The Effect and Regulatory Mechanism of High Mobility Group Box-1 Protein on Immune Cells in Inflammatory Diseases
Abstract
1. Introduction
2. HMGB1 Characteristics
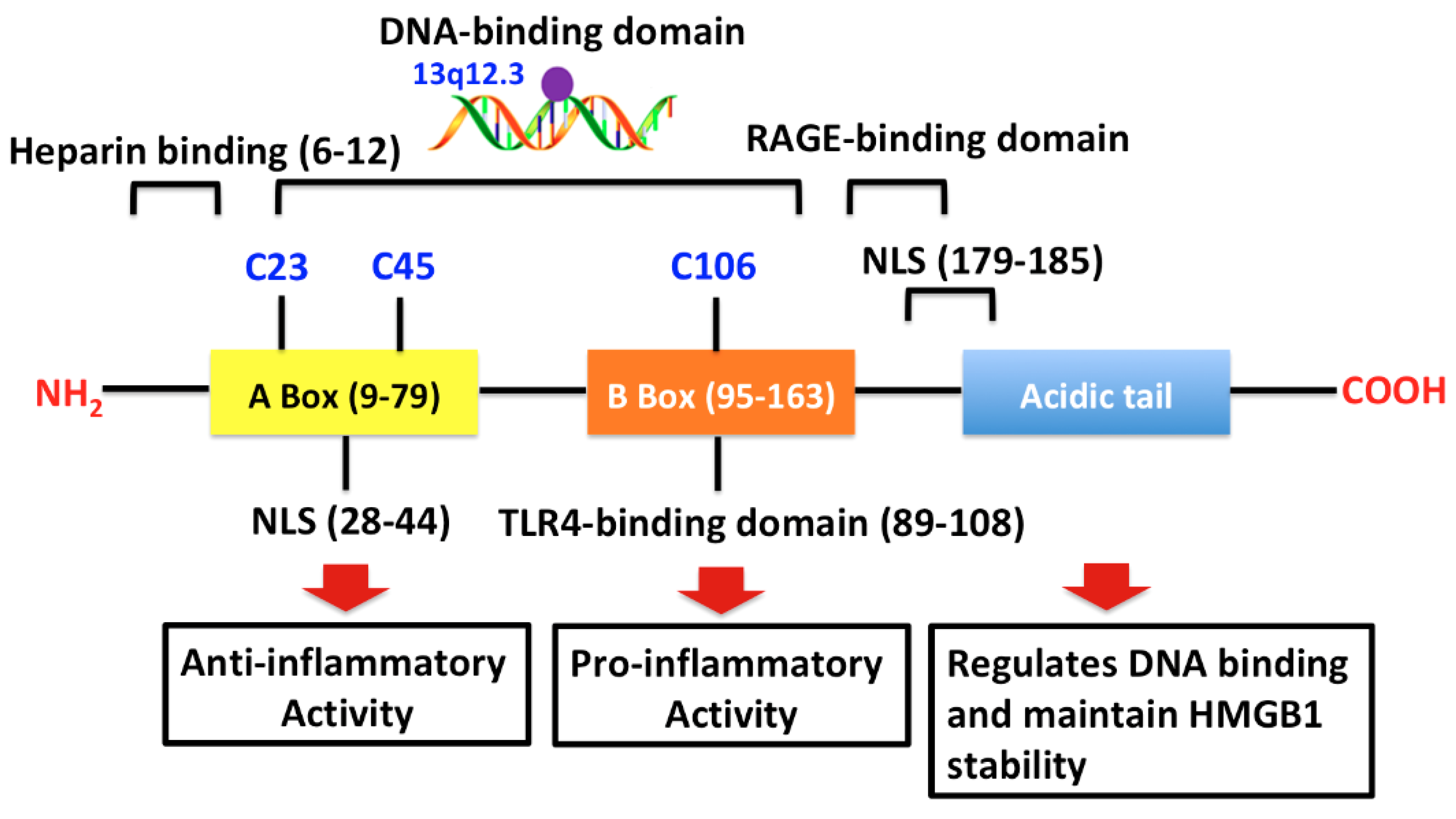
2.1. The HMGB1 Protein
2.2. Regulation of HMGB1 and Its Release Into the Extracellular Space
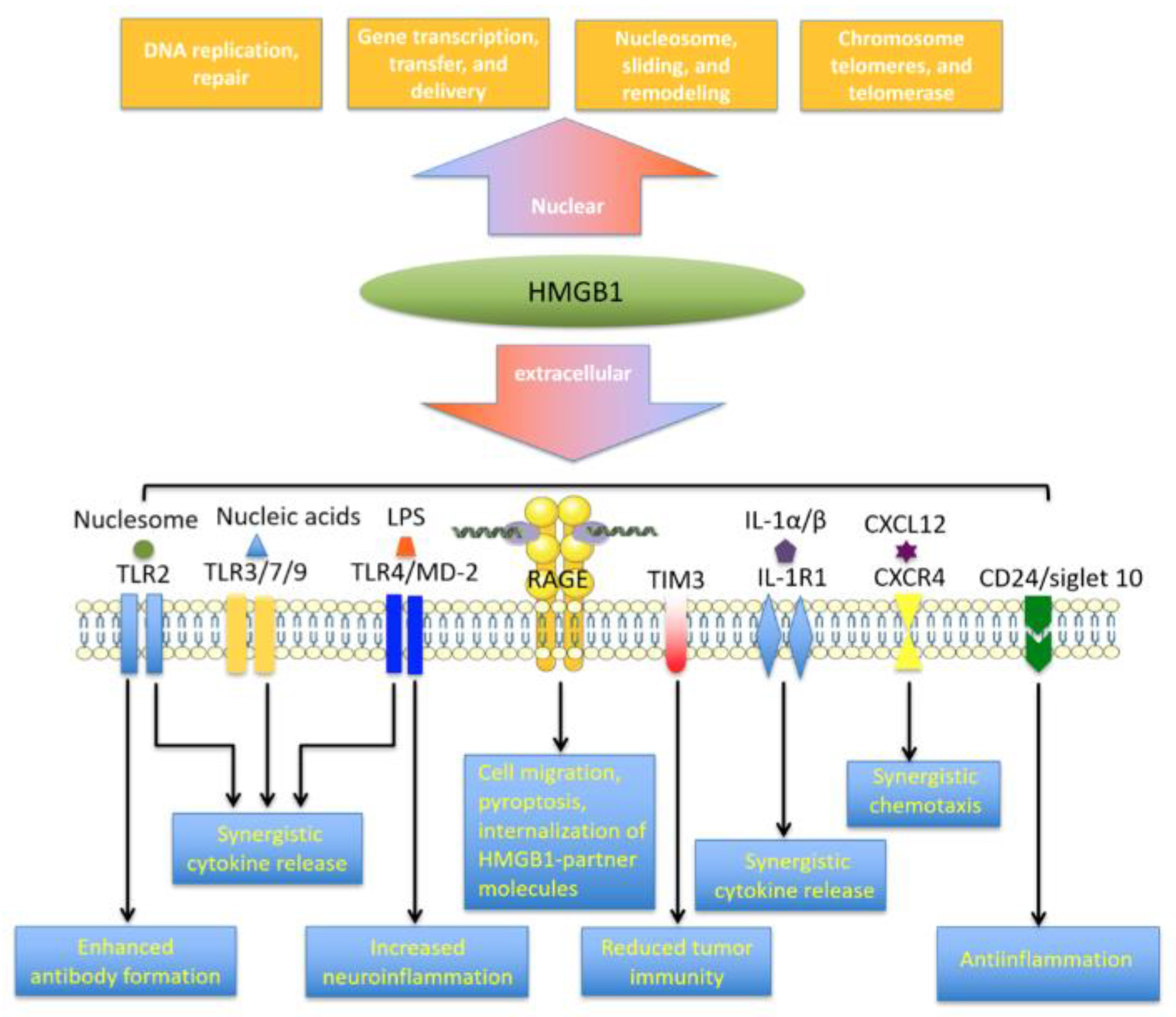
3. HMGB1 Receptor Networks
4. Effects of HMGB1 on Immune Cell Types and the Regulatory Mechanisms Involved
4.1. HMGB1 and Neutrophils
4.2. HMGB1 and Macrophages
4.3. HMGB1 and Dendritic Cells
4.4. HMGB1 and T Lymphocytes
4.5. HMGB1 and Regulatory T Cells
5. Role of HMGB1 in Various Inflammatory Disorders
5.1. Sepsis
5.2. Autoimmune Diseases
5.3. Acute Liver Injury
5.4. Lung Diseases
5.5. Cardiac Injury
5.6. Encephalopathy
5.7. Other Inflammatory Conditions
| Diseases | Year | Authors | Observations or Conclusions | Ref. |
|---|---|---|---|---|
| Sepsis | 1999 | Wang etal. | HMGB1 acts as a late meditator of endotoxin lethality in mice. | [77] |
| 2010 | Lamkanfi et al. | HMGB1 release critical for endotoxin occurs downstream of inflammasome assembly and caspase 1 activation. | [78] | |
| 2015 | Hwang et al. | Theacetylation-dependent interaction between HMGB1 and SIRT1 is critical for LPS-induced lethality in an experimental model of sepsis. | [164] | |
| 2011 | Youn et al. | HMGB1 has two LPS-binding peptide regions that can be utilized to design anti-sepsis or LPS-neutralizing therapeutics in a mouse model. | [165] | |
| 2004 | Yang et al. | Specific inhibition of HMGB1 protects against the development of organ injury and increases survival in septic mice. | [166] | |
| 2016 | Valdes-Ferrer et al. | HMGB1 mediates anemia of inflammation by interfering with erythropoiesis in murine sepsis survivals. | [167] | |
| 2013 | Valdes-Ferrer et al. | HMGB1 mediates splenomegaly and expansion of splenic CD11b+ly-6C(high) inflammatory monocytes in murine sepsis survivors. | [168] | |
| 2017 | Stevens et al. | Anti-HMGB1 antibodies alter inflammation in murine sepsis model and reduce sepsis mortality without potentiating immunosuppression. | [169] | |
| 2017 | Gregoire et al. | HMGB1 induces neutrophil dysfunction in septic mice and in patients who survive septic shock. | [170] | |
| 2016 | Gil et al. | Naringin reduces the release of TNF-α and HMGB1 from LPS-stimulated macrophages and improves survival in a CLP-induced sepsis mice. | [171] | |
| 2006 | Suda et al. | Anti-HMGB1 antibodies improve survival of rats with sepsis. | [172] | |
| Arthritis | 2003 | Taniguchi et al. | HMGB1 is strongly expressed in synovial fluid of RA patients inducing the release of proinflammatory cytokine from synovial fluid macrophages. | [173] |
| 2007 | Goldstein et al. | Cholinergic anti-inflammatory pathway activity and HMGB1 serum levels in patients with RA. | [175] | |
| 2010 | Ostberg et al. | HMGB1 is involved in the pathogenesis of this spontaneous polyarthritis and intervention with an HMGB1 antagonist can mediate beneficial effects. | [177] | |
| 2011 | Schierbeck et al. | Monoclonal anti-HMGB1 antibody significantly ameliorates the clinical courses and partially prevents joint destruction in collagen type II-induced arthritis and spontaneous arthritis model. | [178] | |
| 2003 | Pullerits et al. | HMGB1 triggers joint inflammation by activating macrophages and inducing production of IL-1 via NF-κB activation. | [179] | |
| 2008 | Hamada et al. | HMGB1 is a coupling factor for hypoxia and inflammation in arthritis. | [180] | |
| 2016 | Lundback et al. | HMGB1 is synovial fluid from idiopathic arthritis patients actively released through both acetylation-dependent and nondependent manners. | [181] | |
| 2003 | Kokkola et al. | Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular HMG1 activity. | [182] | |
| 2006 | Wouwer et al. | The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis in mouse model. | [183] | |
| 2008 | Zetterstrom et al. | Gold sodium thiomalate inhibits the extracellular release of HMGB1 from activated macrophages and causes the nuclear retention of this protein, suggesting the anti-rheumatic effects of gold sodium thiomalate in RA. | [184] | |
| SLE | 2012 | Ma et al. | Elevated plasma level of HMGB1 is associated with disease activity and combined alterations with IFN-α and TNF-α in SLE. | [186] |
| 2011 | Abdulahad et al. | Levels of HMGB1 in the sera of SLE patients, in particular in those with active renal disease, are increased. Serum HMGB1 levels are related to SLE disease activity index scores and proteinuria, as well as to levels of anti-HMGB1 antibodies. | [189] | |
| 2014 | Zhang et al. | HMGB1 inhibition attenuates lupus-like disease in BXSB mice. | [190] | |
| 2019 | Whittall-Garcia et al. | In SLE patients, NETs are a source of extracellular HMGB1, which correlates with clinical and histopathological features of active nephritis. | [191] | |
| 2015 | Li et al. | Extracellular HMGB1 facilitates self-DNA induced macrophage activation via promoting DNA accumulation in endosomes and contributes to the pathogenesis of lupus nephritis. | [192] | |
| Liver injury | 2010 | Evankovich et al. | High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histonedeacetylase activity. | [193] |
| 2016 | Lundback et al. | Anti-HMGB1 polyclonal antibody significantly attenuates serum elevations of alanine aminotransferase and abrogates markers of inflammation and improves survival in a model of acetaminophen-acute liver injury. | [197] | |
| 2011 | Dragomir et al. | HMGB1 released by acetaminophen-injured hepatocytes leads to macrophage activation. | [196] | |
| Asthma | 2017 | Di Candia et al. | HMGB1 is upregulated in the airways in asthma and potentiates airway smooth muscle contraction via TLR4. | [201] |
| 2015 | Cuppari et al. | Sputum HMGB1 is increased in asthmatic children and correlates with asthma severity and inversely with lung function indices. | [203] | |
| 2017 | Zhang et al. | Vitamin D reduces inflammatory response in asthmatic mice via HMGB1/TLR4/NF-κB pathway. | [205] | |
| Acute lung injury | 2015 | Sodhi et al | Intestinal epithelial TLR4 activation leads to HMGB1 release from gut and the development of lung injury. | [208] |
| 2005 | Kim et al. | Hemorrhage results in increased HMGB1 expression in the lung primarily via neutrophil sources. | [210] | |
| 2013 | Patel et al. | HMGB1 mediates hyperoxia-induced impairment of Pseudomonas aeruginosa clearance and inflammatory lung injury in mice. | [212] | |
| 2014 | Entezari et al. | Inhibition of extracellular HMGB1 attenuates hyperoxia-induced inflammatory acute lung injury. | [214] | |
| 2004 | Ueno et al. | HMGB1 is increased in plasma and lung epitheliallining fluid of patients with acute lung injury and mice instilled with lipopolysaccharide. | [215] | |
| Cardiac injury | 2009 | Kohno et al. | Elevated serum HMGB1 of is associated with adverse clinical outcomes in patients with myocardial infarction. HMGB1 blockade intramyocardial infarction model aggravated left ventricular remodeling possibly via impairment of the infarct-healing process. | [217] |
| 2008 | Kitahara et al. | HMGB1 enhances angiogenesis, restores cardiac function and improves survival after myocardial infarction in mice. | [218] | |
| Encepha-lopathy | 2014 | Zou et al. | Ethanol alters histone deacetylases that regulate HMGB1 release and that danger signal HMGB1 as endogenous ligand for TLR4 mediates ethanol-induced brain neuroimmune signaling via activation of microglial TLR4. | [220] |
| 2012 | Chavan et al. | Elevated HMGB1 mediates cognitive decline in sepsis survivors in mice. | [221] | |
| 2019 | Liu et al. | Icariin and icaritin ameliorate hippocampus neuroinflammation via inhibiting HMGB1-related pro-inflammatory signals in lipopolysaccharide-induced inflammation model in mice. | [223] | |
| Trauma | 2007 | Levy et al. | HMGB1 levels are transiently elevated just 1 h after injury in both wild-type and TLR4 mutant mice. | [225] |
| 2012 | Shimazaki et al. | Anti-HMGB1 antibody reduces inflammatory reactions and improve survival via blocking extracellular HMGB1 in a rat model of crush injury. | [226] |
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Oppenheim, J.J.; Yang, D. Alarmins: Chemotactic activators of immune responses. Curr. Opin. Immunol. 2005, 17, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Nizet, V.; Ohtake, T.; Lauth, X.; Trowbridge, J.; Rudisill, J.; Dorschner, R.A.; Pestonjamasp, V.; Piraino, J.; Huttner, K.; Gallo, R.L. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 2001, 414, 454–457. [Google Scholar] [CrossRef]
