The Anti-Infectious Role of Sphingosine in Microbial Diseases
Abstract
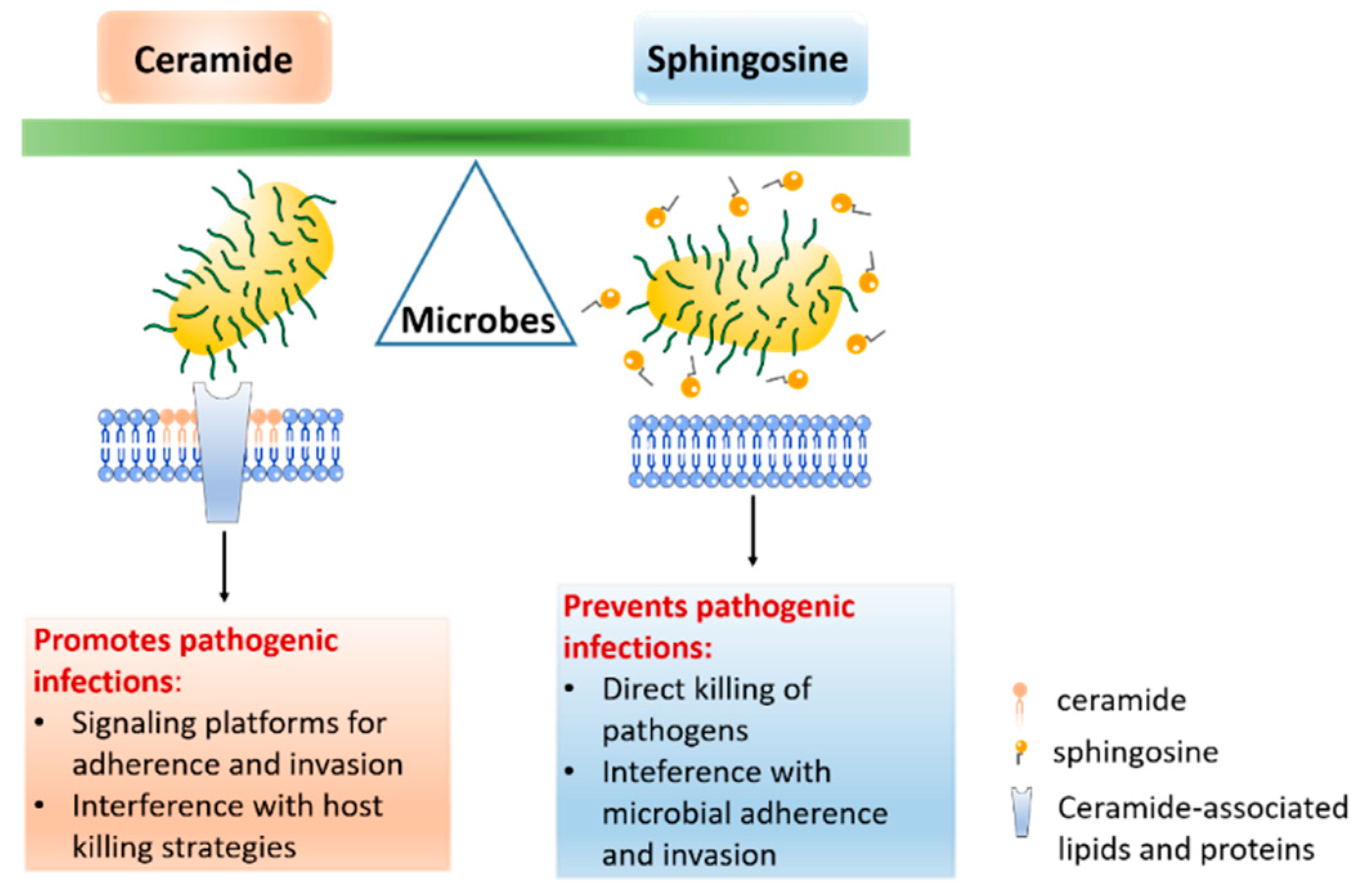
:1. Introduction
2. The Role of Sphingosine in Infectious Diseases
2.1. Sphingosine and Bacteria
2.1.1. Staphylococcus aureus
2.1.2. Pseudomonas aeruginosa
2.1.3. Neisseria gonorrhoeae
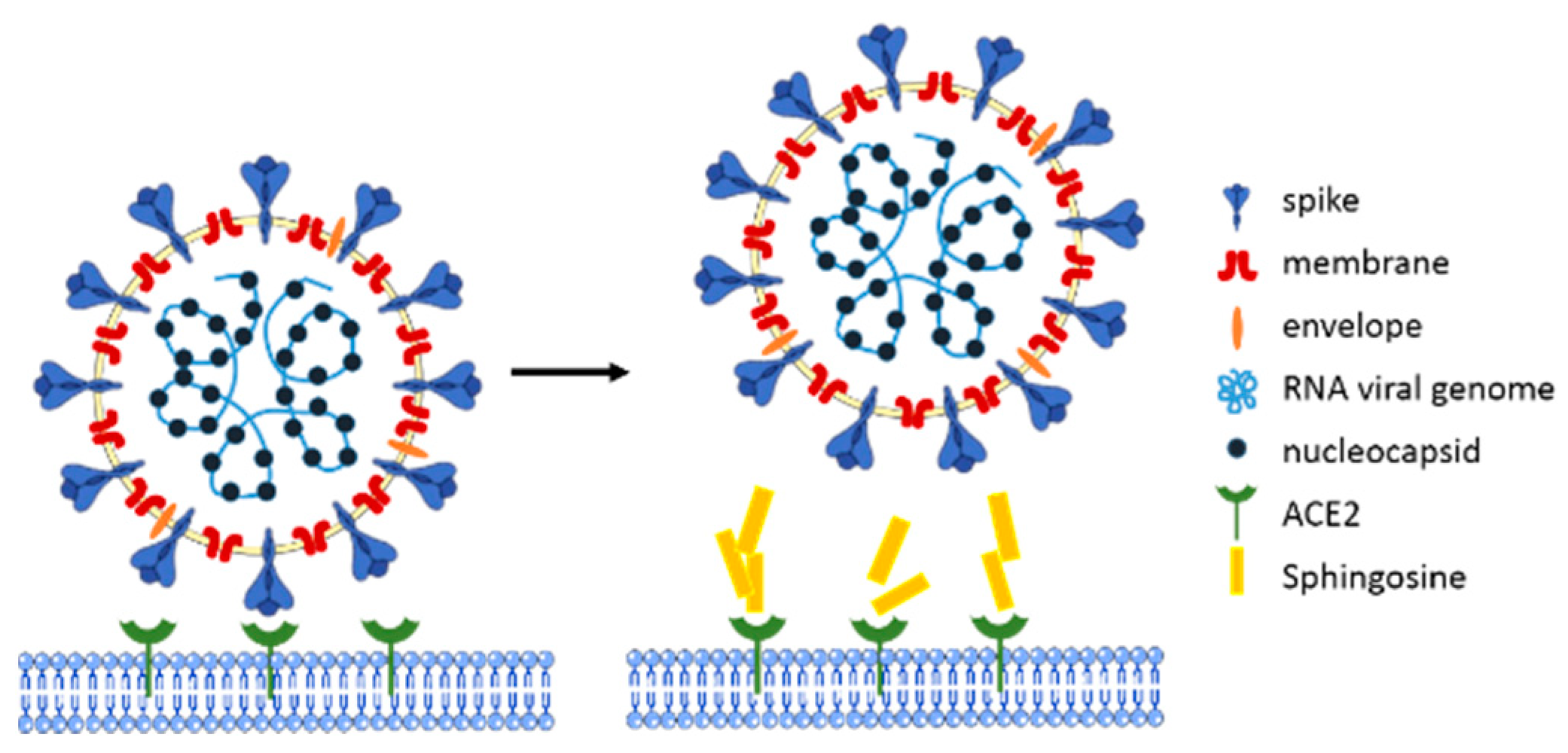
2.2. Sphingosine and Viruses
2.3. Sphingosine and Fungi
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Futerman, A.H.; Hannun, Y.A. The complex life of simple sphingolipids. EMBO Rep. 2004, 5, 777–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, J.S.; Spiegel, S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nat. Cell Biol. 1996, 381, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Merrill, A.H. Sphingolipid metabolism and cell growth regulation. FASEB J. 1996, 10, 1388–1397. [Google Scholar] [CrossRef]
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009, 50, S91–S96. [Google Scholar] [CrossRef] [Green Version]
- Ohanian, J.; Ohanian, V. Sphingolipids in mammalian cell signalling. Cell. Mol. Life Sci. 2001, 58, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Tirodkar, T.S.; Voelkel-Johnson, C. Sphingolipids in apoptosis. Exp. Oncol. 2012, 34, 231–242. [Google Scholar]
- Iessi, E.; Marconi, M.; Manganelli, V.; Sorice, M.; Malorni, W.; Garofalo, T.; Matarrese, P. On the role of sphingolipids in cell survival and death. Int. Rev. Cell Mol. Biol. 2020, 351, 149–195. [Google Scholar] [CrossRef]
- Hanada, K. Sphingolipids in infectious diseases. Jpn. J. Infect. Dis. 2005, 58, 131–148. [Google Scholar]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Olivera, A.; Kohama, T.; Edsall, L.; Nava, V.; Cuvillier, O.; Poulton, S.; Spiegel, S. Sphingosine Kinase Expression Increases Intracellular Sphingosine-1-Phosphate and Promotes Cell Growth and Survival. J. Cell Biol. 1999, 147, 545–558. [Google Scholar] [CrossRef] [Green Version]
- Pyne, N.J.; Pyne, S. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer 2010, 10, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Maceyka, M.; Nava, V.E.; Milstien, S.; Spiegel, S. Aminoacylase 1 is a sphingosine kinase 1-interacting protein. FEBS Lett. 2004, 568, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Arish, M.; Husein, A.; Kashif, M.; Saleem, M.; Akhter, Y.; Rub, A. Sphingosine-1-phosphate signaling: Unraveling its role as a drug target against infectious diseases. Drug Discov. Today 2016, 21, 133–142. [Google Scholar] [CrossRef]
