1:200 |
| TGM2 | CST3557 | Cell Signaling | 1:200 |
| goat anti-rabbit Alexa-Fluor 568 | A-11011 | Invitrogen | 1:200–1:500 |
| goat anti-mouse Alexa-Fluor-568 | A-11004 | Invitrogen | 1:200–1:500 |
| goat anti-rat Alexa-Fluor-568 | A-11077 | Invitrogen | 1:200–1:500 |
| goat anti-mouse Alexa-Fluor-488 | A-11001 | Invitrogen | 1:200–1:500 |
| goat anti-rat Alexa-Fluor-488 | A-11006 | Invitrogen | 1:200–1:500 |
References
- Allison, M.A.; Ho, E.; Denenberg, J.O.; Langer, R.D.; Newman, A.B.; Fabsitz, R.R.; Criqui, M.H. Ethnic-Specific Prevalence of Peripheral Arterial Disease in the United States. Am. J. Prev. Med. 2007, 32, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.; McDermott, M.M.; Norman, P.; Sampson, U.K.; Williams, L.J.; A. Mensah, G.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Fereydooni, A.; Gorecka, J.; Dardik, A. Using the epidemiology of critical limb ischemia to estimate the number of patients amenable to endovascular therapy. Vasc. Med. 2019, 25, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Annex, B.H. Therapeutic angiogenesis for critical limb ischaemia. Nat. Rev. Cardiol. 2013, 10, 387–396. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Man-agement of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [PubMed]
- Grochot-Przeczek, A.; Dulak, J.; Jozkowicz, A. Therapeutic angiogenesis for revascularization in peripheral artery disease. Gene 2013, 525, 220–228. [Google Scholar] [CrossRef]
- Iyer, S.R.; Annex, B.H. Therapeutic Angiogenesis for Peripheral Artery Disease: Lessons Learned in Translational Science. JACC Basic Transl. Sci. 2017, 2, 503–512. [Google Scholar] [CrossRef]
- Kirana, S.; Stratmann, B.; Prante, C.; Prohaska, W.; Koerperich, H.; Lammers, D.; Gastens, M.H.; Quast, T.; Negrean, M.; Stirban, O.A.; et al. Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int. J. Clin. Pract. 2012, 66, 384–393. [Google Scholar] [CrossRef]
- Lu, D.; Chen, B.; Liang, Z.; Deng, W.; Jiang, Y.; Li, S.; Xu, J.; Wu, Q.; Zhang, Z.; Xie, B.; et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res. Clin. Pract. 2011, 92, 26–36. [Google Scholar] [CrossRef]
- Fan, W.; Sun, D.; Liu, J.; Liang, D.; Wang, Y.; Narsinh, K.H.; Li, Y.; Qin, X.; Liang, J.; Tian, J.; et al. Adipose stromal cells amplify angio-genic signaling via the VEGF/mTOR/Akt pathway in a murine hindlimb ischemia model: A 3D multimodality imaging study. PLoS ONE 2012, 7, e45621. [Google Scholar] [CrossRef]
- Powell, R.J.; Marston, W.; Berceli, S.; Guzman, R.J.; Henry, T.D.; Longcore, A.T.; Stern, T.P.; Watling, S.; Bartel, R.L. Cellular Therapy with Ixmyelocel-T to Treat Critical Limb Ischemia: The Randomized, Double-blind, Placebo-controlled RESTORE-CLI Trial. Mol. Ther. 2012, 20, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Guo, L.; Cui, S.; Tong, Z.; Dardik, A.; Gu, Y. Autologous bone marrow-derived mononuclear cell therapy in Chinese pa-tients with critical limb ischemia due to thromboangiitis obliterans: 10-year results. Stem Cell Res. Ther. 2018, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Gorecka, J.; Kostiuk, V.; Fereydooni, A.; Gonzalez, L.; Luo, J.; Dash, B.; Isaji, T.; Ono, S.; Liu, S.; Lee, S.R.; et al. The potential and limita-tions of induced pluripotent stem cells to achieve wound healing. Stem Cell Res. Ther. 2019, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by de-fined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Lee, S.-J.; Sohn, Y.-D.; Andukuri, A.; Kim, S.; Byun, J.; Han, J.W.; Park, I.-H.; Jun, H.-W.; Yoon, Y.-S. Enhanced Therapeutic and Long-Term Dynamic Vascularization Effects of Human Pluripotent Stem Cell–Derived Endothelial Cells Encapsulated in a Nanomatrix Gel. Circulation 2017, 136, 1939–1954. [Google Scholar] [CrossRef] [PubMed]
