Imaging the Renal Microcirculation in Cell Therapy
Abstract
:1. Introduction
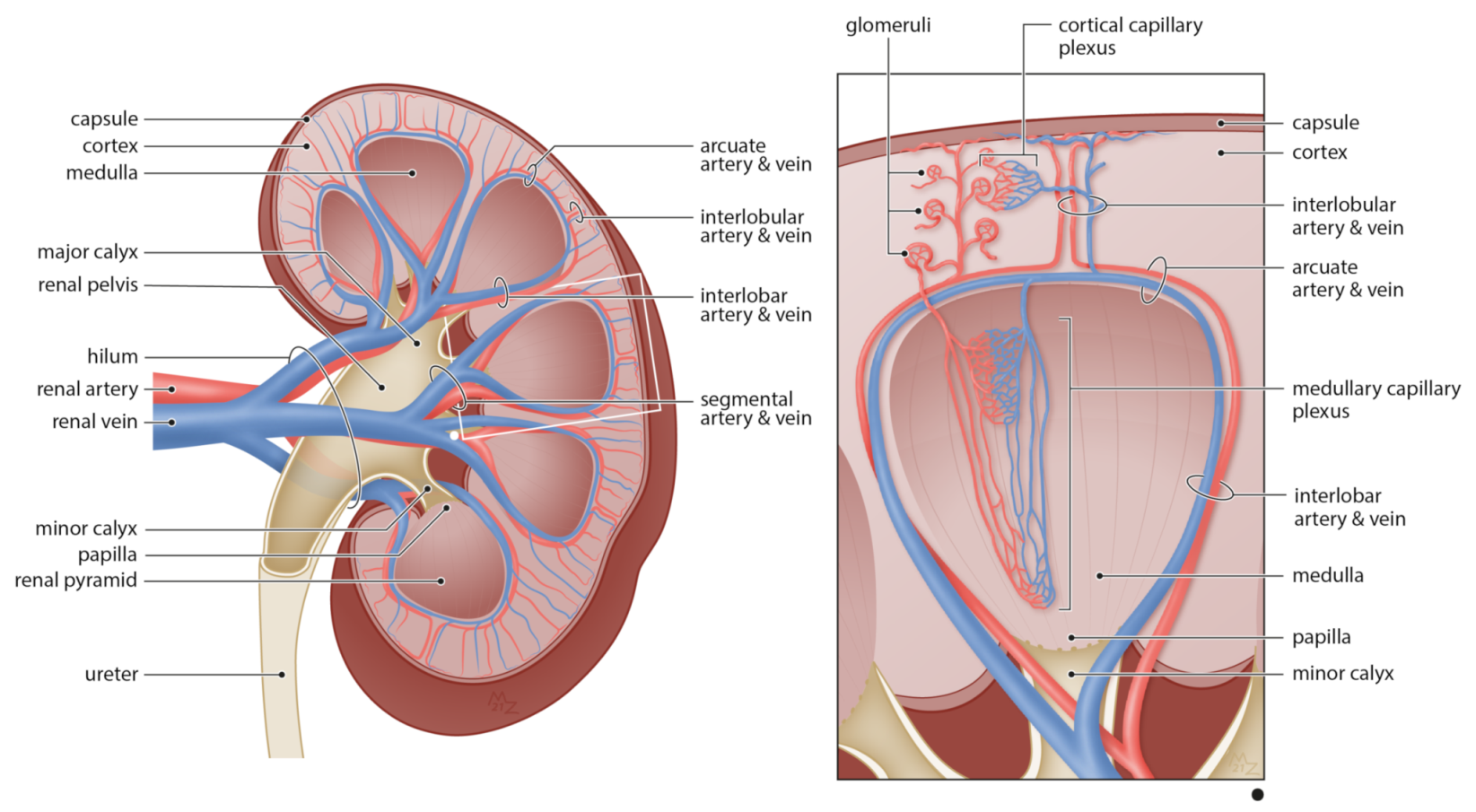
2. The Renal Vasculature
2.1. The Renal Blood Circulation
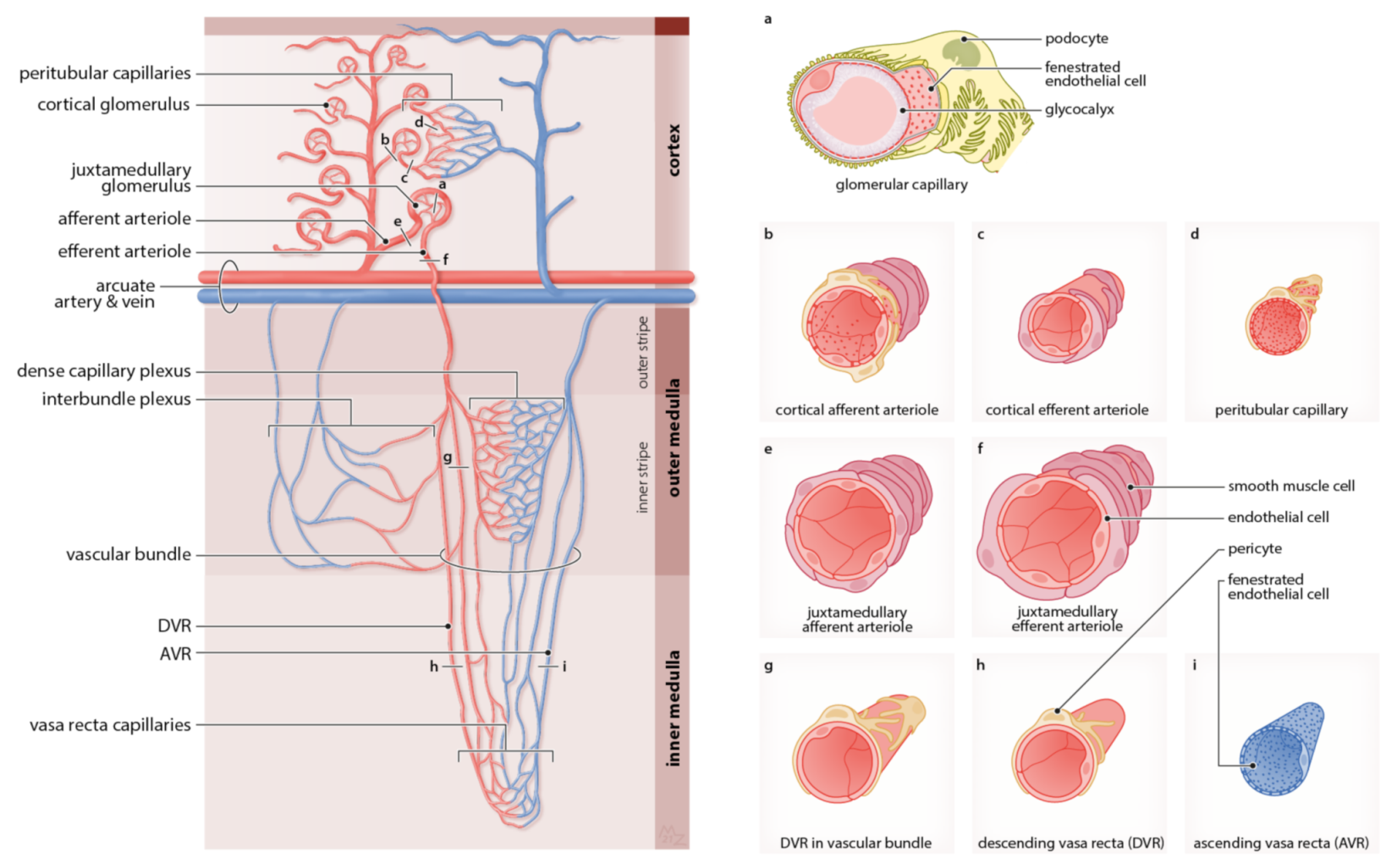
2.2. The Capillary Networks of the Kidney
3. Renal Microvascular Malfunctions
3.1. Endothelial Dysfunction
3.2. Pericyte Involvement in Renal Malfunction
3.3. Endothelial Cell-Pericyte Signaling Interactions
3.4. Pericytes as Precursor of Myofibroblasts
4. Vascular Imaging Modalities
4.1. Ex Vivo
4.1.1. Microcomputed Tomography (Micro-CT)
4.1.2. Light Sheet Fluorescence Microscopy (LSFM)
4.2. In Vivo
4.2.1. Multiphoton Microscopy (MPM)
4.2.2. Computed Tomography (CT)
4.2.3. Magnetic Resonance Imaging (MRI)
4.2.4. Ultrasound
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ang | Angiopoietin |
| AKI | Acute kidney injury |
| AP | Activator protein |
| ATF | Activating transcription factor |
| ATP | Adenosine triphosphate |
| AVR | Ascending vasa recta |
| BOLD | Blood-oxygen-level dependent contrast |
| CD | Cluster of differentiation |
| CKD | Chronic kidney disease |
| CLARITY | Clear lipid-exchanged acrylamide-hybridized rigid imaging |
| CT | Computed tomography |
| CTGF | Connective tissue growth factor |
| CUBIC | Clear, unobstructed brain/body imaging cocktails and computational analysis |
| DISCO | Three-dimensional imaging of solvent-cleared organs |
| DVR | Descending vasa recta |
| ECM | Extracellular matrix |
| ECi | Ethyl cinnamate |
| ESRD | End-stage renal disease |
| Fox | Forkhead box |
| fUS | Functional ultrafast ultrasound |
| GFR | Glomerular filtration rate |
| Gli | Glioma-associated oncogene homologue |
| HIF | Hypoxia-inducible factor |
| HVM | Hand-held vital microscopy |
| hPSC | human pluripotent stem cell |
| IRI | Ischemia-reperfusion injury |
| LSFM | Light sheet fluorescence microscopy |
| MBF | Medullary blood flow |
| micro-CT | Microcomputed tomography |
| MPM | Multiphoton microscopy |
| MRA | Magnetic resonance angiography |
| MRI | Magnetic resonance imaging |
| MSCs | Mesenchymal stromal cells |
| NG | Neuron-glial antigen |
| NO | Nitric oxide |
| OTC | Optical tissue clearing |
| PDGFR | Platelet-derived growth factor receptor |
| PKD | Polycystic kidney disease |
| PTA | Phosphotungstic acid |
| PW | Pulsed-wave |
| RBF | Renal blood flow |
| S1P | Sphingosine-1-phosphate |
| SCL | Stem cell leukemia |
| SD | Standard deviation |
| SHANEL | Small-micelle-mediated human organ efficient clearing and labeling |
| SMA | Smooth muscle actin |
| SMCs | Smooth muscle cells |
| TGF | Transforming growth factor |
| ULM | Ultrasound localization microscopy |
| VEGF | Vascular endothelial growth factor |
| VesSAP | Vessel segmentation & analysis pipeline |
References
- Chade, A.R. Renal vascular structure and rarefaction. Compr. Physiol. 2013, 3, 817–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chade, A.R. Small Vessels, Big Role: Renal Microcirculation and Progression of Renal Injury. Hypertension 2017, 69, 551–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehling, J.; Bábícková, J.; Gremse, F.; Klinkhammer, B.M.; Baetke, S.; Knuechel, R.; Kiessling, F.; Floege, J.; Lammers, T.; Boor, P. Quantitative micro-computed tomography imaging of vascular dysfunction in progressive kidney diseases. J. Am. Soc. Nephrol. 2016, 27, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. Physiol. Behav. 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Foiret, J.; Zhang, H.; Ilovitsh, T.; Mahakian, L.; Tam, S.; Ferrara, K.W. Ultrasound localization microscopy to image and assess microvasculature in a rat kidney. Sci. Rep. 2017, 7, 13662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Yu, J.; Rush, B.M.; Stocker, S.D.; Tan, R.J.; Kim, K. Ultrasound super-resolution imaging provides a noninvasive assessment of renal microvasculature changes during mouse acute kidney injury. Kidney Int. 2020, 98, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Hueper, K.; Gutberlet, M.; Rong, S.; Hartung, D.; Mengel, M.; Lu, X.; Haller, H.; Wacker, F.; Meier, M.; Gueler, F. Acute Kidney Injury: Arterial Spin Labeling to Monitor Renal Perfusion Impairment in Mice—Comparison with Histopathologic Results and Renal Function. Radiology 2014, 270, 117–124. [Google Scholar] [CrossRef]
