Microtubule-Associated Protein ATIP3, an Emerging Target for Personalized Medicine in Breast Cancer
Abstract
:1. ATIP3 and the MTUS1 Gene, a Historical Point of View
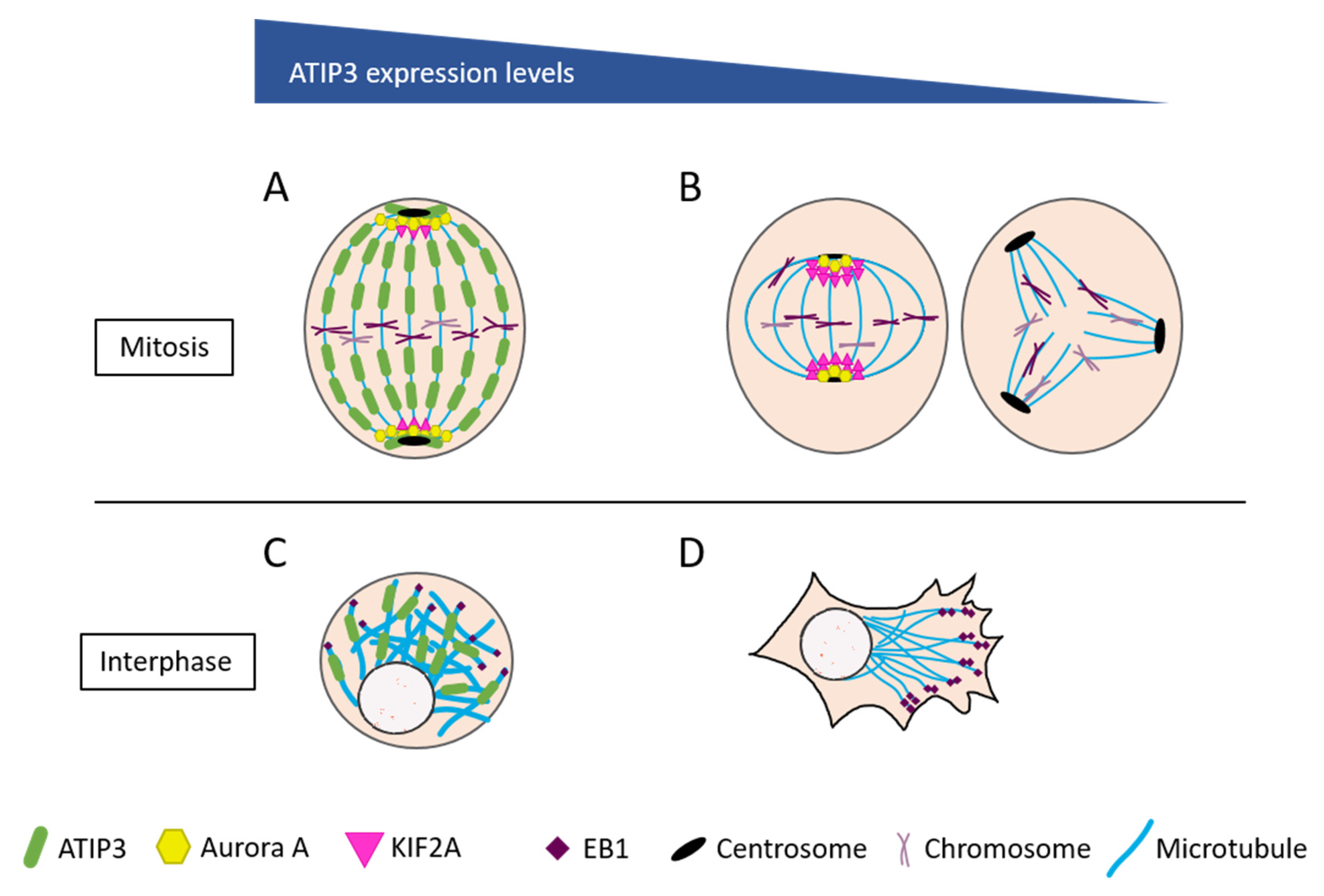
2. ATIP3 Is a Microtubule-Associated Protein
3. Cancer-Related Molecular Mechanisms Controlled by ATIP3
4. ATIP3 Is a Prognostic Biomarker in Breast Cancer
5. ATIP3 Is a Predictive Biomarker of Taxane-Based Chemotherapy in Breast Cancer
6. New ATIP3-Associated Emerging Targets for Breast Cancer Therapy?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seibold, S.; Rudroff, C.; Weber, M.; Galle, J.; Wanner, C.; Marx, M. Identification of a New Tumor Suppressor Gene Located at Chromosome 8p21.3-22. FASEB J. 2003, 17, 1180–1182. [Google Scholar] [CrossRef]
- Kinjo, T.; Isomura, M.; Iwamasa, T.; Nakamura, Y. Molecular Cloning and Characterization of Two Novel Genes on Chromosome 8p21.3. J. Hum. Genet. 2000, 45, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.C.; Radford, D.M.; Holt, M.S.; Helms, C.; Goate, A.; Brandt, W.; Parik, M.; Phillips, N.J.; DeSchryver, K.; Schuh, M.E.; et al. Sequence-Ready Contig for the 1.4-CM Ductal Carcinoma in Situ Loss of Heterozygosity Region on Chromosome 8p22–P23. Genomics 1999, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nouet, S.; Amzallag, N.; Li, J.-M.; Louis, S.; Seitz, I.; Cui, T.-X.; Alleaume, A.-M.; Di Benedetto, M.; Boden, C.; Masson, M.; et al. Trans-Inactivation of Receptor Tyrosine Kinases by Novel Angiotensin II AT2 Receptor-Interacting Protein, ATIP. J. Biol. Chem. 2004, 279, 28989–28997. [Google Scholar] [CrossRef] [Green Version]
- Nouet, S.; Nahmias, C. Signal Transduction from the Angiotensin II AT2 Receptor. Trends Endocrinol. Metab. 2000, 11, 1–6. [Google Scholar] [CrossRef]
- Fujita, T.; Mogi, M.; Min, L.-J.; Iwanami, J.; Tsukuda, K.; Sakata, A.; Okayama, H.; Iwai, M.; Nahmias, C.; Higaki, J.; et al. Attenuation of Cuff-Induced Neointimal Formation by Overexpression of Angiotensin II Type 2 Receptor-Interacting Protein 1. Hypertension 2009, 53, 688–693. [Google Scholar] [CrossRef]
- Kukida, M.; Mogi, M.; Ohshima, K.; Nakaoka, H.; Iwanami, J.; Kanno, H.; Tsukuda, K.; Chisaka, T.; Min, L.-J.; Wang, X.-L.; et al. Angiotensin II Type 2 Receptor Inhibits Vascular Intimal Proliferation With Activation of PPARγ. AJHYPE 2016, 29, 727–736. [Google Scholar] [CrossRef] [Green Version]
- Min, L.-J.; Mogi, M.; Iwanami, J.; Jing, F.; Tsukuda, K.; Ohshima, K.; Horiuchi, M. Angiotensin II Type 2 Receptor-Interacting Protein Prevents Vascular Senescence. J. Am. Soc. Hypertens 2012, 6, 179–184. [Google Scholar] [CrossRef]
- Soda, K.; Nakada, Y.; Iwanari, H.; Hamakubo, T. AT2 Receptor Interacting Protein 1 (ATIP1) Mediates COX-2 Induction by an AT2 Receptor Agonist in Endothelial Cells. Biochem. Biophys. Rep. 2020, 24, 100850. [Google Scholar] [CrossRef]