- Rovere-Querini, P.; Capobianco, A.; Scaffidi, P.; Valentinis, B.; Catalanotti, F.; Giazzon, M.; Dumitriu, I.E.; Muller, S.; Iannacone, M.; Traversari, C.; et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004, 5, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Evans, J.E.; Rock, K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 2003, 425, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, Q.; Yang, H.; Tracey, K.J.; Bustin, M.; Oppenheim, J.J. High mobility group box-1 (HMGB1) protein induces the migration and activation of human dendritic cells and acts as an alarmin. J. Leukoc. Biol. 2006, 81, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Abeyama, K.; Stern, D.M.; Ito, Y.; Kawahara, K.I.; Yoshimoto, Y.; Tanaka, M.; Uchimura, T.; Ida, N.; Yamazaki, Y.; Yamada, S.; et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J. Clin. Invest. 2005, 115, 1267–1274. [Google Scholar] [CrossRef]
- Kang, R.; Chen, R.; Zhang, Q.; Hou, W.; Wu, S.; Cao, L.; Huang, J.; Yu, Y.; Fan, X.G.; Yan, Z.; et al. HMGB1 in health and disease. Mol. Aspects Med. 2014, 40, 1–116. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, H.; Zeng, Q.; Imperato, G.H.; Addorisio, M.E.; Li, J.; He, M.; Cheng, K.F.; Al-Abed, Y.; Harris, H.E.; et al. Inhibition of HMGB1/RAGE-mediated endocytosis by HMGB1 antagonist box A, anti-HMGB1 antibodies, and cholinergic agonists suppresses inflammation. Mol. Med. 2019, 25, 13. [Google Scholar] [CrossRef]
- Christaki, E.; Opal, S.M.; Keith, J.C., Jr.; Kessimian, N.; Palardy, J.E.; Parejo, N.A.; Tan, X.Y.; Piche-Nicholas, N.; Tchistiakova, L.; Vlasuk, G.P.; et al. A monoclonal antibody against RAGE alters gene expression and is protective in experimental models of sepsis and pneumococcal pneumonia. Shock 2011, 35, 492–498. [Google Scholar] [CrossRef]
- Andersson, U.; Tracey, K.J. HMGB1 is a therapeutic target for sterileinflammation and infection. Ann. Rev. Immunol. 2011, 29, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Jin, X.; Sun, J.; Li, F.; Feng, Q.; Zhang, C.; Cao, Y.; Wang, Y. Protective effect of HMGB1 a box on organ injury of acute pancreatitis in mice. Pancreas 2009, 38, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.H.; Johns, E.W. Are the high mobility group non-histone chromosomalproteins associated with ‘active’ chromatin? Biochim. Biophys. Acta 1978, 519, 279–284. [Google Scholar] [CrossRef]
- Romani, M.; Rodman, T.C.; Vidali, G.; Bustin, M. Serological analysis of speciesspecificity in the high mobility group chromosomal proteins. J. Biol. Chem. 1979, 254, 2918–2922. [Google Scholar] [CrossRef]
- Merenmies, J.; Pihlaskari, R.; Laitinen, J.; Wartiovaara, J.; Rauvala, H. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Aminoacid sequence and localization in the filopodia of the advancing plasma membrane. J. Biol. Chem. 1991, 266, 16722–16729. [Google Scholar] [CrossRef]
- Calogero, S.; Grassi, F.; Aguzzi, A.; Voigtlander, T.; Ferrier, P.; Ferrari, S.; Bianchi, M.E. The lack of chromosomal protein hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat. Genet. 1999, 22, 276–280. [Google Scholar] [CrossRef]
- Maher, F.J.; Nathans, D. Multivalent DNA-binding properties of the HMG-1 proteins. Proc. Natl. Acad. Sci. USA. 1996, 93, 6716–6720. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zeng, M.; Wang, W.; Tang, J. The HMGB1 acidic tail regulates HMGB1 DNA binding specificity by a unique mechanism. Biochem. Biophys. Res. Commun. 2007, 360, 14–19. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Hasu, M.; Popov, D.; Zhang, J.H.; Chen, J.; Yan, S.D.; Brett, J.; Cao, R.; Kuwabara, K.; Costache, G.; et al. Receptor for advanced glycation end products (AGEs) has a central role in vessel wall interactions and gene activation in responseto circulating AGE proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 8807–8811. [Google Scholar] [CrossRef]
- Hori, O.; Brett, J.; Slattery, T.; Cao, R.; Zhang, J.; Chen, J.X.; Nagashima, M.; Lundh, E.R.; Vijay, S.; Nitecki, D.; et al. The receptor for advanced glycation endproducts (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J. Biol. Chem. 1995, 270, 25752–25761. [Google Scholar] [CrossRef]
- Bierhaus, A.; Humpert, P.M.; Morcos, M.; Wendt, T.; Chavakis, T.; Arnold, B.; Stern, D.M.; Nawroth, P.P. Understanding RAGE the receptor for advanced glycationend products. J. Mol. Med. 2005, 83, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Sevillano, N.; Giron, M.D.; Salido, M.; Vargas, A.M.; Vilches, J.; Salto, R. Internalization of the receptor for advanced glycation end products (RAGE) is required to mediate intracellular responses. J. Biochem. 2009, 145, 21–30. [Google Scholar] [CrossRef]
- Furlani, D.; Donndorf, P.; Westien, I.; Ugurlucan, M.; Pittermann, E.; Wang, W.; Li, W.; Vollmar, B.; Steinhoff, G.; Kaminski, A.; et al. HMGB-1 induces c-kit+ cell microvascular rolling and adhesion via both toll-likereceptor-2 and toll-like receptor-4 of endothelial cells. J. Cell. Mol. Med. 2012, 16, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, F.; Hawez, A.; Mörgelin, M.; Thorlacius, H. Neutrophil extracellular trap-microparticle complexes trigger neutrophil recruitment via high-mobility group protein 1 (HMGB1)-toll-like receptors (TLR2)/TLR4 signalling. Br. J. Pharmacol. 2019, 176, 3350–3363. [Google Scholar]
- Zhang, C.; Dong, H.; Chen, F.; Wang, Y.; Ma, J.; Wang, G. The HMGB1-RAGE/TLR-TNF-α signaling pathway may contribute to kidney injury induced by hypoxia. Exp. Ther. Med. 2019, 17, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, R.; Peng, Z.; Hu, B.; Rao, X.; Li, J. HMGB1 participates in LPS-induced acute lung injury by activating the AIM2 inflammasome in macrophages and inducing polarization of M1 macrophages via TLR2, TLR4, and RAGE/NF-κB signaling pathways. Int. J. Mol. Med. 2020, 45, 61–80. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Chavan, S.S.; Andersson, U. High Mobility Group Box Protein 1 (HMGB1): The Prototypical Endogenous Danger Molecule. Mol. Med. 2015, 21, S6–S12. [Google Scholar] [CrossRef]
- Yanai, H.; Ban, T.; Wang, Z.; Choi, M.K.; Kawamura, T.; Negishi, H.; Nakasato, M.; Lu, Y.; Hangai, S.; Koshiba, R.; et al. HMGB proteins function as universal sentinels fornucleic-acid-mediated innate immune responses. Nature 2009, 462, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N.; Stockinger, B.; Tak, P.P. The resolution of inflammation. Nat. Rev. Immunol. 2013, 13, 59–66. [Google Scholar] [CrossRef]
- Fan, H.; Tang, H.B.; Chen, Z.; Wang, H.Q.; Zhang, L.; Jiang, Y.; Li, T.; Yang, C.F.; Wang, X.Y.; Li, X.; et al. Inhibiting HMGB1-RAGE axis prevents pro-inflammatory macrophages/microglia polarization and affords neuroprotection after spinal cord injury. J. Neuroinflammation 2020, 17, 295. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Van Houten, B.; Zeh, H.J.; Billiar, T.R.; Lotze, M.T. High mobility group box 1 (HMGB1) phenotypic role revealed with stress. Mol. Med. 2014, 20, 359–362. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tajima, Y. HMGB1 is a promising therapeutic target for acute liver failure. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gauthier, A.; Daley, L.; Dial, K.; Wu, J.; Woo, J.; Lin, M.; Ashby, C.; Mantell, L.L. The Role of HMGB1, a Nuclear Damage-Associated Molecular Pattern Molecule, in the Pathogenesis of Lung Diseases. Antioxid. Redox Signal. 2019, 31, 954–993. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Shaikh, M.F.; Chakraborti, A.; Kumari, Y.; Aledo-Serrano, A.; Aleksovska, K.; Alvim, M.K.M.; Othman, I. HMGB1: A common biomarker and potential target for TBI, neuroinflammation, epilepsy, and cognitive dysfunction. Front. Neurosci. 2018, 12, 628. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, Z.; Barnie, P.A.; Su, Z. Dual faced HMGB1 plays multiple roles in cardiomyocyte senescence and cardiac inflammatory injury. Cytokine Growth Factor Rev. 2019, 47, 74–82. [Google Scholar] [CrossRef]
- Landsman, D.; Bustin, M. A signature for the HMG-1 box DNA-binding proteins. Bioessays 1993, 15, 539–546. [Google Scholar] [CrossRef]
- Thomas, J.O. HMG1 and 2: Architectural DNA-bindingproteins. Biochem. Soc. Trans. 2001, 29, 395–401. [Google Scholar] [CrossRef]