- Levy, B.I.; Schiffrin, E.L.; Mourad, J.J.; Agostini, D.; Vicaut, E.; Safar, M.E.; Struijker-Boudier, H.A. Impaired tissue perfusion a pathology common to hypertension, obesity, and diabetes mellitus. Circulation 2008, 118, 968–976. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Molema, G.; Aird, W.C. Vascular Heterogeneity in the Kidney. Semin. Nephrol. 2012, 32, 145–155. [Google Scholar] [CrossRef]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef] [Green Version]
- Attwell, D.; Mishra, A.; Hall, C.N.; O’Farrell, F.M.; Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab. 2016, 36, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.G.; Eppel, G.A.; Anderson, W.P.; Denton, K.M. Mechanisms underlying the diffential control of blood flow in the renal medulla and cortex. J. Hypertens. 2004, 22, 1439–1451. [Google Scholar] [CrossRef]
- Pallone, T.L.; Silldorff, E.P.; Turner, M.R. Intrarenal blood flow: Microvascular anatomy and the regulation of medullary perfusion. Clin. Exp. Pharmacol. Physiol. 1998, 25, 383–392. [Google Scholar] [CrossRef]
- Pallone, T.L.; Edwards, A.; Mattson, D.L. Renal medullary circulation. Compr. Physiol. 2012, 2, 97–140. [Google Scholar] [CrossRef] [PubMed]
- Guerci, P.; Ergin, B.; Ince, C. The macro- and microcirculation of the kidney. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 315–329. [Google Scholar] [CrossRef]
- Shaw, I.; Rider, S.; Mullins, J.; Hughes, J.; Péault, B. Pericytes in the renal vasculature: Roles in health and disease. Nat. Rev. Nephrol. 2018, 14, 521–534. [Google Scholar] [CrossRef]
- Rosivall, L.; Peti-Peterdi, J.J. Heterogeneity of the afferent arteriole—Correlations between morphology and function. Nephrol. Dial. Transplant. 2006, 21, 2703–2707. [Google Scholar] [CrossRef] [Green Version]
- Stefanska, A.; Kenyon, C.; Christian, H.C.; Buckley, C.; Shaw, I.; Mullins, J.J.; Péault, B. Human kidney pericytes produce renin. Kidney Int. 2016, 90, 1251–1261. [Google Scholar] [CrossRef] [Green Version]
- Schlondorff, D.O. Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney Int. 2008, 74, 860–866. [Google Scholar] [CrossRef] [Green Version]
- Pallone, T.L.; Zhang, Z.; Rhinehart, K. Physiology of the renal medullary microcirculation. Am. J. Physiol. Ren. Physiol. 2003, 284, F253–F266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallone, T.L. Complex vascular bundles, thick ascending limbs, and aquaporins: Wringing out the outer medulla. Am. J. Physiol. Ren. Physiol. 2014, 306, 505–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerhackl, B.L.; Robertson, C.R.; Jamison, R.L. The medullary microcirculation. Kidney Int. 1987, 31, 641–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumas, S.J.; Meta, E.; Borri, M.; Luo, Y.; Li, X.; Rabelink, T.J.; Carmeliet, P. Phenotypic diversity and metabolic. Nat. Rev. Nephrol. 2021, 1–24. [Google Scholar] [CrossRef]
- Dumas, S.J.; García-Caballero, M.; Carmeliet, P. Metabolic Signatures of Distinct Endothelial Phenotypes. Trends Endocrinol. Metab. 2020, 31, 580–595. [Google Scholar] [CrossRef]
- Jourde-Chiche, N.; Fakhouri, F.; Dou, L.; Bellien, J.; Burtey, S.; Frimat, M.; Jarrot, P.A.; Kaplanski, G.; Le Quintrec, M.; Pernin, V.; et al. Endothelium structure and function in kidney health and disease. Nat. Rev. Nephrol. 2019, 15, 87–108. [Google Scholar] [CrossRef]
- Long, D.A.; Norman, J.T.; Fine, L.G. Restoring the renal microvasculature to treat chronic kidney disease. Nat. Rev. Nephrol. 2012, 8, 244–250. [Google Scholar] [CrossRef]
- Choi, Y.J.; Chakraborty, S.; Nguyen, V.; Nguyen, C.; Kim, B.K.; Shim, S.I.; Suki, W.N.; Truong, L.D. Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: Altered expression of vascular endothelial growth factor. Hum. Pathol. 2000, 31, 1491–1497. [Google Scholar] [CrossRef]
- Ishii, Y.; Sawada, T.; Kubota, K.; Fuchinoue, S.; Teraoka, S.; Shimizu, A. Injury and progressive loss of peritubular capillaries in the development of chronic allograft nephropathy. Kidney Int. 2005, 67, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Serón, D.; Alexopoulos, E.; Raftery, M.J.; Hartley, B.; Cameron, J.S. Number of interstitial capillary cross-sections assessed by monoclonal antibodies: Relation to interstitial damage. Nephrol. Dial. Transplant. 1990, 5, 889–893. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [Green Version]