- Li, J.-M.; Mogi, M.; Tsukuda, K.; Tomochika, H.; Iwanami, J.; Min, L.-J.; Nahmias, C.; Iwai, M.; Horiuchi, M. Angiotensin II-Induced Neural Differentiation via Angiotensin II Type 2 (AT2) Receptor-MMS2 Cascade Involving Interaction between AT2 Receptor-Interacting Protein and Src Homology 2 Domain-Containing Protein-Tyrosine Phosphatase 1. Mol. Endocrinol. 2007, 21, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Jing, F.; Mogi, M.; Min, L.-J.; Ohshima, K.; Nakaoka, H.; Tsukuda, K.; Wang, X.; Iwanami, J.; Horiuchi, M. Effect of Angiotensin II Type 2 Receptor-Interacting Protein on Adipose Tissue Function via Modulation of Macrophage Polarization. PLoS ONE 2013, 8, e60067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinemund, J.; Seidel, K.; Steckelings, U.M.; Zaade, D.; Klare, S.; Rompe, F.; Katerbaum, M.; Schacherl, J.; Li, Y.; Menk, M.; et al. Poly(ADP-Ribose) Polymerase-1 (PARP-1) Transcriptionally Regulates Angiotensin AT2 Receptor (AT2R) and AT2R Binding Protein (ATBP) Genes. Biochem. Pharmacol. 2009, 77, 1795–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wruck, C.J.; Funke-Kaiser, H.; Pufe, T.; Kusserow, H.; Menk, M.; Schefe, J.H.; Kruse, M.L.; Stoll, M.; Unger, T. Regulation of Transport of the Angiotensin AT2 Receptor by a Novel Membrane-Associated Golgi Protein. ATVB 2005, 25, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Benedetto, M.; Bièche, I.; Deshayes, F.; Vacher, S.; Nouet, S.; Collura, V.; Seitz, I.; Louis, S.; Pineau, P.; Amsellem-Ouazana, D.; et al. Structural Organization and Expression of Human MTUS1, a Candidate 8p22 Tumor Suppressor Gene Encoding a Family of Angiotensin II AT2 Receptor-Interacting Proteins, ATIP. Gene 2006, 380, 127–136. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Ye, H.; Zhou, X. Genomic Characterization of the Human Mitochondrial Tumor Suppressor Gene 1 (MTUS1): 5’ Cloning and Preliminary Analysis of the Multiple Gene Promoters. BMC Res. Notes 2009, 2, 109. [Google Scholar] [CrossRef] [Green Version]
- Krezel, M.A.; Rezmann, L.A.; Varghayee, N.; Pete, J.; Frauman, A.G.; Louis, S.N.S. Gene Sequencing and Tissue Expression of Unknown Isoforms of an Angiotensin II Type 2 Receptor Interacting Protein, ATIP, in the Rat. Biosci. Biotechnol. Biochem. 2011, 75, 414–418. [Google Scholar] [CrossRef] [Green Version]
- Zuern, C.; Krenacs, L.; Starke, S.; Heimrich, J.; Palmetshofer, A.; Holtmann, B.; Sendtner, M.; Fischer, T.; Galle, J.; Wanner, C.; et al. Microtubule Associated Tumor Suppressor 1 Deficient Mice Develop Spontaneous Heart Hypertrophy and SLE-like Lymphoproliferative Disease. Int. J. Oncol. 2012, 40, 1079–1088. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Wang, C.; Jin, Y.; Wang, Y.; Wang, A.; Zhou, X. P53 Regulates the Expression of Human Angiotensin II AT(2) Receptor Interacting Protein (ATIP1) Gene. Oncol. Lett. 2011, 2, 919–922. [Google Scholar] [CrossRef]
- Rodrigues-Ferreira, S.; Di Tommaso, A.; Dimitrov, A.; Cazaubon, S.; Gruel, N.; Colasson, H.; Nicolas, A.; Chaverot, N.; Molinié, V.; Reyal, F.; et al. 8p22 MTUS1 Gene Product ATIP3 Is a Novel Anti-Mitotic Protein Underexpressed in Invasive Breast Carcinoma of Poor Prognosis. PLoS ONE 2009, 4, e7239. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues-Ferreira, S.; Nahmias, C. An ATIPical Family of Angiotensin II AT2 Receptor-Interacting Proteins. Trends Endocrinol. Metab. 2010, 21, 684–690. [Google Scholar] [CrossRef]