- Verrijdt, G.; Haelens, A.; Schoenmakers, E.; Rombauts, W.; Claessens, F. Comparativeanalysis of the influence of the high-mobility group box 1 protein on DNA bindingand transcriptional activation by the androgen, glucocorticoid, progesterone and mineralocorticoid receptors. Biochem. J. 2002, 361, 97–103. [Google Scholar] [CrossRef]
- Li, J.; Kokkola, R.; Tabibzadeh, S.; Yang, R.; Ochani, M.; Qiang, X.; Harris, H.E.; Czura, C.J.; Wang, H.; Ulloa, L.; et al. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol. Med. 2003, 9, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Stros, M.; Kucirek, M.; Sani, S.A.; Polanska, E. HMGB1-mediated DNA bending: Distinctroles in increasing p53 binding to DNA and the transactivation of p53-responsive gene promoters. Biochim. Biophys. Acta Gen. Regul. Mech. 2018, 1861, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Stros, M. HMGB proteins: Interactions with DNA and chromatin. Biochim. Biophys. Acta 2010, 1799, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Ronfani, L.; Bianchi, M. Regulated expression and subcellular localizationof HMGB1, a chromatin protein with a cytokine function. J. Intern. Med. 2004, 255, 332–343. [Google Scholar] [CrossRef]
- Gardella, S.; Andrei, C.; Ferrera, D.; Lotti, L.V.; Torrisi, M.R.; Bianchi, M.E.; Rubartelli, A. The nuclear protein HMGB1 is secreted by monocytes via a nonclassicalvesicle-mediated secretory pathway. EMBO Rep. 2002, 3, 995–1001. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Gong, Z. Regulation of Acetylation in High Mobility Group Protein B1 Cytosol Translocation. DNA Cell Biol. 2019, 38, 491–499. [Google Scholar] [CrossRef]
- Bonaldi, T.; Talamo, F.; Scaffidi, P.; Ferrera, D.; Porto, A.; Bachi, A.; Rubartelli, A.; Agresti, A.; Bianchi, M.E. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003, 22, 5551–5560. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, X.; Antoine, D.; Xiao, X.; Wang, H.; Andersson, U.; Billiar, T.R.; Tracey, K.J.; Lu, B. Regulation of posttranslational modifications of HMGB1 duringimmune responses. Antioxid. Redox Signal. 2016, 24, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Ugrinova, I.; Zlateva, S.; Pasheva, E. The effect of PKC phosphorylation on the “architectural” properties of HMGB1 protein. Mol. Biol. Rep. 2012, 39, 9947–9953. [Google Scholar] [CrossRef]
- Lu, B.; Nakamura, T.; Inouye, K.; Li, J.; Tang, Y.; Lundback, P.; Valdes-Ferrer, S.I.; Olofsson, P.S.; Kalb, T.; Roth, J. Novel role of PKR in inflammasome activation and HMGB1 release. Nature 2012, 488, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Sowinska, A.; Rensing, M.; Klevenvall, L.; Neog, M.; Lundbäck, P.; Harris, H.E. Cleavage of HMGB1 by proteolytic enzymes associated with inflammatory conditions. Front. Immunol. 2020, 11, 448262. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Shi, Y.; Kang, R.; Li, T.; Xiao, W.; Wang, H.; Xiao, X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J. Leukoc. Biol. 2007, 81, 741–747. [Google Scholar] [CrossRef]
- Yu, Y.; Tang, D.; Kang, R. Oxidative stress-mediated HMGB1 biology. Front. Physiol. 2015, 6, 93. [Google Scholar] [CrossRef]
- Antoine, D.J.; Harris, H.E.; Andersson, U.; Tracey, K.J.; Bianchi, M.E. A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins. Mol. Med. 2014, 20, 135–137. [Google Scholar] [CrossRef]
- Kazama, H.; Ricci, J.E.; Herndon, J.M.; Hoppe, G.; Green, D.R.; Ferguson, T.A. Induction of immunological tolerance by apoptotic cells requires caspase-dependentoxidation of high-mobility group box-1 protein. Immunity 2008, 29, 21–32. [Google Scholar] [CrossRef]
- Venereau, E.; Casalgrandi, M.; Schiraldi, M.; Antoine, D.J.; Cattaneo, A.; Marchis, F.D.; Liu, J.; Antonelli, A.; Preti, A.; Raeli, L. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J. Exp. Med. 2012, 209, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.S.; Kim, H.S.; Lee, B.; Kim, Y.H.; Son, M.; Shin, J.S. Immunological significance of HMGB1 post-translational modification and redox biology. Front. Immunol. 2020, 11, 1189. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necroticcells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Schiller, M.; Heyder, P.; Ziegler, S.; Niessen, A.; Claßen, L.; Lauffer, A.; Lorenz, H.M. During apoptosis HMGB1 is translocated into apoptotic cell-derived membranous vesicles. Autoimmunity 2013, 46, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S. The expression of HMGB1 on microparticles released during cell activation and cell death in vitro and in vivo. Mol. Med. 2014, 20, 158–163. [Google Scholar] [CrossRef]
- Bertheloot, D.; Naumovski, A.L.; Langhoff, P.; Horvath, G.L.; Jin, T.; Xiao, T.S.; Garbi, N.; Agrawal, S.; Kolbeck, R.; Latz, E. RAGE enhances TLR responses through binding and internalization of RNA. J. Immunol. 2016, 197, 4118–4126. [Google Scholar] [CrossRef]
- Liang, Y.; Hou, C.; Kong, J.; Wen, H.; Zheng, X.; Wu, L. HMGB1 binding to receptor for advance dglycation end products enhances inflammatory responses of human bronchial epithelial cells by activating p38 MAPK and ERK1/2. Mol. Cell Biochem. 2015, 405, 63–71. [Google Scholar] [CrossRef]
- Kierdorf, K.; Fritz, G. RAGE regulation and signaling in inflammation and beyond. J. Leukoc. Biol. 2013, 94, 55–68. [Google Scholar] [CrossRef]
- Ito, I.; Fukazawa, J.; Yoshida, M. Post-translational methylation of high mobility group box 1 (HMGB1) causes its cytoplasmic localization in neutrophils. J. Biol. Chem. 2007, 282, 16336–16344. [Google Scholar] [CrossRef] [PubMed]
- Sirois, C.M.; Jin, T.; Miller, A.L.; Bertheloot, D.; Nakamura, H.; Horvath, G.L.; Mian, A.; Jiang, J.; Schrum, J.; Bossaller, L. RAGE is a nucleic acid receptor that promotes inflammatory responses to DNA. J. Exp. Med. 2013, 210, 2447–2463. [Google Scholar] [CrossRef]
- Kokkola, R.; Andersson, A.; Mullins, G.; Ostberg, T.; Treutiger, C.J.; Arnold, B.; Nawroth, P.; Andersson, U.; Harris, R.A.; Harris, H.E. RAGE is the major receptor forthe proinflammatory activity of HMGB1 in rodent macrophages. Scand. J. Immunol. 2005, 61, 1–9. [Google Scholar] [CrossRef]
- Hou, L.; Yang, Z.; Wang, Z.; Zhang, X.; Zhao, Y.; Yang, H.; Zheng, B.; Tian, W.; Wang, S.; He, Z.; et al. NLRP3/ASC-mediated alveolar macrophage pyroptosis enhances HMGB1 secretion in acute lung injury induced by cardiopulmonary bypass. Lab. Invest. 2018, 98, 1052–1064. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, P.M.; Doggett, T.A.; Choi, J.; Hancock, M.A.; Durocher, Y.; Frank, F.; Nagar, B.; Ferguson, T.A.; Saleh, M. An immunogenic peptide in the A-box ofHMGB1 protein reverses apoptosis-induced tolerance through RAGE receptor. J. Biol. Chem. 2014, 289, 7777–7786. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Receptor for AGE (RAGE): Signaling mechanismsin the pathogenesis of diabetes and its complications. Ann. N. Y. Acad. Sci. 2011, 1243, 88–102. [Google Scholar] [CrossRef]
- Akashi-Takamura, S.; Miyake, K. TLR accessory molecules. Curr. Opin. Immunol. 2008, 20, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Deng, M.; Loughran, P.A.; Yang, M.; Lin, M.; Yang, C.; Gao, W.; Jin, S.; Li, S.; Cai, J.; et al. LPS induces active HMGB1 release from hepatocytes into exosomes through the coordinated activities of TLR4 and caspase-11/GSDMD signaling. Front. Immunol. 2020, 11, 229. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, J.; Wang, W.; Zhao, W.; Peng, F.; Xiang, Y.; Chen, G.; Chen, T.; Chai, C.; Zheng, S.; et al. HMGB1-promoted and TLR2/4-dependent NK cell maturation and activation take part in rotavirus-induced murine biliary atresia. PLoS Pathog. 2014, 10, e1004011. [Google Scholar] [CrossRef]