- Pannabecker, T.L. Renal vascular pericytes: Long overlooked and poorly understood, but clearly important, and what about those regulatory pathways? Am. J. Physiol. Ren. Physiol. 2018, 314, F67–F69. [Google Scholar] [CrossRef]
- Sequeira-Lopez, M.L.S.; Lin, E.E.; Li, M.; Hu, Y.; Sigmund, C.D.; Gomez, R.A. The earliest metanephric arteriolar progenitors and their role in kidney vascular development. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R138–R149. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Li, M.; Göthert, J.R.; Gomez, R.A.; Sequeira-Lopez, M.L.S. Hemovascular progenitors in the kidney require sphingosine-1-phosphate receptor 1 for vascular development. J. Am. Soc. Nephrol. 2016, 27, 1984–1995. [Google Scholar] [CrossRef] [Green Version]
- Kramann, R.; Humphreys, B.D. Kidney pericytes: Roles in regeneration and fibrosis. Semin. Nephrol. 2014, 34, 374–383. [Google Scholar] [CrossRef] [Green Version]
- Sequeira Lopez, M.L.S.; Gomez, R.A. Development of the renal arterioles. J. Am. Soc. Nephrol. 2011, 22, 2156–2165. [Google Scholar] [CrossRef] [Green Version]
- Rider, S.A.; Mullins, L.J.; Verdon, R.F.; Macrae, C.A.; Mullins, J.J. Renin expression in developing zebrafish is associated with angiogenesis and requires the notch pathway and endothelium. Am. J. Physiol. Ren. Physiol. 2015, 309, F531–F539. [Google Scholar] [CrossRef] [Green Version]
- Stefanska, A.; Eng, D.; Kaverina, N.; Pippin, J.W.; Gross, K.W.; Duffield, J.S.; Shankland, S.J. Cells of renin lineage express hypoxia inducible factor 2α following experimental ureteral obstruction. BMC Nephrol. 2016, 17, 5. [Google Scholar] [CrossRef] [Green Version]
- Gaengel, K.; Genové, G.; Armulik, A.; Betsholtz, C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 630–638. [Google Scholar] [CrossRef] [Green Version]
- Fiedler, U.; Augustin, H.G. Angiopoietins: A link between angiogenesis and inflammation. Trends Immunol. 2006, 27, 552–558. [Google Scholar] [CrossRef]
- Hammes, H.P.; Lin, J.; Renner, O.; Shani, M.; Lundqvist, A.; Betsholtz, C.; Brownlee, M.; Deutsch, U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 2002, 51, 3107–3112. [Google Scholar] [CrossRef] [Green Version]
- Lindblom, P.; Gerhardt, H.; Liebner, S.; Abramsson, A.; Enge, M.; Hellström, M.; Bäckström, G.; Fredriksson, S.; Landegren, U.; Nyström, H.C.; et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003, 17, 1835–1840. [Google Scholar] [CrossRef] [Green Version]
- Pfister, F.; Feng, Y.; Hagen, F.V.; Hoffmann, S.; Molema, G.; Hillebrands, J.L.; Shani, M.; Deutsch, U.; Hammes, H.P. Pericyte migration: A novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes 2008, 57, 2495–2502. [Google Scholar] [CrossRef] [Green Version]
- Khairoun, M.; van der Pol, P.; de Vries, D.K.; Lievers, E.; Schlagwein, N.; de Boer, H.C.; Bajema, I.M.; Rotmans, J.I.; van Zonneveld, A.J.; Rabelink, T.J.; et al. Renal ischemia-reperfusion induces a dysbalance of angiopoietins, accompanied by proliferation of pericytes and fibrosis. Am. J. Physiol. Ren. Physiol. 2013, 305, F901–F910. [Google Scholar] [CrossRef] [Green Version]
- De Vries, D.K.; Khairoun, M.; Lindeman, J.H.; Bajema, I.M.; de Heer, E.; Roest, M.; van Zonneveld, A.J.; van Kooten, C.; Rabelink, T.J.; Schaapherder, A.F.; et al. Renal ischemia-reperfusion induces release of angiopoietin-2 from human grafts of living and deceased donors. Transplantation 2013, 96, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Khairoun, M.; de Koning, E.J.; van den Berg, B.M.; Lievers, E.; de Boer, H.C.; Schaapherder, A.F.; Mallat, M.J.; Rotmans, J.I.; van der Boog, P.J.; Van Zonneveld, A.J.; et al. Microvascular damage in type 1 diabetic patients is reversed in the first year after simultaneous pancreas-kidney transplantation. Am. J. Transplant. 2013, 13, 1272–1281. [Google Scholar] [CrossRef]
- Sato, T.N.; Tozawa, Y.; Deutsch, U.; Wolburg-Buchholz, K.; Fujiwara, Y.; Gendron-Maguire, M.; Gridley, T.; Wolburg, H.; Risau, W.; Qin, Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 1995, 376, 70–74. [Google Scholar] [CrossRef]
- Goumans, M.J.; Lebrin, F.; Valdimarsdottir, G. Controlling the angiogenic switch: A balance between two distinct TGF-β receptor signaling pathways. Trends Cardiovasc. Med. 2003, 13, 301–307. [Google Scholar] [CrossRef]