- Molina, A.; Velot, L.; Ghouinem, L.; Abdelkarim, M.; Bouchet, B.P.; Luissint, A.-C.; Bouhlel, I.; Morel, M.; Sapharikas, E.; Di Tommaso, A.; et al. ATIP3, a Novel Prognostic Marker of Breast Cancer Patient Survival, Limits Cancer Cell Migration and Slows Metastatic Progression by Regulating Microtubule Dynamics. Cancer Res. 2013, 73, 2905–2915. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues-Ferreira, S.; Nehlig, A.; Monchecourt, C.; Nasr, S.; Fuhrmann, L.; Lacroix-Triki, M.; Garberis, I.; Scott, V.; Delaloge, S.; Pistilli, B.; et al. Combinatorial Expression of Microtubule-Associated EB1 and ATIP3 Biomarkers Improves Breast Cancer Prognosis. Breast Cancer Res. Treat. 2019, 173, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Liu, D.; Deng, M.; Liu, J.; Wang, J.; Zhang, L.; Liu, Q.; Zhang, T.; Chen, Y.; Jin, G. Identification of Differently Expressed Genes with Specific SNP Loci for Breast Cancer by the Integration of SNP and Gene Expression Profiling Analyses. Pathol. Oncol. Res. 2015, 21, 469–475. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, J.-X.; Zhu, Y.-P.; Zhou, L.-Y.; Shu, Q.-A.; Chen, L.-W. Reduced Expression of MTUS1 MRNA Is Correlated with Poor Prognosis in Bladder Cancer. Oncol. Lett. 2012, 4, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Rogler, A.; Hoja, S.; Giedl, J.; Ekici, A.B.; Wach, S.; Taubert, H.; Goebell, P.J.; Wullich, B.; Stöckle, M.; Lehmann, J.; et al. Loss of MTUS1/ATIP Expression Is Associated with Adverse Outcome in Advanced Bladder Carcinomas: Data from a Retrospective Study. BMC Cancer 2014, 14, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuern, C.; Heimrich, J.; Kaufmann, R.; Richter, K.K.; Settmacher, U.; Wanner, C.; Galle, J.; Seibold, S. Down-Regulation of MTUS1 in Human Colon Tumors. Oncol. Rep. 2010, 23, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Melcher, R.; Hartmann, E.; Zopf, W.; Herterich, S.; Wilke, P.; Müller, L.; Rosler, E.; Kudlich, T.; Al-Taie, O.; Rosenwald, A.; et al. LOH and Copy Neutral LOH (CnLOH) Act as Alternative Mechanism in Sporadic Colorectal Cancers with Chromosomal and Microsatellite Instability. Carcinogenesis 2011, 32, 636–642. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, O.; Kara, M.; Yumrutas, O.; Bozgeyik, E.; Bozgeyik, I.; Celik, O.I. MTUS1 and Its Targeting MiRNAs in Colorectal Carcinoma: Significant Associations. Tumour Biol. 2016, 37, 6637–6645. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yang, C.; Qiu, L.; Feng, X.; Sun, K.; Deng, H. Transcriptional Information Underlying the Generation of CSCs and the Construction of a Nine-MRNA Signature to Improve Prognosis Prediction in Colorectal Cancer. Cancer Biol. Ther. 2020, 21, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.; Kim, Y.; Kim, H.; Bang, S.; Jee, S.; Park, S.; Shin, S.