- Tian, J.; Avalos, A.M.; Mao, S.Y.; Chen, B.; Senthil, K.; Wu, H.; Parroche, P.; Drabic, S.; Golenbock, D.; Sirois, C.; et al. Toll-like receptor 9-dependent activation by DNA-containingimmune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007, 8, 487–496. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Ju, Z.; Ragab, A.A.; Lundback, P.; Long, W.; Valdes-Ferrer, S.I.; He, M.; Pribis, J.P.; Li, J.; et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. J. Exp. Med. 2015, 212, 5–14. [Google Scholar] [CrossRef]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Criollo, A.; Ortiz, C.; Lidereau, R.; Mariette, C.; Chaput, N.; Mira, J.P.; Delaloge, S.; et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol. Rev. 2007, 220, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Baghdadi, M.; Akiba, H.; Yoshiyama, H.; Kinoshita, I.; Dosaka-Akita, H.; Fujioka, Y.; Ohba, Y.; Gorman, J.V.; Colgan, J.D.; et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol. 2012, 13, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Balosso, S.; Liu, J.; Bianchi, M.E.; Vezzani, A. Disulfide-containing high mobility group box-1 promotes N-methyl-D-aspartate receptor function and excitotoxicity by activating Toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxid. Redox Signal. 2014, 21, 1726–1740. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Tang, J.; Zheng, P.; Liu, Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 2009, 323, 1722–1725. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Sarkar, A.; Walle, L.V.; Vitari, A.C.; Amer, A.O.; Wewers, M.D.; Tracey, K.J.; Kanneganti, T.D.; Dixit, V.M. Inflammasome-dependent release of thealarmin HMGB1 in endotoxemia. J. Immunol. 2010, 185, 4385–4392. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Zmijewski, J.; Xu, Z.; Abraham, E. HMGB1 develops enhanced proinflammatoryactivity by binding to cytokines. J. Immunol. 2008, 180, 2531–2537. [Google Scholar] [CrossRef]
- Hreggvidsdottir, H.S.; Ostberg, T.; Wahamaa, H.; Schierbeck, H.; Aveberger, A.C.; Klevenvall, L.; Palmblad, K.; Ottosson, L.; Andersson, U.; Harris, H.E. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J. Leukoc. Biol. 2009, 86, 655–662. [Google Scholar] [CrossRef]
- Hreggvidsdottir, H.S.; Lundberg, A.M.; Aveberger, A.C.; Klevenvall, L.; Andersson, U.; Harris, H.E. High mobility group box protein 1 (HMGB1)-partner molecule complexes enhance cytokine production by signaling through the partner molecule receptor. Mol. Med. 2012, 18, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Taniguchi, T. Nucleic acid sensing and beyond: Virtues and vices of high mobility group box 1. J. Intern. Med. 2014, 276, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Wang, H.; Palmblad, K.; Aveberger, A.C.; Bloom, O.; Erlandsson-Harris, H.; Janson, A.; Kokkola, R.; Zhang, M.; Yang, H.; et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000, 192, 565–570. [Google Scholar] [CrossRef]
- Meng, L.; Li, L.; Lu, S.; Li, K.; Su, Z.; Wang, Y.; Fan, X.; Li, X.; Zhao, G. The protective effect of dexmedetomidine on LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB and PI3K/Akt/mTOR pathways. Mol. Immunol. 2018, 94, 7–17. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, H.; Dong, H.; Wu, Y.; Yao, L.; Zou, F.; Cai, S. High-mobility group box 1 impairs airway epithelial barrier function through the activation of the RAGE/ERK pathway. Int. J. Mol. Med. 2016, 37, 1189–1198. [Google Scholar] [CrossRef]
- Vizoso-Vazquez, A.; Barreiro-Alonso, A.; Gonzalez-Siso, M.I.; Rodriguez-Belmonte, E.; Lamas-Maceiras, M.; Cerdan, M.E. HMGB proteins involved in TOR signaling asgeneral regulators of cell growth by controlling ribosome biogenesis. Curr. Genet. 2018, 64, 1205–1213. [Google Scholar] [CrossRef]
- Xie, W.; Zhu, T.; Dong, X.; Nan, F.; Meng, X.; Zhou, P.; Sun, G.; Sun, X. HMGB1-triggered inflammation inhibition of notoginseng leaf triterpenes against cerebral ischemia and reperfusion injury via MAPK and NF-κB signaling pathways. Biomolecules 2019, 9, 512. [Google Scholar] [CrossRef]
- Luan, Y.Y.; Yao, F.H.; Zhang, Q.H.; Zhu, X.M.; Dong, N.; Yao, Y.M. Effect of high mobility group box-1 protein on immune cells and its regulatory mechanism. Chin. J. Appl. Physiol. 2012, 28, 548–554. [Google Scholar]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROS cue: Mechanisms of NADPH oxidase activation and bacterial resistance. Front. Cell Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Reino, D.C.; Palange, D.; Feketeova, E.; Bonitz, R.P.; Xu, D.Z.; Lu, Q.; Sheth, S.U.; Pena, G.; Ulloa, L.; Maio, A.D.; et al. Activation of toll-like receptor 4 is necessary for trauma hemorrhagic shock-induced gut injury and polymorphonuclear neutrophil priming. Shock 2012, 38, 107–114. [Google Scholar] [CrossRef]
- Huebener, P.; Pradere, J.P.; Hernandez, C.; Gwak, G.Y.; Caviglia, J.M.; Mu, X.; Loike, J.D.; Schwabe, R.F. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J. Clin. Invest. 2015, 125, 539–550. [Google Scholar] [CrossRef]
- Bukong, T.N.; Cho, Y.; Iracheta-Vellve, A.; Saha, B.; Lowe, P.; Adejumo, A.; Furi, I.; Ambade, A.; Gyongyosi, B.; Catalano, D.; et al. Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use. J. Hepatol. 2018, 69, 1145–1154. [Google Scholar] [CrossRef]
- Wang, C.; Chang, D.Y.; Chen, M.; Zhao, M.H. HMGB1 contributes to glomerular endothelial cell injury in ANCA-associated vasculitis through enhancing endothelium-neutrophil interactions. J. Cell Mol. Med. 2017, 21, 1351–1360. [Google Scholar] [CrossRef]
- Yan, B.; Chen, F.; Xu, L.; Xing, J.; Wang, X. HMGB1-TLR4-IL23-IL17A axis promotes paraquat-induced acute lung injury by mediating neutrophil infiltration in mice. Sci. Rep. 2017, 4, 597. [Google Scholar] [CrossRef]
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; Metrio, M.D.; Panciroli, C.; Maiuri, L.; Maseri, A.; D’Angelo, A.; Bianchi, M.E.; et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014, 12, 2074–2088. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, H.; Song, J.; Dong, H.; Yao, L.; Liang, Z.; LV, Y.; Zou, F.; Cai, S. Ethyl pyruvate decreases airway neutrophilinfiltration partly through a high mobility group box 1-dependent mechanism in a chemical-induced murine asthma model. Int. Immunopharmacol. 2014, 21, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, G.; Hu, B.; Fang, L.P.; Cao, E.H.; Xin, X.F.; Song, Y.; Shi, Y. Anti-HMGB1 neutralizing antibodyameliorates neutrophilic airway inflammation by suppressingdendritic cell-mediated Th17 polarization. Mediat. Inflamm. 2014, 2014, 257930. [Google Scholar] [CrossRef] [PubMed]
- Tadie, J.M.; Bae, H.B.; Jiang, S.; Park, D.W.; Bell, C.P.; Yang, H.; Pittet, J.F.; Tracey, K.; Thannickal, V.J.; Abraham, E.; et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L342–L349. [Google Scholar] [CrossRef]
- Ma, Y.H.; Ma, T.T.; Wang, C.; Wang, H.; Chang, D.Y.; Chen, M.; Zhao, M.H. High-mobility group box 1 potentiates antineutrophil cytoplasmic antibody-inducing neutrophil extracellular traps formation. Arthritis Res. Ther. 2016, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Y.; Lai, D.; Zhang, P.; Yang, Y.; Li, Y.; Fei, K.; Jiang, G.; Fan, J. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 2018, 22, 597. [Google Scholar] [CrossRef]
- Grégoire, M.; Uhel, F.; Lesouhaitier, M.; Gacouin, A.; Guirriec, M.; Mourcin, F.; Dumontet, E.; Chalin, A.; Samson, M.; Berthelot, L.L.; et al. Impaired efferocytosis and neutrophil extracellular trap clearance by macrophages in ARDS. Eur. Respir. J. 2018, 52, 1702590. [Google Scholar] [CrossRef]
- Kim, S.W.; Lee, H.; Lee, H.K.; Kim, I.D.; Lee, J.K. Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol. Commun. 2019, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.; Maueröder, C.; Hove, L.V.; Catrysse, L.; Vikkula, H.K.; Sze, M.; Maes, B.; Karjosukarso, D.; Martens, L.; Gonçalves, A.; et al. Epithelial HMGB1 Delays Skin Wound Healing and Drives Tumor Initiation by Priming Neutrophils for NET Formation. Cell Rep. 2019, 29, 2689–2701.e4. [Google Scholar] [CrossRef]
- Cicco, S.; Cicco, G.; Racanelli, V.; Vacca, A. Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): Two Potential Targets for COVID-19 Treatment. Mediat. Inflamm. 2020, 2020, 7527953. [Google Scholar] [CrossRef]
- Ren, D.; Sun, R.; Wang, S. Role of inducible nitric oxidesynthase expressed by alveolar macrophages in high mobility group box 1-induced acute lung injury. Inflamm. Res. 2006, 55, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gorasiya, S.; Antoine, D.J.; Sitapara, R.A.; Wu, W.; Sharma, L.; Yang, H.; Ashby, C.R.; Vasudevan, D.; Zur, M.; et al. The compromise of macrophage functions by hyperoxia is attenuated by ethacrynic acid via inhibition of NF-κB-mediated release of high-mobility group box-1. Am. J. Respir. Cell Mol. Biol. 2015, 52, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, Y.; Xiong, P.; Tan, Z.; Gong, F.; Hou, X.; Zheng, F. Effects of mimicked acetylated HMGB1 on macrophages and dendritic cells. Mol. Med. Rep. 2018, 18, 5527–5535. [Google Scholar] [CrossRef]
- Friggeri, A.; Yang, Y.; Banerjee, S.; Park, Y.J.; Liu, G.; Abraham, E. HMGB1 inhibits macrophage activity in efferocytosis through binding to the avb3-integrin. Am. J. Physiol. Cell Physiol. 2010, 299, C1267–C1276. [Google Scholar] [CrossRef]