- Benjamin, L.E.; Hemo, I.; Keshet, E. A plasticity for blood vessel remodelling is defined by pericyte coverage of the performed endothelial network and is regulated by PDGF-B and VEGF: Comment. Development 1998, 125, 1591–1598. [Google Scholar] [CrossRef]
- Chae, S.S.; Paik, J.H.; Allende, M.L.; Proia, R.L.; Hla, T. Regulation of limb development by the sphingosine 1-phosphate receptor S1p1/EDG-1 occurs via the hypoxia/VEGF axis. Dev. Biol. 2004, 268, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Humphreys, B.D.; Lin, S.L.; Kobayashi, A.; Hudson, T.E.; Nowlin, B.T.; Bonventre, J.V.; Valerius, M.T.; McMahon, A.P.; Duffield, J.S. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 2010, 176, 85–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrimpf, C.; Duffield, J.S. Mechanisms of fibrosis: The role of the pericyte. Curr. Opin. Nephrol. Hypertens. 2011, 20, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kuppe, C.; Ibrahim, M.M.; Kranz, J.; Zhang, X.; Ziegler, S.; Perales-Patón, J.; Jansen, J.; Reimer, K.C.; Smith, J.R.; Dobie, R.; et al. Decoding myofibroblast origins in human kidney fibrosis. Nature 2021, 589, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Kramann, R.; Schneider, R.K.; Dirocco, D.P.; Machado, F.; Fleig, S.; Bondzie, P.A.; Henderson, J.M.; Ebert, B.L.; Humphreys, B.D. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 2015, 16, 51–66. [Google Scholar] [CrossRef] [Green Version]
- Kramann, R.; Wongboonsin, J.; Chang-Panesso, M.; Machado, F.G.; Humphreys, B.D. Gli1+ pericyte loss induces capillary rarefaction and proximal tubular injury. J. Am. Soc. Nephrol. 2017, 28, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.A.; El-Badri, N. Pericytes: The role of multipotent stem cells in vascular maintenance and regenerative medicine. Adv. Exp. Med. Biol. 2018, 1079, 69–86. [Google Scholar] [CrossRef]
- Dar, A.; Domev, H.; Ben-Yosef, O.; Tzukerman, M.; Zeevi-Levin, N.; Novak, A.; Germanguz, I.; Amit, M.; Itskovitz-Eldor, J. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation 2012, 125, 87–99. [Google Scholar] [CrossRef] [Green Version]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chang, F.C.; Wu, C.F.; Chou, Y.H.; Hsu, H.L.; Chiang, W.C.; Shen, J.; Chen, Y.M.; Wu, K.D.; Tsai, T.J.; et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011, 80, 1170–1181. [Google Scholar] [CrossRef] [Green Version]
- Buhl, E.M.; Djudjaj, S.; Klinkhammer, B.M.; Ermert, K.; Puelles, V.G.; Lindenmeyer, M.T.; Cohen, C.D.; He, C.; Borkham-Kamphorst, E.; Weiskirchen, R.; et al. Dysregulated mesenchymal PDGFR-β drives kidney fibrosis. EMBO Mol. Med. 2020, 12, e11021. [Google Scholar] [CrossRef]
- Lin, S.L.; Chang, F.C.; Schrimpf, C.; Chen, Y.T.; Wu, C.F.; Wu, V.C.; Chiang, W.C.; Kuhnert, F.; Kuo, C.J.; Chen, Y.M.; et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am. J. Pathol. 2011, 178, 911–923. [Google Scholar] [CrossRef] [Green Version]
- Angelotti, M.L.; Antonelli, G.; Conte, C.; Romagnani, P. Imaging the kidney: From light to super-resolution microscopy. Nephrol. Dial. Transplant. 2021, 36, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Florijn, B.W.; Duijs, J.M.; Levels, J.H.; Dallinga-Thie, G.M.; Wang, Y.; Boing, A.N.; Yuana, Y.; Stam, W.; Limpens, R.W.; Au, Y.W.; et al. Diabetic nephropathy alters the distribution of circulating angiogenic MicroRNAs among extracellular vesicles, HDL, and Ago-2. Diabetes 2019, 68, 2287–2300. [Google Scholar] [CrossRef]
- Groeneweg, K.E.; Au, Y.W.; Duijs, J.M.; Florijn, B.W.; van Kooten, C.; de Fijter, J.W.; Reinders, M.E.; van Zonneveld, A.J.; Bijkerk, R. Diabetic nephropathy alters circulating long noncoding RNA levels that normalize following simultaneous pancreas–kidney transplantation. Am. J. Transplant. 2020, 20, 3451–3461. [Google Scholar] [CrossRef]
- Wu, H.; Uchimura, K.; Donnelly, E.L.; Kirita, Y.; Morris, S.A.; Humphreys, B.D. Comparative Analysis and Refinement of Human PSC-Derived Kidney Organoid Differentiation with Single-Cell Transcriptomics. Cell Stem Cell 2018, 23, 869–881.e8. [Google Scholar] [CrossRef] [Green Version]
- Maniatis, S.; Äijö, T.; Vickovic, S.; Braine, C.; Kang, K.; Mollbrink, A.; Fagegaltier, D.; Andrusivová, Ž.; Saarenpää, S.; Saiz-Castro, G.; et al. Spatiotemporal Dynamics of Molecular Pathology in Amyotrophic Lateral Sclerosis. Science 2019, 364, 89–93. [Google Scholar] [CrossRef]