- Hudlicky, T.; Rouden, J.; Luna, H.; Allen, S. Microbial Oxidation of Aromatics in Enantiocontrolled Synthesis. 2. Rational Design of Aza Sugars (endo-Nitrogenous). Total Synthesis of (+)-Kifunensine, Mannojirimycin, and Other Glycosidase Inhibitors. J. Am. Chem. Soc. 1994, 116, 5099–5107. [Google Scholar] [CrossRef]
- Tommasino, C.; Marconi, M.; Ciarlo, L.; Matarrese, P.; Malorni, W. Autophagic flux and autophagosome morphogenesis require the participation of sphingolipids. Apoptosis 2015, 20, 645–657. [Google Scholar] [CrossRef]
- Olivera, A.; Rosenthal, J.; Spiegel, S. Effect of acidic phospholipids on sphingosine kinase. J. Cell. Biochem. 1996, 60, 529–537. [Google Scholar] [CrossRef]
- Smith, E.R.; Merrill, A.H.; Obeid, L.M.; Hannun, Y.A. Effects of Sphingosine and Other Sphingolipids on Protein Kinase C. Methods Enzymol. 2000, 312, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.L.; Drake, D.R.; Dawson, D.V.; Blanchette, D.R.; Brogden, K.A.; Wertz, P.W. Antibacterial Activity of Sphingoid Bases and Fatty Acids against Gram-Positive and Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2011, 56, 1157–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, H.; Okamoto, K.; Aoki, M.; Kato, H.; Katsume, A.; Ohta, A.; Tsukuda, T.; Shimma, N.; Aoki, Y.; Arisawa, M.; et al. Host sphingolipid biosynthesis as a target for hepatitis C virus therapy. Nat. Chem. Biol. 2005, 1, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Rollin-Pinheiro, R.; Singh, A.; Barreto-Bergter, E.; Del Poeta, M. Sphingolipids as targets for treatment of fungal infections. Futur. Med. Chem. 2016, 8, 1469–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pewzner-Jung, Y.; Tabazavareh, S.T.; Grassmé, H.; Becker, K.A.; Japtok, L.; Steinmann, J.; Joseph, T.; Lang, S.; Tuemmler, B.; Schuchman, E.H.; et al. Sphingoid long chain bases prevent lung infection by Pseudomonas aeruginosa. EMBO Mol. Med. 2014, 6, 1205–1214. [Google Scholar] [CrossRef]
- Grassmé, H.; Henry, B.; Ziobro, R.; Becker, K.A.; Riethmüller, J.; Gardner, A.; Seitz, A.P.; Steinmann, J.; Lang, S.; Ward, C.; et al. β1-Integrin Accumulates in Cystic Fibrosis Luminal Airway Epithelial Membranes and Decreases Sphingosine, Promoting Bacterial Infections. Cell Host Microbe 2017, 21, 707–718.e8. [Google Scholar] [CrossRef] [Green Version]
- Tabazavareh, S.T.; Seitz, A.; Jernigan, P.; Sehl, C.; Keitsch, S.; Lang, S.; Kahl, B.C.; Edwards, M.; Grassmé, H.; Gulbins, E.; et al. Lack of Sphingosine Causes Susceptibility to Pulmonary Staphylococcus Aureus Infections in Cystic Fibrosis. Cell. Physiol. Biochem. 2016, 38, 2094–2102. [Google Scholar] [CrossRef]