- Davis, K.; Banerjee, S.; Friggeri, A.; Bell, C.; Abraham, E.; Zerfaoui, M. Poly (ADP-ribosyl) ation of high mobility group box 1 (HMGB1) protein enhances inhibition of efferocytosis. Mol. Med. 2012, 18, 359–369. [Google Scholar] [CrossRef]
- Schaer, C.A.; Laczko, E.; Schoedon, G.; Schaer, D.J.; Vallelian, F. Chloroquine interference with hemoglobin endocytic trafficking suppresses adaptive heme andiron homeostasis in macrophages: The paradox of an antimalarial agent. Oxid. Med. Cell. Longev. 2013, 2013, 870472. [Google Scholar] [CrossRef]
- Gao, T.; Chen, Z.; Chen, H.; Yuan, H.; Wang, Y.; Peng, X.; Wei, C.; Yang, J.; Xu, C. Inhibition of HMGB1 mediates neuroprotection of traumatic brain injury by modulating the microglia/macrophage polarization. Biochem. Biophys. Res. Commun. 2018, 497, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Irie, Y.; Tsubota, M.; Ishikura, H.; Sekiguchi, F.; Terada, Y.; Tsujiuchi, T.; Liu, K.; Nishibori, M.; Kawabata, A. Macrophage-derived HMGB1 as a Pain Mediator in the Early Stage of Acute Pancreatitis in Mice: Targeting RAGE and CXCL12/CXCR4 Axis. J. Neuroimmune Pharmacol. 2017, 12, 693–707. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, R.; Shao, X.; Ji, X.; Lu, H.; Zhou, S.; Zong, G.; Xu, H.; Su, Z. HMGB1 silencing in macrophages prevented their functional skewing and ameliorated EAM development: Nuclear HMGB1 may be a checkpoint molecule of macrophage reprogramming. Int. Immunopharmacol. 2018, 56, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Suarez, J.S.; Minaai, M.; Li, S.; Gaudino, G.; Pass, H.I.; Carbone, M.; Yang, H. HMGB1 as a therapeutic target in disease. J. Cell Physiol. 2020. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- VanPatten, S.; Al-Abed, Y. High Mobility Group Box-1 (HMGb1): Current Wisdom and Advancement as a Potential Drug Target. J. Med. Chem. 2018, 61, 5093–5107. [Google Scholar] [CrossRef]
- Yang, H.; Hreggvidsdottir, H.S.; Palmblad, K.; Wang, H.; Ochani, M.; Li, J.; Lu, B.; Chavan, S.; Rosas-Ballina, M.; Al-Abed, Y.; et al. A critical cysteine is required for HMGB1 binding to toll-like receptor 4 and activation of macrophage cytokine release. Proc. Natl. Acad. Sci. USA 2010, 107, 11942–11947. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Porat, A.; He, M.; Suurmond, J.; Santiago-Schwarz, F.; Andersson, U.; Coleman, T.R.; Volpe, B.T.; Tracey, K.J.; Al-Abed, Y.; et al. C1q and HMGB1 reciprocally regulate human macrophage polarization. Blood 2016, 128, 2218–2228. [Google Scholar] [CrossRef]
- Liu, T.; Xiang, A.; Peng, T.; Doran, A.C.; Tracey, K.J.; Barnes, B.J.; Tabas, I.; Son, M.; Diamond, B. HMGB1-C1q complexes regulate macrophage function by switching between leukotriene and specialized proresolving mediator biosynthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 23254–23263. [Google Scholar] [CrossRef]
- Campana, L.; Bosurgi, L.; Bianchi, M.E.; Manfredi, A.A.; Rovere-Querini, P. Requirement of HMGB1 for stromal cell-derived factor-1/CXCL12-dependent migration of macrophages and dendritic cells. J. Leukoc. Biol. 2009, 86, 609–615. [Google Scholar] [CrossRef]
- Qin, S.; Wang, H.; Yuan, R.; Li, H.; Ochani, M.; Ochani, K.; Rosas-Ballina, M.; Czura, C.J.; Huston, J.M.; Miller, E.; et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 2006, 203, 1637–1642. [Google Scholar] [CrossRef]
- Zhu, X.; Messer, J.S.; Wang, Y.; Lin, F.; Cham, C.M.; Chang, J.; Billiar, T.R.; Lotze, M.T.; Boone, D.L.; Chang, E.B. Cytosolic hmgb1 controls the cellular autophagy/apoptosis checkpoint during inflammation. J. Clin. Invest. 2015, 125, 1098–1110. [Google Scholar] [CrossRef]
- Yang, H.; Antoine, D.J.; Andersson, U.; Tracey, K.J. The many faces of HMGB1: Molecular structure-functional activity in inflammation apoptosis, and chemotaxis. J. Leukoc. Biol. 2013, 93, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, Z.; Chen, J.Z.; Pei, L.G.; Xie, J.; Wei, Z.H.; Kang, L.N.; Wang, L.; Xu, B. High mobility group B-1 (HMGB-1) promotes apoptosis of macrophage-derived foam cells by inducing endoplasmic reticulum stress. Cell Physiol. Biochem. 2018, 48, 1019–1029. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Y.; Wang, J.; Shi, X.; Liu, Q.; Liu, Z.; Li, Y.; Scott, M.J.; Xiao, G.; Li, S.; et al. Macrophage endocytosis of high-mobility group box 1 triggers pyroptosis. Cell Death Differ. 2014, 21, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, S.; Antoine, D.J.; Lundback, P.; Lock, J.G.; Nita, A.F.; Hogstrand, K.; Grandien, A.; Erlandsson-Harris, H.; Andersson, U.; Applequist, S.E. TLR activation regulates damage-associated molecular pattern isoforms released during pyroptosis. EMBO J. 2013, 32, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kim, Y.M. HMGB1: LPS delivery vehicle for caspase-11-mediated pyroptosis. Immunity 2018, 49, 582–584. [Google Scholar] [CrossRef]
- Li, R.; Zou, X.; Huan, H.; Yu, Y.; Zhang, H.; Liu, P.; Pan, S.; Ouyang, Y.; Shang, Y. HMGB1/PI3K/Akt/mTOR signaling participates in the pathological process of acute lung injury by regulating the maturation and function of dendritic cells. Front. Immunol. 2020, 11, 1104. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Huang, L.; Ji, X.; Yao, S.; Abdelaziz, M.H.; Cai, W.; Wang, H.; Cheng, J.; Dineshkumar, K.; Aparna, V.; et al. HMGB1-induced ILC2s activate dendritic cells by producing IL-9 in asthmatic mouse model. Cell Immunol. 2020, 352, 104085. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, Y.M.; Yan, Y.H.; Dong, N.; Sheng, Z.Y. High mobility group box 1 protein suppresses T cell-mediated immunity via CD11c (low) CD45RB (high) dendritic cell differentiation. Cytokine 2011, 54, 205–211. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, F.L.; Wang, H.B.; Dong, N.; Zhu, X.M.; Wu, Y.; Wang, Y.T.; Yao, Y.M. TNF-α mRNA is negatively regulated by microRNA-181a-5p in maturation of dendritic cells induced by high mobility group box-1 protein. Sci. Rep. 2017, 7, 12239. [Google Scholar] [CrossRef]
- Wakabayashi, A.; Shimizu, M.; Shinya, E.; Takahashi, H. HMGB1 released from intestinal epithelia damaged by cholera toxin adjuvant contributes to activation of mucosal dendritic cells and induction of intestinal cytotoxic T lymphocytes and IgA. Cell Death Dis. 2018, 9, 631. [Google Scholar] [CrossRef]
- Saenz, R.; Futalan, D.; Leutenez, L.; Eekhout, F.; Fecteau, J.F.; Sundelius, S.; Sundqvist, S.; Larsson, M.; Hayashi, T.; Minev, B.; et al. TLR4-dependent activation of dendritic cells by an HMGB1-derived peptide adjuvant. J. Transl. Med. 2014, 12, 211. [Google Scholar] [CrossRef]
- Zhang, L.T.; Yao, Y.M.; Yao, F.H.; Huang, L.F.; Dong, N.; Yu, Y.; Sheng, Z.Y. Association between high-mobility group box-1 protein release and immune function of dendritic cells in thermal injury. J. Interferon Cytokine Res. 2010, 30, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Zhu, X.M.; Luo, Y.N.; Wu, Y.; Dong, N.; Tong, Y.L.; Yao, Y.M. Sestrin2 protects dendritic cells against endoplasmic reticulum stress-related apoptosis induced by high mobility group box-1 protein. Cell Death Dis. 2020, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, J.; Li, R.; Zhu, F.; Xu, W.; Zha, G.; He, G.; Cao, H.; Wang, Y.; Yang, J. ATP/P2X7-NLRP3 axis of dendritic cells participates in the regulation of airway inflammation and hyper-responsiveness in asthma by mediating HMGB1 expression and secretion. Exp. Cell Res. 2018, 366, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Su, X.; Huang, G.; Xin, X.F.; Cao, E.H.; Shi, Y.; Song, Y. sRAGE alleviates neutrophilic asthma by blocking HMGB1/RAGE signalling in airway dendritic cells. Sci. Rep. 2017, 7, 14268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, G.; Hu, B.; Qian, G.; Song, Y. Recombinant HMGB1 A box protein inhibits Th17 responses in mice with neutrophilic asthma by suppressing dendritic cell-mediated Th17 polarization. Int. Immunopharmacol. 2015, 24, 110–118. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Khan, M.N.; Shi, L.; Wang, Z.; Zheng, F.; Gong, F.; Fang, M. HMGB1 exacerbates experimental mouse colitis by enhancing innate lymphoid cells 3 inflammatory responses via promoted IL-23 production. Innate Immun. 2016, 22, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liang, Q.; Zhang, Y.; Yang, J.; Li, M.; Wang, K.; Cui, M.; Chen, H.; Fu, Z.F.; Zhao, L.; et al. An optimized HMGB1 expressed by recombinant rabies virus enhances immunogenicity through activation of dendritic cells in mice. Oncotarget 2017, 8, 83539–83554. [Google Scholar] [CrossRef]