- Torres Crigna, A.; Daniele, C.; Gamez, C.; Balbuena, S.M.; Pastene, D.O.; Nardozi, D.; Brenna, C.; Yard, B.; Gretz, N.; Bieback, K. Stem/stromal cells for treatment of kidney injuries with focus on preclinical models. Front. Med. 2018, 5, 179. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.Y.; Krier, J.D.; Tang, H.; Jordan, K.L.; Grande, J.P.; Lerman, A.; Textor, S.C.; Lerman, L.O. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells 2012, 30, 1030–1041. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, B.; Eirin, A.; Li, Z.; Zhu, X.Y.; Zhang, X.; Lerman, A.; Textor, S.C.; Lerman, L.O. Mesenchymal Stem Cells Improve Medullary Inflammation and Fibrosis after Revascularization of Swine Atherosclerotic Renal Artery Stenosis. PLoS ONE 2013, 8, e67474. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.Y.; Urbieta-Caceres, V.; Krier, J.D.; Textor, S.C.; Lerman, A.; Lerman, L.O. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells 2013, 31, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Franchi, F.; Peterson, K.M.; Xu, R.; Miller, B.; Psaltis, P.J.; Harris, P.C.; Lerman, L.O.; Rodriguez-Porcel, M. Mesenchymal stromal cells improve renovascular function in polycystic kidney disease. Cell Transplant. 2015, 24, 1687–1698. [Google Scholar] [CrossRef] [Green Version]
- Imafuku, A.; Oka, M.; Miyabe, Y.; Sekiya, S.; Nitta, K.; Shimizu, T. Rat Mesenchymal Stromal Cell Sheets Suppress Renal Fibrosis via Microvascular Protection. Stem Cells Transl. Med. 2019, 8, 1330–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eirin, A.; Zhu, X.Y.; Jonnada, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Mesenchymal Stem Cell-Derived Extracellular Vesicles Improve the Renal Microvasculature in Metabolic Renovascular Disease in Swine. Cell Transplant. 2018, 27, 1080–1095. [Google Scholar] [CrossRef] [Green Version]
- Flannery, B.P.; Deckman, H.W.; Roberge, W.G.; D’Amico, K.L. Three-dimensional X-ray microtomography. Science 1987, 237, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.M.; Demirkaya, O.; Ritman, E.L. Three-dimensional imaging of vasculature and parenchyma in intact rodent organs with X-ray micro-CT. JAMA J. Am. Med. Assoc. 1998, 275, H1103–H1114. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Sanz, A.; Rodnguez-Barbero, A.; Bentley, M.D.; Rltman, E.L.; Romero, J.C. Three-dimensional microcomputed tomography of renal vasculature in rats. Hypertension 1998, 31, 440–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentley, M.D.; Rodriguez-Porcel, M.; Lerman, A.; Hershman Sarafov, M.; Romero, J.C.; Pelaez, L.I.; Grande, J.P.; Ritman, E.L.; Lerman, L.O. Enhanced renal cortical vascularization in experimental hypercholesterolemia. Kidney Int. 2002, 61, 1056–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentley, M.D.; Jorgensen, S.M.; Lerman, L.O.; Ritman, E.L.; Romero, J.C. Visualization of three-dimensional nephron structure with microcomputed tomography. Anat. Rec. (Hoboken) 2007, 290, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Hillman, B.J.; Lee, S.M.; Tracey, P.; Swindell, W.; Long, D.M. CT determination of renal and hepatic microvascular volumes in experimental acute renal failure. Investig. Radiol. 1982, 17, 41–45. [Google Scholar] [CrossRef]
- Xu, R.; Franchi, F.; Miller, B.; Crane, J.A.; Peterson, K.M.; Psaltis, P.J.; Harris, P.C.; Lerman, L.O.; Rodriguez-Porcel, M. Polycystic Kidneys Have Decreased Vascular Density: A Micro-CT Study. Microcirculation 2013, 20, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz, M.C.; García-Sanz, A.; Bentley, M.D.; Fortepiani, L.A.; García-Esta n, J.; Ritman, E.L.; Romero, J.C.; Juncos, L.A. Microcomputed tomography of kidneys following chronic bile duct ligation. Kidney Int. 2000, 58, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Chade, A.R.; Rodriguez-Porcel, M.; Bentley, M.D.; Ritman, E.L.; Lerman, A.; Lerman, L.O. Cortical microvascular remodeling in the stenotic kidney: Role of increased oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1854–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, J.P.; Le, B.; Khan, Z.; Kett, M.M.; Gardiner, B.S.; Smith, D.W.; Melhem, M.M.; Maksimenko, A.; Pearson, J.T.; Evans, R.G. Micro-computed tomographic analysis of the radial geometry of intrarenal artery-vein pairs in rats and rabbits: Comparison with light microscopy. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, R.; Fernandez, S.R.; Kelsen, S.; Maric, C.; Chade, A.R. Role of renal microcirculation in experimental renovascular disease. Nephrol. Dial. Transplant. 2010, 25, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- Huisken, J.; Swoger, J.; Del Bene, F.; Wittbrodt, J.; Stelzer, E.H. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 2004, 305, 1007–1009. [Google Scholar] [CrossRef] [Green Version]