- Carstens, H.; Schumacher, F.; Keitsch, S.; Kramer, M.; Kühn, C.; Sehl, C.; Soddemann, M.; Wilker, B.; Herrmann, D.; Swaidan, A.; et al. Clinical Development of Sphingosine as Anti-Bacterial Drug: Inhalation of Sphingosine in Mini Pigs has no Adverse Side Effects. Cell. Physiol. Biochem. 2019, 53, 1015–1028. [Google Scholar] [CrossRef]
- Bibel, D.J.; Aly, R.; Shinefield, H.R. Antimicrobial Activity of Sphingosines. J. Investig. Dermatol. 1992, 98, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, C.L.; Walters, K.S.; Drake, D.R.; Blanchette, D.R.; Dawson, D.V.; Brogden, K.A.; Wertz, P.W. Sphingoid Bases Are Taken Up byEscherichia coliandStaphylococcus aureusand Induce Ultrastructural Damage. Ski. Pharmacol. Physiol. 2013, 26, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhaegh, R.; Becker, K.A.; Edwards, M.J.; Gulbins, E. Sphingosine kills bacteria by binding to cardiolipin. J. Biol. Chem. 2020, 295, 7686–7696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; Byun, J.; Jung, M.; Yang, J.J.; Park, K.-H.; Moon, S.-Y.; Lee, H.J.; Lee, M.S. Disseminated Gonococcal Infection Presenting as Bacteremia and Liver Abscesses in a Healthy Adult. Infect. Chemother. 2015, 47, 60–63. [Google Scholar] [CrossRef]
- Seitz, A.P.; Schumacher, F.; Baker, J.; Soddemann, M.; Wilker, B.; Caldwell, C.C.; Gobble, R.M.; Kamler, M.; Becker, K.A.; Beck, S.; et al. Sphingosine-coating of plastic surfaces prevents ventilator-associated pneumonia. J. Mol. Med. 2019, 97, 1195–1211. [Google Scholar] [CrossRef] [Green Version]
- Rice, T.C.; Pugh, A.M.; Seitz, A.P.; Gulbins, E.; Nomellini, V.; Caldwell, C.C. Sphingosine rescues aged mice from pulmonary pseudomonas infection. J. Surg. Res. 2017, 219, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Rice, T.C.; Seitz, A.P.; Edwards, M.J.; Gulbins, E.; Caldwell, C.C. Frontline Science: Sphingosine rescues burn-injured mice from pulmonaryPseudomonas aeruginosainfection. J. Leukoc. Biol. 2016, 100, 1233–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckmann, N.; Pugh, A.M.; Auteri, N.J.; Edwards, M.J.; Gulbins, E.; Caldwell, C.C. Therapeutic Inhaled Sphingosine for Treating Lung Infection in a Mouse Model of Critical Illness. Cell. Physiol. Biochem. 2020, 54, 1054–1067. [Google Scholar] [CrossRef]
- Bibel, D.J.; Aly, R.; Shah, S.; Shinefield, H.R. Sphingosines: Antimicrobial barriers of the skin. Acta Derm. Venereol. 1993, 73, 407–411. [Google Scholar] [PubMed]