- Melki, M.T.; Saïdi, H.; Dufour, A.; Olivo-Marin, J.C.; Gougeon, M.L. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk--a pivotal role of HMGB1. PLoS Pathog. 2010, 15, e1000862. [Google Scholar] [CrossRef]
- Dumitriu, I.E.; Baruah, P.; Valentinis, B.; Voll, R.E.; Herrmann, M.; Nawroth, P.P.; Arnold, B.; Bianchi, M.E.; Manfredi, A.A.; Rovere-Querini, P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J. Immunol. 2005, 174, 7506–7515. [Google Scholar] [CrossRef]
- Zhang, L.T.; Yao, Y.M.; Dong, Y.Q.; Dong, N.; Yu, Y.; Sheng, Z.Y. Relationship between high-mobility group box 1 protein release and T-cell suppression in rats after thermal injury. Shock 2008, 30, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Bandala-Sanchez, E.; Bediaga, N.G.; Goddard-Borger, E.D.; Ngui, K.; Naselli, G.; Stone, N.L.; Neale, A.M.; Pearce, L.A.; Wardak, A.; Czabotar, P.; et al. CD52 glycan binds the proinflammatory B box of HMGB1 to engage the Siglec-10 receptor and suppress human T cell function. Proc. Natl. Acad. Sci. USA 2018, 115, 7783–7788. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, E.; Fasth, A.E.R.; Palmblad, K.; Harris, H.E.; Andersson, U. High mobility group box chromosomal protein 1 acts as a proliferation signal for activated T lymphocytes. Immunobiology 2009, 214, 303–309. [Google Scholar] [CrossRef]
- Wang, R.; Wang, P.; DU, G. HMGB1 promotes myocardial ischemic injury and regulates the proportion of CD4+, CD8+ T cells and Th17 cells in spleen through TLR4. Chin. J. Cell. Mol. Immunol. 2018, 34, 794–799. [Google Scholar]
- Xia, Q.; Duan, L.; Shi, L.; Zheng, F.; Gong, F.; Fang, M. High-mobility group box 1 accelerates early acute allograft rejection via enhancing IL-17+ γδ T-cell response. Transpl. Int. 2014, 27, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Palani, K.; Zhang, S.; Lepsenyi, M.; Hwaiz, R.; Rahman, M.; Syk, I.; Jeppsson, B.; Thorlacius, H. Rho kinase regulates induction of T-cell immune dysfunction in abdominal sepsis. Infect. Immun. 2013, 81, 2499–2506. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Zhu, F.; Li, R.; Liu, B.; Xu, W.; He, G.; Cao, H.; Wang, Y.; Yang, J. HMGB1 regulates T helper 2 and T helper17 cell differentiation both directly and indirectly in asthmatic mice. Mol. Immunol. 2018, 97, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.Y.; Jia, M.; Zhang, H.; Zhu, F.J.; Dong, N.; Feng, Y.W.; Wu, M.; Tong, Y.L.; Yao, Y.M. The potential mechanism of extracellular high mobility group box-1 protein mediated p53 expression in immune dysfunction of T lymphocytes. Oncotarget 2017, 8, 112959–112971. [Google Scholar] [CrossRef]
- Ren, C.; Li, X.H.; Wu, Y.; Dong, N.; Tong, Y.L.; Yao, Y.M. Inhibition of Cerebral High-Mobility Group Box 1 protein attenuates multiple organ damage and improves T cell-mediated immunity in septic rats. Mediat. Inflamm. 2019, 2019, 6197084. [Google Scholar] [CrossRef]
- Wu, Z.S.; Yao, Y.M.; Hong, G.L.; Xu, X.P.; Liu, Y.; Dong, N.; Zheng, J.Y.; Lu, Z.Q.; Zhao, G.J.; Zhu, X.M.; et al. Role of mitofusin-2 in high mobility group box-1 protein-mediated apoptosis of T cells in vitro. Cell Physiol. Biochem. 2014, 33, 769–783. [Google Scholar] [CrossRef]
- Lu, Z.Q.; Tang, L.M.; Zhao, G.J.; Yao, Y.M.; Zhu, X.M.; Dong, N.; Yu, Y. Overactivation of mitogen-activated protein kinase and suppression of mitofusin-2 expression are two independent events in high mobility group box 1 protein-mediated T cell immune dysfunction. J. Interferon Cytokine Res. 2013, 33, 529–541. [Google Scholar] [CrossRef]
- Huang, L.F.; Yao, Y.M.; Meng, H.D.; Zhao, X.D.; Dong, N.; Yu, Y.; Sheng, Z.Y. The effect of high mobility group box-1 protein on immune function of human T lymphocytes in vitro. Chin. Crit. Care Med. 2008, 20, 7–13. [Google Scholar]
- Ruan, X.C.; Darwiche, S.S.; Cai, C.C.; Scott, M.J.; Pape, H.C.; Billiar, T.R. Anti-HMGB1 monoclonal antibody ameliorates immunosuppression after peripheral tissue trauma: Attenuated T-lymphocyte response and increased splenic CD11b (+) Gr-1 (+) myeloid-derived suppressor cells require HMGB1. Mediat. Inflamm. 2015, 2015, 458626. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, Y.M.; Huang, L.F.; Dong, N.; Yu, Y.; Sheng, Z.Y. The potential effect and mechanism of high-mobility group box 1 protein on regulatory T cell-mediated immunosuppression. J. Interferon Cytokine Res. 2011, 31, 249–257. [Google Scholar] [CrossRef]
- Wild, C.A.; Bergmann, C.; Fritz, G.; Schuler, P.; Hoffmann, T.K.; Lotfi, R.; Westendorf, A.; Brandau, S.; Lang, S. HMGB1 conveys immunosuppressive characteristics on regulatory and conventional T cells. Int. Immunol. 2012, 24, 485–494. [Google Scholar] [CrossRef]
- Huang, L.F.; Yao, Y.M.; Zhang, L.T.; Dong, N.; Yu, Y.; Sheng, Z.Y. The effect of high-mobility group box 1 protein on activity of regulatory T cells after thermal injury in rats. Shock 2009, 31, 322–329. [Google Scholar] [CrossRef]
- Zhu, X.M.; Yao, Y.M.; Liang, H.P.; Xu, C.T.; Dong, N.; Yu, Y.; Sheng, Z.Y. High mobility group box-1 protein regulate immunosuppression of regulatory T cells through toll-like receptor 4. Cytokine 2011, 54, 296–304. [Google Scholar] [CrossRef]
- Luo, C.; Liu, H.; Wang, H.; Wang, J. Toll-like receptor 4 signaling in high mobility group box-1 protein 1 mediated the suppression of regulatory T-cells. Med. Sci. Monit. 2017, 23, 300–308. [Google Scholar] [CrossRef][Green Version]
- Zhou, M.; Fang, H.; Du, M.; Li, C.; Tang, R.; Liu, H.; Gao, Z.; Ji, Z.; Ke, B.; Chen, X.L. The modulation of regulatory T cells via HMGB1/PTEN/β-catenin axis in LPS induced acute lung injury. Front. Immunol. 2019, 10, 1612. [Google Scholar] [CrossRef]
- Cheng, L.S.; Li, J.; Liu, Y.; Wang, F.P.; Wang, S.Q.; She, W.M.; Wu, S.D.; Qi, X.L.; Zhou, Y.P.; Jiang, W. HMGB1-induced autophagy: A new pathway to maintain Treg function during chronic hepatitis B virus infection. Clin. Sci. 2017, 131, 381–394. [Google Scholar] [CrossRef]
- Xu, Y.J.; Li, L.; Chen, Y.; Fu, B.; Wu, D.S.; Li, X.L.; Zhao, X.L.; Chen, F.P. Role of HMGB1 in regulation of STAT3 expression in CD4 (+) T cells from patients with aGVHD after allogeneic hematopoietic stem cell transplantation. Clin. Immunol. 2015, 161, 278–283. [Google Scholar] [CrossRef][Green Version]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef]
- Hwang, J.S.; Choi, H.S.; Ham, S.A.; Yoo, T.; Lee, W.J.; Paek, K.S.; Seo, H.G. Deacetylation-mediated interaction of SIRT1-HMGB1 improves survival in amouse model of endotoxemia. Sci. Rep. 2015, 5, 15971. [Google Scholar] [CrossRef]
- Youn, J.H.; Kwak, M.S.; Wu, J.; Kim, E.S.; Ji, Y.; Min, H.J.; Yoo, J.H.; Choi, J.E.; Cho, H.S.; Shin, J.S. Identification of lipopolysaccharide-binding peptide regionswithin HMGB1 and their effects on subclinical endotoxemia in a mouse model. Eur. J. Immunol. 2011, 41, 2753–2762. [Google Scholar] [CrossRef]
- Yang, H.; Ochani, M.; Li, J.; Qiang, X.; Tanovic, M.; Harris, H.E.; Susarla, S.M.; Ulloa, L.; Wang, H.; DiRaimo, R.; et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 2004, 101, 296–301. [Google Scholar] [CrossRef]
- Valdes-Ferrer, S.I.; Papoin, J.; Dancho, M.E.; Olofsson, P.S.; Li, J.; Lipton, J.M.; Avancena, P.; Yang, H.; Zou, Y.R.; Chavan, S.S.; et al. HMGB1 mediates anemia of inflammation in murine sepsis survivors. Mol. Med. 2016, 21, 951–958. [Google Scholar] [CrossRef]
- Valdes-Ferrer, S.I.; Rosas-Ballina, M.; Olofsson, P.S.; Lu, B.; Dancho, M.E.; Ochani, M.; Li, J.H.; Scheinerman, J.A.; Katz, D.A.; Levine, Y.A.; et al. HMGB1 mediates splenomegaly and expansion of splenicCD11b+ Ly-6C (high) inflammatory monocytes in murine sepsis survivors. J. Intern. Med. 2013, 274, 381–390. [Google Scholar] [CrossRef]
- Stevens, N.E.; Chapman, M.J.; Fraser, C.K.; Kuchel, T.R.; Hayball, J.D.; Diener, K.R. Therapeutic targeting of HMGB1 during experimental sepsis modulates the inflammatorycytokine profile to one associated with improved clinical outcomes. Sci. Rep. 2017, 7, 5850. [Google Scholar] [CrossRef]
- Gregoire, M.; Tadie, J.M.; Uhel, A.; Gacouin, K.; Piau, C.; Bone, N.; Tulzo, Y.L.; Abraham, J.W.; Tarte, K.; Zmijewski, J.W. Frontline science HMGB1 induces neutrophil dysfunctionin experimental sepsis and in patients who survive septic shock. J. Leukoc. Biol. 2017, 101, 1281–1287. [Google Scholar] [CrossRef]