-J.; Jang, K. Loss of MTUS1 Expression Is Associated With Poor Prognosis in Patients With Gallbladder Carcinoma. In Vivo 2020, 34, 125–132. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Yu, T.; Dong, Z.; Tang, L.; Sun, X. Loss of MTUS1 in Gastric Cancer Promotes Tumor Growth and Metastasis. Neoplasma 2014, 61, 128–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Li, X.; Fu, L.; Zhang, N.; Yang, J.; Cai, J. LncRNA LIFR‑AS1 Inhibits Gastric Carcinoma Cell Proliferation, Migration and Invasion by Sponging MiR‑4698. Mol. Med. Rep. 2021, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Parbin, S.; Pradhan, N.; Das, L.; Saha, P.; Deb, M.; Sengupta, D.; Patra, S.K. DNA Methylation Regulates Microtubule-Associated Tumor Suppressor 1 in Human Non-Small Cell Lung Carcinoma. Exp. Cell Res. 2019, 374, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhang, N.; Cai, Y.; Li, S.; Zheng, C.; Jin, Y.; Yu, T.; Wang, A.; Zhou, X. Down-Regulation of Tumor Suppressor MTUS1/ATIP Is Associated with Enhanced Proliferation, Poor Differentiation and Poor Prognosis in Oral Tongue Squamous Cell Carcinoma. Mol. Oncol. 2012, 6, 73–80. [Google Scholar] [CrossRef]
- Ribeiro, I.P.; Marques, F.; Caramelo, F.; Ferrão, J.; Prazeres, H.; Julião, M.J.; Rifi, W.; Savola, S.; de Melo, J.B.; Baptista, I.P.; et al. Genetic Imbalances Detected by Multiplex Ligation-Dependent Probe Amplification in a Cohort of Patients with Oral Squamous Cell Carcinoma—The First Step towards Clinical Personalized Medicine. Tumor Biol. 2014. [Google Scholar] [CrossRef]
- Zhao, T.; Ding, X.; Chang, B.; Zhou, X.; Wang, A. MTUS1/ATIP3a down-Regulation Is Associated with Enhanced Migration, Invasion and Poor Prognosis in Salivary Adenoid Cystic Carcinoma. BMC Cancer 2015, 15, 203. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; He, Q.; Liu, Z.; Ding, X.; Zhou, X.; Wang, A. Angiotensin II Type 2 Receptor-Interacting Protein 3a Suppresses Proliferation, Migration and Invasion in Tongue Squamous Cell Carcinoma via the Extracellular Signal-Regulated Kinase-Snai2 Pathway. Oncol. Lett. 2016, 11, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Mahjabeen, I.; Kayani, M.A. Loss of Mitochondrial Tumor Suppressor Genes Expression Is Associated with Unfavorable Clinical Outcome in Head and Neck Squamous Cell Carcinoma: Data from Retrospective Study. PLoS ONE 2016, 11, e0146948. [Google Scholar] [CrossRef] [Green Version]
- Bozgeyik, I.; Yumrutas, O.; Bozgeyik, E. MTUS1, a Gene Encoding Angiotensin-II Type 2 (AT2) Receptor-Interacting Proteins, in Health and Disease, with Special Emphasis on Its Role in Carcinogenesis. Gene 2017, 626, 54–63. [Google Scholar] [CrossRef]
- Lv, Q.; Dong, F.; Zhou, Y.; Cai, Z.; Wang, G. RNA-Binding Protein SORBS2 Suppresses Clear Cell Renal Cell Carcinoma Metastasis by Enhancing MTUS1 MRNA Stability. Cell Death Dis. 2020, 11, 1056. [Google Scholar] [CrossRef]