- Ueda, H.R.; Ertürk, A.; Chung, K.; Gradinaru, V.; Chédotal, A.; Tomancak, P.; Keller, P.J. Tissue clearing and its applications in neuroscience. Nat. Rev. Neurosci. 2020, 21, 61–79. [Google Scholar] [CrossRef]
- Klingberg, A.; Hasenberg, A.; Ludwig-Portugall, I.; Medyukhina, A.; Männ, L.; Brenzel, A.; Engel, D.R.; Figge, M.T.; Kurts, C.; Gunzer, M. Fully automated evaluation of total glomerular number and capillary tuft size in nephritic kidneys using lightsheet microscopy. J. Am. Soc. Nephrol. 2017, 28, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Stelzer, E.H. Light-sheet fluorescence microscopy for quantitative biology. Nat. Methods 2014, 12, 23–26. [Google Scholar] [CrossRef]
- Huang, J.; Brenna, C.; Khan, A.u.M.; Daniele, C.; Rudolf, R.; Heuveline, V.; Gretz, N. A cationic near infrared fluorescent agent and ethyl-cinnamate tissue clearing protocol for vascular staining and imaging. Sci. Rep. 2019, 9, 521. [Google Scholar] [CrossRef]
- Saritas, T.; Puelles, V.G.; Su, X.T.; Ellison, D.H.; Kramann, R. Optical clearing and imaging of immunolabeled kidney tissue. J. Vis. Exp. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Todorov, M.I.; Cai, R.; Maskari, R.A.; Steinke, H.; Kemter, E.; Mai, H.; Rong, Z.; Warmer, M.; Stanic, K.; et al. Cellular and Molecular Probing of Intact Human Organs. Cell 2020, 180, 796–812. [Google Scholar] [CrossRef]
- Todorov, M.I.; Paetzold, J.C.; Schoppe, O.; Tetteh, G.; Shit, S.; Efremov, V.; Todorov-Völgyi, K.; Düring, M.; Dichgans, M.; Piraud, M.; et al. Machine learning analysis of whole mouse brain vasculature. Nat. Methods 2020, 17, 442–449. [Google Scholar] [CrossRef]
- Tögel, F.; Hu, Z.; Weiss, K.; Isaac, J.; Lange, C.; Westenfelder, C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am. J. Physiol. Ren. Physiol. 2005, 289, F31–F42. [Google Scholar] [CrossRef] [Green Version]
- Eirin, A.; Zhu, X.Y.; Urbieta-Caceres, V.H.; Grande, J.P.; Lerman, A.; Textor, S.C.; Lerman, L.O. Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am. J. Physiol. Ren. Physiol. 2011, 300, F1394–F1401. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhu, X.; Zhang, L.; Ferguson, C.M.; Song, T.; Jiang, K.; Conley, S.M.; Krier, J.D.; Tang, H.; Saadiq, I.; et al. Mesenchymal Stem/Stromal Cells and their Extracellular Vesicle Progeny Decrease Injury in Poststenotic Swine Kidney through Different Mechanisms. Stem Cells Dev. 2020, 29, 1190–1200. [Google Scholar] [CrossRef]
- Saad, A.; Dietz, A.B.; Herrmann, S.M.; Hickson, L.J.; Glockner, J.F.; McKusick, M.A.; Misra, S.; Bjarnason, H.; Armstrong, A.S.; Gastineau, D.A.; et al. Autologous mesenchymal stem cells increase cortical perfusion in renovascular disease. J. Am. Soc. Nephrol. 2017, 28, 2777–2785. [Google Scholar] [CrossRef] [Green Version]
- Peti-Peterdi, J. Multiphoton imaging of renal tissues in vitro. Am J Physiol Ren. Physiol 2005, 288, F1079–F1083. [Google Scholar] [CrossRef] [Green Version]
- Bábíčková, J.; Klinkhammer, B.M.; Buhl, E.M.; Djudjaj, S.; Hoss, M.; Heymann, F.; Tacke, F.; Floege, J.; Becker, J.U.; Boor, P. Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int. 2017, 91, 70–85. [Google Scholar] [CrossRef]
- Van den Berg, C.W.; Ritsma, L.; Avramut, M.C.; Wiersma, L.E.; van den Berg, B.M.; Leuning, D.G.; Lievers, E.; Koning, M.; Vanslambrouck, J.M.; Koster, A.J.; et al. Renal Subcapsular Transplantation of PSC-Derived Kidney Organoids Induces Neo-vasculogenesis and Significant Glomerular and Tubular Maturation In Vivo. Stem Cell Rep. 2018, 10, 751–765. [Google Scholar] [CrossRef] [Green Version]
- Van den Berg, C.W.; Koudijs, A.; Ritsma, L.; Rabelink, T.J. In vivo assessment of size-selective glomerular sieving in transplanted human induced pluripotent stem cell-derived kidney organoids. J. Am. Soc. Nephrol. 2020, 31, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Ritsma, L.; Steller, E.J.; Ellenbroek, S.I.; Kranenburg, O.; Borel Rinkes, I.H.; van Rheenen, J. Surgical implantation of an abdominal imaging window for intravital microscopy. Nat. Protoc. 2013, 8, 583–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prunier, C.; Chen, N.; Ritsma, L.; Vrisekoop, N. Procedures and applications of long-term intravital microscopy. Methods 2017, 128, 52–64. [Google Scholar] [CrossRef]