- Gil, M.; Kim, Y.K.; Hong, S.B.; Lee, K.J. Naringin decreases TNF-a and HMGB1 release from LPS-stimulated macrophages and improves survival in a CLP-induced sepsis mice. PLoS ONE 2016, 11, e0164186. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Kitagawa, Y.; Ozawa, S.; Saikawa, Y.; Ueda, M.; Ebina, M.; Yamada, S.; Hashimoto, S.; Fukata, S.; Abraham, E.; et al. Anti-high mobility group box chromosomal protein 1 antibodies improve survival ofrats with sepsis. World J. Surg. 2006, 30, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Kawahara, K.; Yone, K.; Hashiguchi, T.; Yamakuchi, M.; Goto, M.; Inoue, K.; Yamada, S.; Ijiri, K.; Matsunaga, S.; et al. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003, 48, 971–981. [Google Scholar] [CrossRef]
- Klint, E.; Grundtman, C.; Engstrom, M.; Catrina, A.I.; Makrygiannakis, D.; Klareskog, L.; Andersson, U.; Ulfgren, A.K. Intraarticular glucocorticoid treatment reduces inflammation in synovial cell infiltrations more efficiently than in synovial blood vessels. Arthritis Rheum. 2005, 52, 3880–3889. [Google Scholar] [CrossRef]
- Goldstein, R.S.; Bruchfeld, A.; Yang, L.; Qureshi, A.R.; Gallowitsch-Puerta, M.; Patel, N.B.; Huston, B.J.; Chavan, S.; Rosas-Ballina, M.; Gregersen, P.K.; et al. Cholinergic anti-inflammatory pathway activityand High Mobility Group box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol. Med. 2007, 13, 210–215. [Google Scholar] [CrossRef]
- Pullerits, R.; Urbonaviciute, V.; Voll, R.E.; Forsblad-D’Elia, H.; Carlsten, H. Serum levels of HMGB1 in postmenopausal patients with rheumatoid arthritis: Associations with proinflammatory cytokines, acute-phase reactants, and clinical disease characteristics. J. Rheumatol. 2011, 38, 1523–1525. [Google Scholar] [CrossRef][Green Version]
- Ostberg, T.; Kawane, K.; Nagata, S.; Yang, H.; Chavan, S.; Klevenvall, L.; Bianchi, M.E.; Harris, H.E.; Andersson, U.; Palmblad, K. Protective targeting of high mobility group box chromosomal protein 1 in a spontaneous arthritis model. Arthritis Rheum. 2010, 62, 2963–2972. [Google Scholar] [CrossRef]
- Schierbeck, H.; Lundback, P.; Palmblad, K.; Klevenvall, L.; Erlandsson-Harris, H.; Andersson, U.; Ottosson, L. Monoclonal anti-HMGB1 (high mobility group boxchromosomal protein 1) antibody protection in two experimental arthritis models. Mol. Med. 2011, 17, 1039–1044. [Google Scholar] [CrossRef]
- Pullerits, R.; Jonsson, I.M.; Verdrengh, M.; Bokarewa, M.; Andersson, U.; Erlandsson-Harris, H.; Tarkowski, A. High mobility group box chromosomal protein1 a DNA binding cytokine, induces arthritis. Arthritis Rheum. 2003, 48, 1693–1700. [Google Scholar] [CrossRef]
- Hamada, T.; Torikai, M.; Kuwazuru, A.; Tanaka, M.; Horai, N.; Fukuda, T.; Yamada, S.; Nagayama, S.; Hashiguchi, K.; Sunahara, N.; et al. Extracellular high mobility group boxchromosomal protein 1 is a coupling factor for hypoxia and inflammation in arthritis. Arthritis Rheum. 2008, 58, 2675–2685. [Google Scholar] [CrossRef]
- Lundback, P.; Stridh, P.; Klevenvall, L.; Jenkins, R.E.; Fischer, M.; Sundberg, E.; Andersson, U.; Antoine, D.J.; Harris, H.E. Characterization of the inflammatory properties of actively released HMGB1 in juvenile idiopathic arthritis. Antioxid. Redox Signal. 2016, 24, 605–619. [Google Scholar] [CrossRef]
- Kokkola, R.; Li, J.; Sundberg, E.; Aveberger, A.C.; Palmblad, K.; Yang, H.; Tracey, K.J.; Andersson, U.; Harris, H.E. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003, 48, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Wouwer, M.V.D.; Plaisance, S.; Vriese, A.D.; Waelkens, E.; Collen, D.; Persson, J.; Daha, M.R.; Conway, E.M. The lectin-like domain of thrombomodulin interfereswith complement activation and protects against arthritis. J. Thromb. Haemost. 2006, 4, 1813–1824. [Google Scholar] [CrossRef]
- Zetterstrom, C.K.; Jiang, W.; Wahamaa, H.; Ostberg, T.; Aveberger, A.C.; Schierbeck, H.; Lotze, M.T.; Andersson, U.; Pisetsky, D.S.; Harris, H.E. Pivotal advance: Inhibition of HMGB1 nuclear translocation as a mechanism for the anti-rheumatic effects of gold sodium thiomalate. J. Leukoc. Biol. 2008, 83, 31–38. [Google Scholar] [CrossRef]
- Schaper, F.; Westra, J.; Bijl, M. Recent developments in the role of high-mobility group box 1 in systemic lupus erythematosus. Mol. Med. 2014, 20, 72–79. [Google Scholar] [CrossRef]
- Ma, C.Y.; Jiao, Y.L.; Zhang, J.; Yang, Q.R.; Zhang, Z.F.; Shen, Y.J.; Chen, Z.J.; Zhao, Y.R. Elevated plasma level of HMGB1 is associated with disease activity andcombined alterations with IFN-alpha and TNF-alpha in systemic lupus erythematosus. Rheumatol. Int. 2012, 32, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Xu, L.; Chen, X.; Xu, W.; Yin, Z.; Gao, X.; Xiong, S. Autoantibody induction by DNA-containing immune complexes requires HMGB1 with the TLR2/microRNA-155 pathway. J. Immunol. 2013, 190, 5411–5422. [Google Scholar] [CrossRef]
- Garcia-Romo, G.S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra20. [Google Scholar] [CrossRef] [PubMed]
- Abdulahad, D.A.; Westra, J.; Bijzet, J.; Limburg, P.C.; Kallenberg, C.G.; Bijl, M. High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation todisease characteristics in systemic lupus erythematosus. Arthritis. Res. Ther. 2011, 13, R71. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Jia, S.; Yao, P.; Yang, Q.; Zhang, Y. High-mobility group box 1 inhibition alleviates lupus-like disease in BXSB mice. Scand. J. Immunol. 2014, 79, 333–337. [Google Scholar] [CrossRef]
- Whittall-García, L.P.; Torres-Ruiz, J.; Zentella-Dehesa, A.; Tapia-Rodríguez, M.; Alcocer-Varela, J.; Mendez-Huerta, N.; Gómez-Martín, D. Neutrophil extracellular traps are a source of extracellular HMGB1 in lupus nephritis: Associations with clinical and histopathological features. Lupus 2019, 28, 1549–1557. [Google Scholar] [CrossRef]
- Li, X.Y.; Yue, Y.; Zhu, Y.Y.; Xiong, S.D. Extracellular, but not intracellular HMGB1, facilitates self-DNA induced macrophage activation via promoting DNA accumulation in endosomes and contributes to the pathogenesis of lupus nephritis. Mol. Immunol. 2015, 65, 177–188. [Google Scholar] [CrossRef]
- Evankovich, J.; Cho, S.W.; Zhang, R.; Cardinal, J.; Dhupar, R.; Zhang, L.; Klune, J.R.; Zlotnicki, J.; Billiar, T.; Tsung, A. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histonede acetylase activity. J. Biol. Chem. 2010, 285, 398888–398897. [Google Scholar] [CrossRef] [PubMed]
- Lea, J.D.; Clarke, J.I.; McGuire, N.; Antoine, D.J. Redox-dependent HMGB1 isoformsas pivotal coordinators of drug-induced liver injury: Mechanistic biomarkers andtherapeutic targets. Antioxid. Redox Signal. 2016, 24, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Minsart, C.; Liefferinckx, C.; Lemmers, A.; Dressen, C.; Quertinmont, E.; Leclercq, I.; Devière, J.; Moreau, R.; Gustot, T. New insights in acetaminophen toxicity: HMGB1 contributes by itself to amplify hepatocyte necrosis in vitro through the TLR4-TRIF-RIPK3 axis. Sci. Rep. 2020, 10, 5557. [Google Scholar] [CrossRef]
- Dragomir, A.C.; Laskin, J.D.; Laskin, D.L. Macrophage activation by factors released from acetaminophen-injured hepatocytes: Potential role of HMGB1. Toxicol. Appl. Pharmacol. 2011, 253, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Lundback, P.; Lea, J.D.; Sowinska, A.; Ottosson, L.; Furst, C.M.; Steen, J.; Aulin, C.; Clarke, J.I.; Kipar, A.; Klevenvall, L.; et al. A novel high mobility group box 1 neutralizing chimeric antibody attenuates drug-induced liver injury and post injury inflammation in mice. Hepatology 2016, 64, 1699–1710. [Google Scholar] [CrossRef]
- Morbini, P.; Villa, C.; Campo, I.; Zorzetto, M.; Inghilleri, S.; Luisetti, M. The receptor for advanced glycation endproducts and its ligands: A new inflammatory pathway in lung disease? Mod. Pathol. 2006, 19, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Ma, L.; Zeng, J.; Mo, B.; Wang, C.; Huang, J.; Sun, Y.; Yu, Y.; Liu, S. High mobility group box 1: A novel mediator of Th2-type response- induced airway inflammation of acuteallergic asthma. J. Thorac. Dis. 2015, 7, 1732–1741. [Google Scholar]
- Di Candia, L.; Gomez, E.; Venereau, E.; Chachi, L.; Kaur, D.; Bianchi, M.E.; Challiss, R.A.J.; Brightling, C.E.; Saunders, R.M. HMGB1 is upregulated in the airways in asthma and potentiates airway smooth muscle contraction via TLR 4. J. Allergy Clin. Immunol. 2017, 140, 584–587.e8. [Google Scholar] [CrossRef]