- Sim, J.; Wi, Y.C.; Park, H.Y.; Park, S.Y.; Yoon, Y.E.; Bang, S.; Kim, Y.; Jang, K.; Paik, S.S.; Shin, S.-J. Clinicopathological Significance of MTUS1 Expression in Patients With Renal Cell Carcinoma. Anticancer Res. 2020, 40, 2961–2967. [Google Scholar] [CrossRef]
- Luo, H.; Ma, C. Identification of Prognostic Genes in Uveal Melanoma Microenvironment. PLoS ONE 2020, 15, e0242263. [Google Scholar] [CrossRef]
- Louis, S.N.S.; Chow, L.T.C.; Varghayee, N.; Rezmann, L.A.; Frauman, A.G.; Louis, W.J. The Expression of MTUS1/ATIP and Its Major Isoforms, ATIP1 and ATIP3, in Human Prostate Cancer. Cancers 2011, 3, 3824–3837. [Google Scholar] [CrossRef] [Green Version]
- Guimond, M.-O.; Battista, M.-C.; Nikjouitavabi, F.; Carmel, M.; Barres, V.; Doueik, A.A.; Fazli, L.; Gleave, M.; Sabbagh, R.; Gallo-Payet, N. Expression and Role of the Angiotensin II AT2 Receptor in Human Prostate Tissue: In Search of a New Therapeutic Option for Prostate Cancer. Prostate 2013, 73, 1057–1068. [Google Scholar] [CrossRef]
- Mitchison, T.; Kirschner, M. Dynamic Instability of Microtubule Growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Akhmanova, A.; Steinmetz, M.O. Control of Microtubule Organization and Dynamics: Two Ends in the Limelight. Nat. Rev. Mol. Cell Biol. 2015, 16, 711–726. [Google Scholar] [CrossRef]
- Guesdon, A.; Bazile, F.; Buey, R.M.; Mohan, R.; Monier, S.; García, R.R.; Angevin, M.; Heichette, C.; Wieneke, R.; Tampé, R.; et al. EB1 Interacts with Outwardly Curved and Straight Regions of the Microtubule Lattice. Nat. Cell Biol. 2016, 18, 1102–1108. [Google Scholar] [CrossRef]
- Maurer, S.P.; Cade, N.I.; Bohner, G.; Gustafsson, N.; Boutant, E.; Surrey, T. EB1 Accelerates Two Conformational Transitions Important for Microtubule Maturation and Dynamics. Curr. Biol. 2014, 24, 372–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velot, L.; Molina, A.; Rodrigues-Ferreira, S.; Nehlig, A.; Bouchet, B.P.; Morel, M.; Leconte, L.; Serre, L.; Arnal, I.; Braguer, D.; et al. Negative Regulation of EB1 Turnover at Microtubule plus Ends by Interaction with Microtubule-Associated Protein ATIP3. Oncotarget 2015, 6, 43557–43570. [Google Scholar] [CrossRef] [Green Version]
- Nehlig, A.; Molina, A.; Rodrigues-Ferreira, S.; Honoré, S.; Nahmias, C. Regulation of End-Binding Protein EB1 in the Control of Microtubule Dynamics. Cell Mol. Life Sci. 2017, 74, 2381–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehlig, A.; Seiler, C.; Steblyanko, Y.; Dingli, F.; Arras, G.; Loew, D.; Welburn, J.; Prigent, C.; Barisic, M.; Nahmias, C. Reciprocal Regulation of Aurora Kinase A and ATIP3 in the Control of Metaphase Spindle Length. Cell Mol. Life Sci. 2021, 78, 1765–1779. [Google Scholar] [CrossRef]
- Ganem, N.J.; Compton, D.A. Functional Roles of Poleward Microtubule Flux during Mitosis. Cell Cycle 2006, 5, 481–485. [Google Scholar] [CrossRef]
- Cirak, Y.; Furuncuoglu, Y.; Yapicier, O.; Aksu, A.; Cubukcu, E. Aurora A Overexpression in Breast Cancer Patients Induces Taxane Resistance and Results in Worse Prognosis. J. BUON 2015, 20, 1414–1419. [Google Scholar]