- Rufaihah, A.J.; Huang, N.F.; Jamé, S.; Lee, J.C.; Nguyen, H.N.; Byers, B.; De, A.; Okogbaa, J.; Rollins, M.; Reijo-Pera, R.; et al. Endothelial Cells Derived from Human iPSCS Increase Capillary Density and Improve Perfusion in a Mouse Model of Peripheral Arterial Disease. Arter. Thromb. Vasc. Biol. 2011, 31, e72–e79. [Google Scholar] [CrossRef]
- Lian, Q.; Zhang, Y.; Zhang, J.; Zhang, H.K.; Wu, X.; Zhang, Y.; Lam, F.F.-Y.; Kang, S.; Xia, J.C.; Lai, W.-H.; et al. Functional Mesenchymal Stem Cells Derived from Human Induced Pluripotent Stem Cells Attenuate Limb Ischemia in Mice. Circulation 2010, 121, 1113–1123. [Google Scholar] [CrossRef]
- Gorecka, J.; Gao, X.; Fereydooni, A.; Dash, B.C.; Luo, J.; Lee, S.R.; Taniguchi, R.; Hsia, H.C.; Qyang, Y.; Dardik, A. Induced pluripotent stem cell-derived smooth muscle cells increase angiogenesis and accelerate diabetic wound healing. Regen. Med. 2020, 15, 1277–1293. [Google Scholar] [CrossRef]
- Klein, D. iPSCs-based generation of vascular cells: Reprogramming approaches and applications. Cell. Mol. Life Sci. 2017, 75, 1411–1433. [Google Scholar] [CrossRef]
- Guo, J.; Hu, H.; Gorecka, J.; Bai, H.; He, H.; Assi, R.; Isaji, T.; Wang, T.; Setia, O.; Lopes, L.; et al. Adipose-derived mesenchymal stem cells accelerate diabetic wound healing in a similar fashion as bone marrow-derived cells. Am. J. Physiol. Physiol. 2018, 315, C885–C896. [Google Scholar] [CrossRef]
- Gui, L.; Dash, B.C.; Luo, J.; Qin, L.; Zhao, L.; Yamamoto, K.; Hashimoto, T.; Wu, H.; Dardik, A.; Tellides, G.; et al. Implantable tis-sue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials 2016, 102, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.C.; Levi, K.; Schwan, J.; Luo, J.; Bartulos, O.; Wu, H.; Qiu, C.; Yi, T.; Ren, Y.; Campbell, S.; et al. Tissue-Engineered Vascular Rings from Human iPSC-Derived Smooth Muscle Cells. Stem Cell Rep. 2016, 7, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Qin, L.; Zhao, L.; Gui, L.; Ellis, M.W.; Huang, Y.; Kural, M.H.; Clark, J.A.; Ono, S.; Wang, J.; et al. Tissue-Engineered Vascular Grafts with Advanced Mechanical Strength from Human iPSCs. Cell Stem Cell 2020, 26, 251–261.e8. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Dardik, A. A Murine Model of Hind Limb Ischemia to Study Angiogenesis and Arteriogenesis. Methods Mol. Biol. 2018, 1717, 135–143. [Google Scholar] [CrossRef]
- Brenes, R.A.; Jadlowiec, C.C.; Bear, M.; Hashim, P.; Protack, C.D.; Li, X.; Lv, W.; Collins, M.J.; Dardik, A. Toward a mouse model of hind limb ischemia to test therapeutic angiogenesis. J. Vasc. Surg. 2012, 56, 1669–1679. [Google Scholar] [CrossRef]
- Tarlov, I.M. Spinal cord compression studies. III. Time limits for recovery after gradual compression in dogs. AMA Arch. Neurol. Psychiatry 1954, 71, 588–597. [Google Scholar] [CrossRef]
- Faber, J.E.; Zhang, H.; Lassance-Soares, R.M.; Prabhakar, P.; Najafi, A.H.; Burnett, M.S.; Epstein, S.E. Aging Causes Collateral Rarefaction and Increased Severity of Ischemic Injury in Multiple Tissues. Arter. Thromb. Vasc. Biol. 2011, 31, 1748–1756. [Google Scholar] [CrossRef]
- Parikh, P.P.; Castilla, D.; Lassance-Soares, R.M.; Shao, H.; Regueiro, M.; Li, Y.; Vazquez-Padron, R.; Webster, K.A.; Liu, Z.-J.; Velazquez, O.C. A Reliable Mouse Model of Hind limb Gangrene. Ann. Vasc. Surg. 2018, 48, 222–232. [Google Scholar] [CrossRef]