- Guven, G.; Hilty, M.P.; Ince, C. Microcirculation: Physiology, Pathophysiology, and Clinical Application. Blood Purif. 2020, 49, 143–150. [Google Scholar] [CrossRef]
- Hilty, M.P.; Guerci, P.; Ince, Y.; Toraman, F.; Ince, C. MicroTools enables automated quantification of capillary density and red blood cell velocity in handheld vital microscopy. Commun. Biol. 2019, 2, 217. [Google Scholar] [CrossRef] [PubMed]
- Missbach-Guentner, J.; Pinkert-Leetsch, D.; Dullin, C.; Ufartes, R.; Hornung, D.; Tampe, B.; Zeisberg, M.; Alves, F. 3D virtual histology of murine kidneys -high resolution visualization of pathological alterations by micro computed tomography. Sci. Rep. 2018, 8, 1407. [Google Scholar] [CrossRef]
- Andreucci, M.; Faga, T.; Pisani, A.; Sabbatini, M.; Michael, A. Acute kidney injury by radiographic contrast media: Pathogenesis and prevention. BioMed Res. Int. 2014, 2014, 362725. [Google Scholar] [CrossRef]
- Susantitaphong, P.; Eiam-Ong, S. Nonpharmacological strategies to prevent contrast-induced acute kidney injury. BioMed Res. Int. 2014, 2014, 463608. [Google Scholar] [CrossRef]
- Weisbord, S.D.; du Cheryon, D. Contrast-associated acute kidney injury is a myth: No. Intensive Care Med. 2018, 44, 107–109. [Google Scholar] [CrossRef] [Green Version]
- Schieda, N.; Blaichman, J.I.; Costa, A.F.; Glikstein, R.; Hurrell, C.; James, M.; Jabehdar Maralani, P.; Shabana, W.; Tang, A.; Tsampalieros, A.; et al. Gadolinium-Based Contrast Agents in Kidney Disease: Comprehensive Review and Clinical Practice Guideline Issued by the Canadian Association of Radiologists. Can. Assoc. Radiol. J. 2018, 69, 136–150. [Google Scholar] [CrossRef] [Green Version]
- Logothetis, N.K. What we can do and what we cannot do with fMRI. Nature 2008, 453, 869–878. [Google Scholar] [CrossRef]
- Grenier, N.; Basseau, F.; Ries, M.; Tyndal, B.; Jones, R.; Moonen, C. Functional MRI of the kidney. Abdom. Imaging 2003, 28, 164–175. [Google Scholar] [CrossRef]
- Michaely, H.J.; Sourbron, S.; Dietrich, O.; Attenberger, U.; Reiser, M.F.; Schoenberg, S.O. Functional renal MR imaging: An overview. Abdom. Imaging 2007, 32, 758–771. [Google Scholar] [CrossRef]
- Dujardin, M.; Sourbron, S.; Luypaert, R.; Verbeelen, D.; Stadnik, T. Quantification of renal perfusion and function on a voxel-by-voxel basis: A feasibility study. Magn. Reson. Med. 2005, 54, 841–849. [Google Scholar] [CrossRef]
- Kramer, U.; Nael, K.; Laub, G.; Nyborg, G.K.; Fenchel, M.; Miller, S.; Claussen, C.D.; Finn, J.P. High-resolution magnetic resonance angiography of the renal arteries using parallel imaging acquisition techniques at 3.0 T: Initial experience. Investig. Radiol. 2006, 41, 125–132. [Google Scholar] [CrossRef]
- Sadick, M.; Schock, D.; Kraenzlin, B.; Gretz, N.; Schoenberg, S.O.; Michaely, H.J. Morphologic and dynamic renal imaging with assessment of glomerular filtration rate in a pcy-mouse model using a clinical 3.0 tesla scanner. Investig. Radiol. 2009, 44, 469–475. [Google Scholar] [CrossRef]
- Glover, G.H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Iacobellis, F.; Segreto, T.; Berritto, D.; Nettuno, F.; Cozzolino, S.; Di Napoli, D.; Montella, M.; Natella, R.; Cappabianca, S.; Brunese, L.; et al. A rat model of acute kidney injury through systemic hypoperfusion evaluated by micro-US, color and PW-Doppler. Radiol. Medica 2019, 124, 323–330. [Google Scholar] [CrossRef]
- Tanter, M.; Fink, M. Ultrafast imaging in biomedical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 102–119. [Google Scholar] [CrossRef]
- Baranger, J.; Arnal, B.; Perren, F.; Baud, O.; Tanter, M.; Demene, C. Adaptive Spatiotemporal SVD Clutter Filtering for Ultrafast Doppler Imaging Using Similarity of Spatial Singular Vectors. IEEE Trans. Med. Imaging 2018, 37, 1574–1586. [Google Scholar] [CrossRef] [Green Version]