- Hinks, T.S.C.; Brown, T.; Lau, L.C.K.; Rupani, H.; Barber, C.; Elliott, S.; Ward, J.A.; Ono, J.; Ohta, S.; Izuhara, K.; et al. Multidimensional endotyping in patients with severeasthma reveals inflammatory heterogeneity in matrixmetallo proteinases and chitinase 3-like protein 1. J. Allergy Clin. Immunol. 2016, 138, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Cuppari, C.; Manti, S.; Chirico, V.; Caruso, R.; Salpietro, V.; Giacchi, V.; Lagana, F.; Arrigo, T.; Salpietro, C.; Leonardi, S. Sputum high mobility group box-1 inasthmatic children: A noninvasive sensitive biomarker reflecting disease status. Ann. Allergy Asthma Immunol. 2015, 115, 103–107. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Lee, Y.; Paik, M.J.; Yee, S.T. Inhibitions of HMGB1 and TLR4 alleviate DINP-induced asthma in mice. Toxicol. Res. 2019, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, N.; Wang, T.; Dai, B.; Shang, Y. Vitamin D reduces inflammatory response in asthmatic mice through HMGB1/TLR4/NF-kB signaling pathway. Mol. Med. Rep. 2017, 17, 2915–2920. [Google Scholar]
- Dong, W.W.; Liu, Y.J.; Lv, Z.; Mao, Y.F.; Wang, Y.W.; Zhu, X.Y.; Jiang, L. Lung endothelial barrier protection by resveratrol involves inhibition of HMGB1 release and HMGB1-induced mitochondrial oxidative damage via a Nrf2-dependent mechanism. Free Radic. Biol. Med. 2015, 88, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute lung injury: A clinical and molecular review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Jia, H.; Yamaguchi, Y.; Lu, P.; Good, M.; Egan, C.; Ozolek, J.; Zhu, X.; Billiar, T.R.; Hackam, D.J. Intestinal epithelial TLR-4 activation is required for the development of acute lung injury after trauma/hemorrhagic shock via the release of HMGB1 from the gut. J. Immunol. 2015, 194, 4931–4939. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Iwasaka, H.; Togo, K.; Noguchi, T. A neutrophil elastase inhibitor, sivelestat, reduces lung injury following endotoxin-induced shock in rats by inhibiting HMGB1. Inflammation 2008, 31, 227–234. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.S.; Strassheim, D.; Douglas, I.; Diaz del Valle, F.; Asehnoune, K.; Mitra, S.; Kwak, S.H.; Yamada, S.; Maruyama, I.; et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 288, L958–L965. [Google Scholar] [CrossRef]
- Liu, X.; Yu, D.; Chen, M.; Sun, T.; Li, G.; Huang, W.; Nie, H.; Wang, C.; Zhang, Y.; Gong, Q.; et al. Hesperidin ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting HMGB1 release. Int. Immunopharmacol. 2015, 25, 370–376. [Google Scholar] [CrossRef]
- Patel, V.S.; Sitapara, R.A.; Gore, A.; Phan, B.; Sharma, L.; Sampat, V.; Li, J.H.; Yang, H.; Chavan, S.S.; Wang, H.; et al. High mobility group box-1 mediates hyperoxia-induced impairment of Pseudomonas aeruginosa clearance and inflammatory lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2013, 48, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Wang, B.; Wang, G.; Xu, J. Blocking HMGB1 signal pathway protects early radiation-induced lung injury. Int. J. Clin. Exp. Pathol. 2015, 8, 4815–4822. [Google Scholar] [PubMed]
- Entezari, M.; Javdan, M.; Antoine, D.J.; Morrow, D.M.; Sitapara, R.A.; Patel, V.; Wang, M.; Sharma, L.; Gorasiya, S.; Zur, M.; et al. Inhibition of extracellular HMGB1 attenuates hyperoxia-induced inflammatory acute lung injury. Redox Biol. 2014, 2, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Matsuda, T.; Hashimoto, S.; Amaya, F.; Kitamura, Y.; Tanaka, M.; Kobayashi, A.; Maruyama, I.; Yamada, S.; Hasegawa, N.; et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am. J. Respir. Crit. Care Med. 2004, 170, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, L.; Ochani, M.; Yang, H.; Tanovic, M.; Halperin, D.; Yang, R.; Czura, C.J.; Fink, M.P.; Tracey, K.J. Ethyl pyruvate prevents lethality in mice with established lethalsepsis and systemic inflammation. Proc. Natl. Acad. Sci. USA 2002, 99, 12351–12356. [Google Scholar] [CrossRef]
- Kohno, T.; Anzai, T.; Naito, K.; Miyasho, T.; Okamoto, M.; Yokota, H.; Yamada, S.; Maekawa, Y.; Takahashi, T.; Yoshikawa, T.; et al. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc. Res. 2009, 81, 565–573. [Google Scholar] [CrossRef]
- Kitahara, T.; Takeishi, Y.; Harada, M.; Niizeki, T.; Suzuki, S.; Sasaki, T.; Ishino, M.; Bilim, O.; Nakajima, O.; Kubota, I. High mobility group box 1 restores cardiac function after myocardial infarction intransgenic mice. Cardiovasc. Res. 2008, 80, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Han, Q.F.; Gao, L.; Sun, Y.; Tang, Z.W.; Wang, M.; Wang, W.; Yao, H.C. HMGB1 protects the heart against ischemia-reperfusion injury via PI3K/AkT pathway-mediated upregulation of VEGF expression. Front. Physiol. 2020, 10, 1595. [Google Scholar] [CrossRef]
- Zou, J.Y.; Crews, F.T. Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS ONE 2014, 9, e87915. [Google Scholar] [CrossRef]
- Chavan, S.S.; Huerta, P.T.; Robbiati, S.; Valdes-Ferrer, S.I.; Ochani, M.; Dancho, M.; Frankfurt, M.; Volpe, B.T.; Tracey, K.J.; Diamond, B. HMGB1 mediates cognitive impairment in sepsis survivors. Mol. Med. 2012, 18, 930–937. [Google Scholar] [CrossRef]
- Wang, F.; Ji, S.; Wang, M.; Liu, L.; Li, Q.; Jiang, F.; Cen, J.; Ji, B. HMGB1 promoted P-glycoprotein at the blood-brain barrier in MCAO rats via TLR4/NF-κB signaling pathway. Eur. J. Pharmacol. 2020, 880, 173189. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Z.; Lu, L.; Liu, J.; Sun, J.; Wu, X.; Dong, J. Icariin and icaritin ameliorated hippocampus neuroinflammation via inhibiting HMGB1-related pro-inflammatory signals in lipopolysaccharide-induced inflammation model in C57BL/6 J mice. Int. Immunopharmacol. 2019, 68, 95–105. [Google Scholar] [CrossRef]
- Hayakawa, K.; Qiu, J.; Lo, E.H. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann. N. Y. Acad. Sci. 2010, 1207, 50–57. [Google Scholar] [CrossRef]
- Levy, R.M.; Mollen, K.P.; Prince, J.M.; Kaczorowski, D.J.; Vallabhaneni, R.; Liu, S.; Tracey, K.J.; Lotze, M.T.; Hackam, D.J.; Fink, M.P.; et al. Systemic inflammation and remote organ injury following trauma require HMGB1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1538–R1544. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J.; Matsumoto, N.; Ogura, H.; Muroya, T.; Kuwagata, Y.; Nakagawa, J.; Yamakawa, K.; Hosotsubo, H.; Imamura, Y.; Shimazu, T. Systemic involvement of high-mobility group box 1 protein and therapeutic effect of anti-high-mobility group box 1 protein antibody in a rat model of crush injury. Shock 2012, 37, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Tenhunen, J.; Tonnessen, T.I. HMGB1 and histones play a significant role in inducing systemic inflammation and multiple organ dysfunctions in severe acute pancreatitis. Int. J. Inflam. 2017, 2017, 1817564. [Google Scholar] [CrossRef]
- Kang, R.; Tang, D.; Schapiro, N.E.; Loux, T.; Livesey, K.M.; Billiar, T.R.; Wang, H.; Van Houten, B.; Lotze, M.T.; Zeh, H.J. The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene 2014, 33, 567–577. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zha, J.; Wang, Y.; Liu, W.; Yang, X.; Yu, P. Tissue damage-associated danger ‘‘signals’’ influence T-cell responses that promote the progression of preneoplasia to cancer. Cancer Res. 2013, 73, 629–639. [Google Scholar] [CrossRef]
- Kusume, A.; Sasahira, T.; Luo, Y.; Isobe, M.; Nakagawa, N.; Tatsumoto, N.; Fujii, K.; Ohmori, H.; Kuniyasu, H. Suppression of dendritic cells by HMGB1 is associated with lymph node metastasis of human colon cancer. Pathobiology 2009, 76, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Haruna, M.; Hirata, M.; Iwahori, K.; Kanazawa, T.; Yamamoto, Y.; Goto, K.; Kawashima, A.; Morimoto-Okazawa, A.; Funaki, S.; Shintani, Y.; et al. Docetaxel upregulates HMGB1 levels in non-small cell lung cancer. Biol. Pharm. Bull. 2020, 43, 399–403. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Y.; Huang, M.; Yao, Y.-m. The Effect and Regulatory Mechanism of High Mobility Group Box-1 Protein on Immune Cells in Inflammatory Diseases. Cells 2021, 10, 1044. https://doi.org/10.3390/cells10051044
Ge Y, Huang M, Yao Y-m. The Effect and Regulatory Mechanism of High Mobility Group Box-1 Protein on Immune Cells in Inflammatory Diseases. Cells. 2021; 10(5):1044. https://doi.org/10.3390/cells10051044
Chicago/Turabian StyleGe, Yun, Man Huang, and Yong-ming Yao. 2021. "The Effect and Regulatory Mechanism of High Mobility Group Box-1 Protein on Immune Cells in Inflammatory Diseases" Cells 10, no. 5: 1044. https://doi.org/10.3390/cells10051044
APA StyleGe, Y., Huang, M., & Yao, Y.-m. (2021). The Effect and Regulatory Mechanism of High Mobility Group Box-1 Protein on Immune Cells in Inflammatory Diseases. Cells, 10(5), 1044. https://doi.org/10.3390/cells10051044