- Vrselja, Z.; Daniele, S.G.; Silbereis, J.; Talpo, F.; Morozov, Y.M.; Sousa, A.M.M.; Tanaka, B.S.; Skarica, M.; Pletikos, M.; Kaur, N.; et al. Restoration of brain circulation and cellular functions hours post-mortem. Nature 2019, 568, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.W.; Huang, Y.; Ju, R.; Maxfield, M.W.; Ren, Y.; Wang, X.; Wang, X.; Stacy, M.R.; Hwa, J. Molecular Imaging of Factor XIII Ac-tivity for the Early Detection of Mouse Coronary Microvascular Disease. Theranostics 2019, 9, 1474–1489. [Google Scholar] [CrossRef]
- Assi, R.; Foster, T.R.; He, H.; Stamati, K.; Bai, H.; Huang, Y.; Hyder, F.; Rothman, D.; Shu, C.; Homer-Vanniasinkam, S.; et al. Delivery of mesenchymal stem cells in biomimetic engineered scaffolds promotes healing of diabetic ulcers. Regen. Med. 2016, 11, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Aicher, A.; Rentsch, M.; Sasaki, K.; Ellwart, J.W.; Fandrich, F.; Siebert, R.; Cooke, J.P.; Dimmeler, S.; Heeschen, C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ. Res. 2007, 100, 581–589. [Google Scholar] [CrossRef]
- Tateishi-Yuyama, E.; Matsubara, H.; Murohara, T.; Ikeda, U.; Shintani, S.; Masaki, H.; Amano, K.; Kishimoto, Y.; Yoshimoto, K.; Akashi, H.; et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002, 360, 427–435. [Google Scholar] [CrossRef]
- Clayton, Z.E.; Yuen, G.S.; Sadeghipour, S.; Hywood, J.D.; Wong, J.W.; Huang, N.F.; Ng, M.K.; Cooke, J.P.; Patel, S. A comparison of the pro-angiogenic potential of human induced pluripotent stem cell derived endothelial cells and induced endothelial cells in a murine model of peripheral arterial disease. Int. J. Cardiol. 2017, 234, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.W.; Li, Q.; Niu, X.; Hu, B.; Liu, J.; Zhou, S.M.; Guo, S.C.; Lang, H.L.; Zhang, C.Q.; Wang, Y.; et al. Exosomes secreted by hu-man-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res. Ther. 2015, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Kwon, Y.W.; Kim, J.W.; Park, G.T.; Yoon, J.W.; Kim, Y.S.; Kim, D.S.; Kwon, S.M.; Bae, S.S.; Ko, K.; et al. Coadministration of endo-thelial and smooth muscle cells derived from human induced pluripotent stem cells as a therapy for critical limb ischemia. Stem Cells Transl. Med. 2021, 10, 414–426. [Google Scholar] [CrossRef]
- Takeda, Y.; Costa, S.; Delamarre, E.; Roncal, C.; De Oliveira, R.L.; Squadrito, M.L.; Finisguerra, V.; Deschoemaeker, S.; Bruyère, F.; Wenes, M.; et al. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nat. Cell Biol. 2011, 479, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Ganta, V.C.; Choi, M.H.; Kutateladze, A.; Fox, T.E.; Farber, C.R.; Annex, B.H. A MicroRNA93-Interferon Regulatory Fac-tor-9-Immunoresponsive Gene-1-Itaconic Acid Pathway Modulates M2-Like Macrophage Polarization to Revascularize Is-chemic Muscle. Circulation 2017, 135, 2403–2425. [Google Scholar] [CrossRef] [PubMed]
- Nucera, S.; Biziato, D.; De Palma, M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int. J. Dev. Biol. 2011, 55, 495–503. [Google Scholar] [CrossRef]
- Pilny, E.; Smolarczyk, R.; Jarosz-Biej, M.; Hadyk, A.; Skorupa, A.; Ciszek, M.; Krakowczyk, Ł.; Kułach, N.; Gillner, D.; Sokół, M.; et al. Human ADSC xenograft through IL-6 secretion activates M2 macrophages responsible for the repair of damaged muscle tissue. Stem Cell Res. Ther. 2019, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Johnson, T.K.; Wang, Y.; Thomas, M.; Huynh, K.; Yang, Q.; Bond, V.C.; Chen, Y.E.; Liu, D. Macrophage M2 polarization induced by exosomes from adipose-derived stem cells contributes to the exosomal proangiogenic effect on mouse ischemic hindlimb. Stem Cell Res. Ther. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Mohler, E.R., 3rd; Lederman, R.J.; Mendelsohn, F.O.; Saucedo, J.F.; Goldman, C.K.; Blebea, J.; Macko, J.; Kessler, P.D.; Rasmussen, H.S.; et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: A phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation 2003, 108, 1933–1938. [Google Scholar] [PubMed]