- Parsons, J.B.; Yao, J.; Frank, M.W.; Jackson, P.; Rock, C.O. Membrane Disruption by Antimicrobial Fatty Acids Releases Low-Molecular-Weight Proteins from Staphylococcus aureus. J. Bacteriol. 2012, 194, 5294–5304. [Google Scholar] [CrossRef] [Green Version]
- Beck, S.; Sehl, C.; Voortmann, S.; Verhasselt, H.L.; Edwards, M.J.; Buer, J.; Hasenberg, M.; Gulbins, E.; Becker, K.A. Sphingosine is able to prevent and eliminate Staphylococcus epidermidis biofilm formation on different orthopedic implant materials in vitro. J. Mol. Med. 2019, 98, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, J.; Bohn, P.; Bhat, H.; Jastrow, H.; Walkenfort, B.; Cansiz, F.; Fink, J.; Bauer, M.; Olszewski, D.; Ramos-Nascimento, A.; et al. Acid ceramidase of macrophages traps herpes simplex virus in multivesicular bodies and protects from severe disease. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Edwards, M.J.; Becker, K.A.; Gripp, B.; Hoffmann, M.; Keitsch, S.; Wilker, B.; Soddemann, M.; Gulbins, A.; Carpinteiro, E.; Patel, S.H.; et al. Sphingosine prevents binding of SARS–CoV-2 spike to its cellular receptor ACE2. J. Biol. Chem. 2020, 295, 15174–15182. [Google Scholar] [CrossRef]
- Burtenshaw, J.M.L. The Mechanism of Self-Disinfection of the Human Skin and its Appendages. J. Hyg. 1942, 42, 184–210. [Google Scholar] [CrossRef] [Green Version]
- Arikawa, J.; Ishibashi, M.; Kawashima, M.; Takagi, Y.; Ichikawa, Y.; Imokawa, G. Decreased Levels of Sphingosine, a Natural Antimicrobial Agent, may be Associated with Vulnerability of the Stratum Corneum from Patients with Atopic Dermatitis to Colonization by Staphylococcus aureus. J. Investig. Dermatol. 2002, 119, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.E.; Boudreau, R.M.; Couch, C.; Becker, K.A.; Edwards, M.J.; Caldwell, C.C.; Gulbins, E.; Seitz, A. Sphingosine’s role in epithelial host defense: A natural antimicrobial and novel therapeutic. Biochimie 2017, 141, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Jr, V.G.F. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paharik, A.E.; Horswill, A.R. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, T.F. Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2009, 15, 138–142. [Google Scholar] [CrossRef]
- Martínez-Solano, L.; Macia, M.D.; Fajardo, A.; Oliver, A.; Martinez, J.L. ChronicPseudomonas aeruginosaInfection in Chronic Obstructive Pulmonary Disease. Clin. Infect. Dis. 2008, 47, 1526–1533. [Google Scholar] [CrossRef] [Green Version]