- Yan, M.; Wang, C.; He, B.; Yang, M.; Tong, M.; Long, Z.; Liu, B.; Peng, F.; Xu, L.; Zhang, Y.; et al. Aurora-A Kinase: A Potent Oncogene and Target for Cancer Therapy. Med. Res. Rev. 2016, 36, 1036–1079. [Google Scholar] [CrossRef]
- Ohi, R.; Coughlin, M.L.; Lane, W.S.; Mitchison, T.J. An Inner Centromere Protein That Stimulates the Microtubule Depolymerizing Activity of a KinI Kinesin. Dev. Cell 2003, 5, 309–321. [Google Scholar] [CrossRef] [Green Version]
- Knowlton, A.L.; Vorozhko, V.V.; Lan, W.; Gorbsky, G.J.; Stukenberg, P.T. ICIS and Aurora B Coregulate the Microtubule Depolymerase Kif2a. Curr. Biol. 2009, 19, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Ping, H.; Guo, L.; Xi, J.; Wang, D. Angiotensin II Type 2 Receptor-Interacting Protein 3a Inhibits Ovarian Carcinoma Metastasis via the Extracellular HMGA2-Mediated ERK/EMT Pathway. Tumour Biol. 2017, 39, 1010428317713389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues-Ferreira, S.; Nehlig, A.; Moindjie, H.; Monchecourt, C.; Seiler, C.; Marangoni, E.; Chateau-Joubert, S.; Dujaric, M.-E.; Servant, N.; Asselain, B.; et al. Improving Breast Cancer Sensitivity to Paclitaxel by Increasing Aneuploidy. Proc. Natl. Acad. Sci. USA 2019, 116, 23691–23697. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene Expression Patterns of Breast Carcinomas Distinguish Tumor Subclasses with Clinical Implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Ou, L.; Xiang, T.-Y.; Hao, X.-Y.; Wang, D.-Z.; Zeng, Q. Reduced Long Non-Coding RNA PTENP1 Contributed to Proliferation and Invasion via MiR-19b/MTUS1 Axis in Patients with Cervical Cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4132–4144. [Google Scholar] [CrossRef]
- Kara, M.; Kaplan, M.; Bozgeyik, I.; Ozcan, O.; Celik, O.I.; Bozgeyik, E.; Yumrutas, O. MTUS1 Tumor Suppressor and Its MiRNA Regulators in Fibroadenoma and Breast Cancer. Gene 2016, 587, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Liu, S.; Zhang, X.; Chen, G.; Liang, H.; Yu, M.; Liao, Z.; Zhou, Y.; Zhang, C.-Y.; Wang, T.; et al. Oncogenic MiR-19a and MiR-19b Co-Regulate Tumor Suppressor MTUS1 to Promote Cell Proliferation and Migration in Lung Cancer. Protein Cell 2017, 8, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Lv, D.-B.; Zhang, J.-Y.; Gao, K.; Yu, Z.-H.; Sheng, W.-C.; Yang, G.; Gao, Y.-Z. MicroRNA-765 Targets MTUS1 to Promote the Progression of Osteosarcoma via Mediating ERK/EMT Pathway. Eur. Rev. Med Pharmacol. Sci. 2019, 23, 4618–4628. [Google Scholar] [CrossRef]
- Di Benedetto, M.; Pineau, P.; Nouet, S.; Berhouet, S.; Seitz, I.; Louis, S.; Dejean, A.; Couraud, P.O.; Strosberg, A.D.; Stoppa-Lyonnet, D.; et al. Mutation Analysis of the 8p22 Candidate Tumor Suppressor Gene ATIP/MTUS1 in Hepatocellular Carcinoma. Mol. Cell Endocrinol. 2006, 252, 207–215. [Google Scholar] [CrossRef]