- Couture, O.; Hingot, V.; Heiles, B.; Muleki-Seya, P.; Tanter, M. Ultrasound localization microscopy and super-resolution: A state of the art. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 1304–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wink, M.H.; Wijkstra, H.; De La Rosette, J.J.; Grimbergen, C.A. Ultrasound imaging and contrast agents: A safe alternative to MRI? Minim. Invasive Ther. Allied Technol. 2006, 15, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Errico, C.; Pierre, J.; Pezet, S.; Desailly, Y.; Lenkei, Z.; Couture, O.; Tanter, M. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 2015, 527, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.L. Ultrasound Contrast: Gas Microbubbles in the Vasculature. Investig. Radiol. 2021, 56, 50–61. [Google Scholar] [CrossRef]
- Demené, C.; Robin, J.; Dizeux, A.; Heiles, B.; Pernot, M.; Tanter, M.; Perren, F. Transcranial ultrafast ultrasound localization microscopy of brain vasculature in patients. Nat. Biomed. Eng. 2021, 5, 219–228. [Google Scholar] [CrossRef]
- Hansen, K.B.; Villagómez-Hoyos, C.A.; Brasen, J.C.; Diamantis, K.; Sboros, V.; Sørensen, C.M.; Jensen, J.A. Robust microbubble tracking for super resolution imaging in ultrasound. In Proceedings of the 2016 IEEE International Ultrasonics Symposium (IUS), Tours, France, 18–21 September 2016; pp. 1–4. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Trzasko, J.D.; Manduca, A.; Huang, R.; Kadirvel, R.; Kallmes, D.F.; Chen, S. Improved Super-Resolution Ultrasound Microvessel Imaging with Spatiotemporal Nonlocal Means Filtering and Bipartite Graph-Based Microbubble Tracking HHS Public Access. IEEE Trans Ultrason Ferroelectr Freq Control 2018, 65, 149–167. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Cui, S.; Yang, L.; Wu, C.; Liu, J.; Yang, F.; Liu, Y.; Bin, J.; Hou, F.F. Contrast-Enhanced Ultrasound for Assessing Renal Perfusion Impairment and Predicting Acute Kidney Injury to Chronic Kidney Disease Progression. Antioxidants Redox Signal. 2017, 27, 1397–1411. [Google Scholar] [CrossRef]
- Fischer, T.; Dieckhöfer, J.; Mühler, M.; Lembcke, A.; Morgera, S.; Budde, K.; Neumayer, H.H.; Ebeling, V.; Thomas, A.; Filimonow, S. The use of contrast-enhanced US in renal transplant: First results and potential clinical benefit. Eur. Radiol. Suppl. 2005, 15, E109–E116. [Google Scholar] [CrossRef]
- Kalantarinia, K.; Belcik, J.T.; Patrie, J.T.; Wei, K. Real-time measurement of renal blood flow in healthy subjects using contrast-enhanced ultrasound. Am. J. Physiol. Ren. Physiol. 2009, 297, F1129–F1134. [Google Scholar] [CrossRef]
- Schneider, A.G.; Hofmann, L.; Wuerzner, G.; Glatz, N.; Maillard, M.; Meuwly, J.Y.; Eggimann, P.; Burnier, M.; Vogt, B. Renal perfusion evaluation with contrast-enhanced ultrasonography. Nephrol. Dial. Transplant. 2012, 27, 674–681. [Google Scholar] [CrossRef]
- Schneider, A.G.; Goodwin, M.D.; Schelleman, A.; Bailey, M.; Johnson, L.; Bellomo, R. Contrast-enhanced ultrasound to evaluate changes in renal cortical perfusion around cardiac surgery: A pilot study. Crit. Care 2013, 17, R138. [Google Scholar] [CrossRef] [Green Version]
- Schneider, A.G.; Goodwin, M.D.; Schelleman, A.; Bailey, M.; Johnson, L.; Bellomo, R. Contrast-enhanced ultrasonography to evaluate changes in renal cortical microcirculation induced by noradrenaline: A pilot study. Crit. Care 2014, 18, 653. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Yang, C.; Wu, S.; Zhou, S.; Ji, Z.; Zhu, T.; He, W. A novel simple noninvasive index to predict renal transplant acute rejection by contrast-enhanced ultrasonography. Transplantation 2015, 99, 636–641. [Google Scholar] [CrossRef]
- Harrois, A.; Duranteau, J. Contrast-enhanced ultrasound: A new vision of microcirculation in the intensive care unit. Crit. Care 2013, 17, 449. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apelt, K.; Bijkerk, R.; Lebrin, F.; Rabelink, T.J. Imaging the Renal Microcirculation in Cell Therapy. Cells 2021, 10, 1087. https://doi.org/10.3390/cells10051087
Apelt K, Bijkerk R, Lebrin F, Rabelink TJ. Imaging the Renal Microcirculation in Cell Therapy. Cells. 2021; 10(5):1087. https://doi.org/10.3390/cells10051087
Chicago/Turabian StyleApelt, Katerina, Roel Bijkerk, Franck Lebrin, and Ton J. Rabelink. 2021. "Imaging the Renal Microcirculation in Cell Therapy" Cells 10, no. 5: 1087. https://doi.org/10.3390/cells10051087
APA StyleApelt, K., Bijkerk, R., Lebrin, F., & Rabelink, T. J. (2021). Imaging the Renal Microcirculation in Cell Therapy. Cells, 10(5), 1087. https://doi.org/10.3390/cells10051087