- Teichgräber, V.; Ulrich, M.; Endlich, N.; Riethmüller, J.; Wilker, B.; De Oliveira–Munding, C.C.; Van Heeckeren, A.M.; Barr, M.L.; Von Kürthy, G.; Schmid, K.W.; et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat. Med. 2008, 14, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- O’Sullivan, B.P.; Freedman, S.D. Cystic fibrosis. Lancet 2009, 373, 1891–1904. [Google Scholar] [CrossRef]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.L.; et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef]
- Kerem, B.; Rommens, J.M.; A Buchanan, J.; Markiewicz, D.; Cox, T.K.; Chakravarti, A.; Buchwald, M.; Tsui, L.C. Identification of the cystic fibrosis gene: Genetic analysis. Science 1989, 245, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Imundo, L.; Barasch, J.; Prince, A.; Al-Awqati, Q. Cystic fibrosis epithelial cells have a receptor for pathogenic bacteria on their apical surface. Proc. Natl. Acad. Sci. USA 1995, 92, 3019–3023. [Google Scholar] [CrossRef] [Green Version]
- Becker, K.A.; Riethmüller, J.; Lüth, A.; Döring, G.; Kleuser, B.; Gulbins, E. Acid Sphingomyelinase Inhibitors Normalize Pulmonary Ceramide and Inflammation in Cystic Fibrosis. Am. J. Respir. Cell Mol. Biol. 2010, 42, 716–724. [Google Scholar] [CrossRef]
- A Kalanuria, A.; Zai, W.; Mirski, M. Ventilator-associated pneumonia in the ICU. Crit. Care 2014, 18, 208. [Google Scholar] [CrossRef] [Green Version]
- Chastre, J.; Fagon, J.-Y. Ventilator-associated Pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O. Treatment of Infections Associated with Surgical Implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- McManus, A.T.; Mason, A.D.; McManus, W.F.; Pruitt, B.A. Twenty-five year review ofPseudomonas aeruginosa bacteremia in a burn center. Eur. J. Clin. Microbiol. Infect. Dis. 1985, 4, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Brewer, S.C.; Wunderink, R.G.; Jones, C.B.; Leeper, K.V. Ventilator-Associated Pneumonia Due to Pseudomonas Aeruginosa. Chest 1996, 109, 1019–1029. [Google Scholar] [CrossRef]
- Vidal, F.; Mensa, J.; Almela, M.; A Martínez, J.; Marco, F.; Casals, C.; Gatell, J.M.; Soriano, E.; De Anta, M.T.J. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch. Intern. Med. 1996, 156, 8862105. [Google Scholar] [CrossRef]
- Society, A.T.; America, I.D.S.O. Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef]
- Morrison, A.J.; Wenzel, R.P. Epidemiology of Infections Due to Pseudomonas aeruginosa. Clin. Infect. Dis. 1984, 6, S627–S642. [Google Scholar] [CrossRef]