- Frank, B.; Bermejo, J.L.; Hemminki, K.; Sutter, C.; Wappenschmidt, B.; Meindl, A.; Kiechle-Bahat, M.; Bugert, P.; Schmutzler, R.K.; Bartram, C.R.; et al. Copy Number Variant in the Candidate Tumor Suppressor Gene MTUS1 and Familial Breast Cancer Risk. Carcinogenesis 2007, 28, 1442–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Liu, F.; Sun, L.; Liu, M.; Li, D.; Su, D.; Zhu, Z.; Dong, J.-T.; Fu, L.; Zhou, J. Oncogenic Function of Microtubule End-Binding Protein 1 in Breast Cancer: EB1 in Breast Cancer. J. Pathol. 2010, 220, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Ferreira, S.; Molina, A.; Nahmias, C. Microtubule-Associated Tumor Suppressors as Prognostic Biomarkers in Breast Cancer. Breast Cancer Res. Treat. 2020, 179, 267–273. [Google Scholar] [CrossRef]
- Wang, J.; Ma, S.; Ma, R.; Qu, X.; Liu, W.; Lv, C.; Zhao, S.; Gong, Y. KIF2A Silencing Inhibits the Proliferation and Migration of Breast Cancer Cells and Correlates with Unfavorable Prognosis in Breast Cancer. BMC Cancer 2014, 14, 461. [Google Scholar] [CrossRef] [Green Version]
- Li, T.-F.; Zeng, H.-J.; Shan, Z.; Ye, R.-Y.; Cheang, T.-Y.; Zhang, Y.-J.; Lu, S.-H.; Zhang, Q.; Shao, N.; Lin, Y. Overexpression of Kinesin Superfamily Members as Prognostic Biomarkers of Breast Cancer. Cancer Cell Int. 2020, 20, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadler, Y.; Camp, R.L.; Schwartz, C.; Rimm, D.L.; Kluger, H.M.; Kluger, Y. Expression of Aurora A (but Not Aurora B) Is Predictive of Survival in Breast Cancer. Clin. Cancer Res. 2008, 14, 4455–4462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, H.R.; Dawson, S.-J.; Blows, F.M.; Provenzano, E.; Pharoah, P.D.; Caldas, C. Aurora Kinase A Outperforms Ki67 as a Prognostic Marker in ER-Positive Breast Cancer. Br. J. Cancer 2012, 106, 1798–1806. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/Paclitaxel Kills Cancer Cells. MBoC 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Rodrigues-Ferreira, S.; Moindjie, H.; Haykal, M.M.; Nahmias, C. Predicting and Overcoming Taxane Chemoresistance. Trends Mol. Med. 2021, 27, 138–151. [Google Scholar] [CrossRef]
- Masuda, H.; Baggerly, K.A.; Wang, Y.; Zhang, Y.; Gonzalez-Angulo, A.M.; Meric-Bernstam, F.; Valero, V.; Lehmann, B.D.; Pietenpol, J.A.; Hortobagyi, G.N.; et al. Differential Response to Neoadjuvant Chemotherapy among 7 Triple-Negative Breast Cancer Molecular Subtypes. Clin. Cancer Res. 2013, 19, 5533–5540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues-Ferreira, S.; Nehlig, A.; Kacem, M.; Nahmias, C. ATIP3 Deficiency Facilitates Intracellular Accumulation of Paclitaxel to Reduce Cancer Cell Migration and Lymph Node Metastasis in Breast Cancer Patients. Sci. Rep. 2020, 10, 13217. [Google Scholar] [CrossRef]
- Rai, A.; Liu, T.; Glauser, S.; Katrukha, E.A.; Estévez-Gallego, J.; Rodríguez-García, R.; Fang, W.-S.; Díaz, J.F.; Steinmetz, M.O.; Altmann, K.-H.; et al. Taxanes Convert Regions of Perturbed Microtubule Growth into Rescue Sites. Nat. Mater. 2020, 19, 355–365. [Google Scholar] [CrossRef]
- Rodrigues-Ferreira, S.; Nahmias, C. From Tumorigenesis to Cell Death: The Aneuploidy Paradox. Mol. Cell. Oncol. 2020, 7, 1709390. [Google Scholar] [CrossRef]