- Lederman, R.J.; Mendelsohn, F.O.; Anderson, R.D.; Saucedo, J.F.; Tenaglia, A.N.; Hermiller, J.B.; Hillegass, W.B.; Rocha-Singh, K.; Moon, T.E.; Whitehouse, M.J.; et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudica-tion (the TRAFFIC study): A randomised trial. Lancet 2002, 359, 2053–2058. [Google Scholar] [CrossRef]
- Belch, J.; Hiatt, W.R.; Baumgartner, I.; Driver, I.V.; Nikol, S.; Norgren, L.; Van Belle, E. Effect of fibroblast growth factor NV1FGF on amputation and death: A randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet 2011, 377, 1929–1937. [Google Scholar] [CrossRef]
- Powell, R.J.; Simons, M.; Mendelsohn, F.O.; Daniel, G.; Henry, T.D.; Koga, M.; Morishita, R.; Annex, B.H. Results of a Double-Blind, Placebo-Controlled Study to Assess the Safety of Intramuscular Injection of Hepatocyte Growth Factor Plasmid to Improve Limb Perfusion in Patients with Critical Limb Ischemia. Circulation 2008, 118, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.H.; Ho, J.C.; Chan, Y.C.; Ng, J.H.; Au, K.W.; Wong, L.Y.; Siu, C.W.; Tse, H.F. Attenuation of hind-limb ischemia in mice with endothelial-like cells derived from different sources of human stem cells. PLoS ONE 2013, 8, e57876. [Google Scholar] [CrossRef]
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013, 19, 998–1004. [Google Scholar] [CrossRef]
- Lund, R.J.; Närvä, E.; Lahesmaa, R. Genetic and epigenetic stability of human pluripotent stem cells. Nat. Rev. Genet. 2012, 13, 732–744. [Google Scholar] [CrossRef]








| Score | Description |
|---|---|
| Tarlov score | |
| 0 | No movement |
| 1 | Barely perceptible movement, non–weight-bearing |
| 2 | Frequent movement, non–weight-bearing |
| 3 | Supports weight, partial weight-bearing |
| 4 | Walks with mild deficit |
| 5 | Normal but slow walking |
| 6 | Full and fast walking |
| Faber ischemia score | |
| 0 | Normal |
| 1–5 | Cyanosis or loss of nail(s) |
| 6–10 | Partial or complete atrophy of digit(s) |
| 11–12 | Partial atrophy or gangrene of forefoot |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Gao, M.; Gorecka, J.; Langford, J.; Liu, J.; Luo, J.; Taniguchi, R.; Matsubara, Y.; Liu, H.; Guo, L.; et al. Human-Induced Pluripotent Stem-Cell-Derived Smooth Muscle Cells Increase Angiogenesis to Treat Hindlimb Ischemia. Cells 2021, 10, 792. https://doi.org/10.3390/cells10040792
Gao X, Gao M, Gorecka J, Langford J, Liu J, Luo J, Taniguchi R, Matsubara Y, Liu H, Guo L, et al. Human-Induced Pluripotent Stem-Cell-Derived Smooth Muscle Cells Increase Angiogenesis to Treat Hindlimb Ischemia. Cells. 2021; 10(4):792. https://doi.org/10.3390/cells10040792
Chicago/Turabian StyleGao, Xixiang, Mingjie Gao, Jolanta Gorecka, John Langford, Jia Liu, Jiesi Luo, Ryosuke Taniguchi, Yutaka Matsubara, Hao Liu, Lianrui Guo, and et al. 2021. "Human-Induced Pluripotent Stem-Cell-Derived Smooth Muscle Cells Increase Angiogenesis to Treat Hindlimb Ischemia" Cells 10, no. 4: 792. https://doi.org/10.3390/cells10040792
APA StyleGao, X., Gao, M., Gorecka, J., Langford, J., Liu, J., Luo, J., Taniguchi, R., Matsubara, Y., Liu, H., Guo, L., Gu, Y., Qyang, Y., & Dardik, A. (2021). Human-Induced Pluripotent Stem-Cell-Derived Smooth Muscle Cells Increase Angiogenesis to Treat Hindlimb Ischemia. Cells, 10(4), 792. https://doi.org/10.3390/cells10040792