- Nebbioso, A.; Tambaro, F.P.; Dell’Aversana, C.; Altucci, L. Cancer Epigenetics: Moving Forward. PLoS Genet. 2018, 14, e1007362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda Furtado, C.L.; Dos Santos Luciano, M.C.; Silva Santos, R.D.; Furtado, G.P.; Moraes, M.O.; Pessoa, C. Epidrugs: Targeting Epigenetic Marks in Cancer Treatment. Epigenetics 2019, 14, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of MicroRNAs in Vivo with “Antagomirs”. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef]
- Forterre, A.; Komuro, H.; Aminova, S.; Harada, M. A Comprehensive Review of Cancer MicroRNA Therapeutic Delivery Strategies. Cancers 2020, 12, 1852. [Google Scholar] [CrossRef] [PubMed]
| Cancer Type | MTUS1 Isoform | Detection Method | Expression Level | Prognosis * | Reference |
|---|---|---|---|---|---|
| Bladder | N.D. | IHC | Underexpressed | OS | [25] |
| N.D. | RT-qPCR | Underexpressed | DFS | [24] | |
| Breast | N.D. | Microarray | Underexpressed | N.D. | [23] |
| ATIP3 | Microarray | OS/MFS | [21] | ||
| ATIP3 | Microarray/IHC | N.D. | [19] | ||
| ATIP3 | Microarray/IHC | OS | [22] | ||
| Colorectal | N.D. | RNA-seq | Underexpressed | OS | [29] |
| N.D. | IHC | N.D. | [27] | ||
| N.D. | RT-qPCR/WB | N.D. | [26] | ||
| N.D. | RT-qPCR | N.D. | [28] | ||
| Gallbladder | N.D. | Microarray/IHC | Underexpressed | DFS | [30] |
| Gastric | N.D. | RT-qPCR | Underexpressed | N.D. | [32] |
| Non small cell lung | N.D. | Microarray | Underexpressed | OS | [33] |
| Oral | N.D. | RT-qPCR | Underexpressed | N.D. | [38] |
| ATIP3 | IHC | OS | [36] | ||
| N.D. | Microarray/IHC | OS | [34] | ||
| Prostate | ATIP1/ATIP3 | RT-qPCR/IHC | Overexpressed | N.D. | [43] |
| Renal | N.D. | IHC | Underexpressed | N.D. | [41] |
| Uveal melanoma | N.D. | Microarray | Underexpressed | MFS | [42] |
| Human ATIP3 | Reference | Xenopus ICIS | Reference | |
|---|---|---|---|---|
| Localization | Microtubule Mitotic Spindle Centrosome | [19] | Centromere Centrosome | [55] |
| Interacts with | EB1 KIF2A DDA3 | [49,51] | XKCM1 KIF2A AURKB INCENP TD-60 | [55,56] |
| Signaling | ERK AURKA KIF2C CDC25B CDK1 | [4,32,51,57] | ||
| Function | Microtubule dynamics Spindle size Centrosome number Proliferation Migration Polarization EMT | [19,21,32,49,51,57,58] | Microtubule dynamics Mitotic spindle integrity | [55,56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haykal, M.M.; Rodrigues-Ferreira, S.; Nahmias, C. Microtubule-Associated Protein ATIP3, an Emerging Target for Personalized Medicine in Breast Cancer. Cells 2021, 10, 1080. https://doi.org/10.3390/cells10051080
Haykal MM, Rodrigues-Ferreira S, Nahmias C. Microtubule-Associated Protein ATIP3, an Emerging Target for Personalized Medicine in Breast Cancer. Cells. 2021; 10(5):1080. https://doi.org/10.3390/cells10051080
Chicago/Turabian StyleHaykal, Maria M., Sylvie Rodrigues-Ferreira, and Clara Nahmias. 2021. "Microtubule-Associated Protein ATIP3, an Emerging Target for Personalized Medicine in Breast Cancer" Cells 10, no. 5: 1080. https://doi.org/10.3390/cells10051080