- Currie, A.J.; Speert, D.P.; Davidson, N.J. Pseudomonas aeruginosa:Role in the Pathogenesis of the CF Lung Lesion. Semin. Respir. Crit. Care Med. 2003, 24, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Rao, S. New insights into pulmonary inflammation in cystic fibrosis. Arch. Dis. Child. 2006, 91, 786–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebenezer, D.L.; Fu, P.; Krishnan, Y.; Maienschein-Cline, M.; Hu, H.; Jung, S.; Madduri, R.; Arbieva, Z.; Harijith, A.; Natarajan, V. Genetic deletion of Sphk2 confers protection against Pseudomonas aeruginosa mediated differential expression of genes related to virulent infection and inflammation in mouse lung. BMC Genom. 2019, 20, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strub, G.M.; Maceyka, M.; Hait, N.C.; Milstien, S.; Spiegel, S. Extracellular and Intracellular Actions of Sphingosine-1-Phosphate. Adv. Exp. Med. Biol. 2010, 688, 141–155. [Google Scholar] [CrossRef] [Green Version]
- Muraglia, K.A.; Chorghade, R.S.; Kim, B.R.; Tang, X.X.; Shah, V.S.; Grillo, A.S.; Daniels, P.N.; Cioffi, A.G.; Karp, P.H.; Zhu, L.; et al. Small-molecule ion channels increase host defences in cystic fibrosis airway epithelia. Nat. Cell Biol. 2019, 567, 405–408. [Google Scholar] [CrossRef]
- Dowhan, W. MOLECULAR BASIS FOR MEMBRANE PHOSPHOLIPID DIVERSITY:Why Are There So Many Lipids? Annu. Rev. Biochem. 1997, 66, 199–232. [Google Scholar] [CrossRef] [Green Version]
- Britigan, B.E.; Cohen, M.S.; Sparling, P.F. Gonococcal Infection: A Model of Molecular Pathogenesis. N. Engl. J. Med. 1985, 312, 1683–1694. [Google Scholar] [CrossRef]
- Solger, F.; Kunz, T.C.; Fink, J.; Paprotka, K.; Pfister, P.; Hagen, F.; Schumacher, F.; Kleuser, B.; Seibel, J.; Rudel, T. A Role of Sphingosine in the Intracellular Survival of Neisseria gonorrhoeae. Front. Cell. Infect. Microbiol. 2020, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Greber, U.F. Principles of Virus Uncoating: Cues and the Snooker Ball. Traffic 2016, 17, 569–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virgin, H.W. The Virome in Mammalian Physiology and Disease. Cell 2014, 157, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, P.D.; Pogany, J. The dependence of viral RNA replication on co-opted host factors. Nat. Rev. Genet. 2011, 10, 137–149. [Google Scholar] [CrossRef]
- Ng, C.G.; Griffin, D.E. Acid sphingomyelinase deficiency increases susceptibility to fatal alphavirus encephalomyelitis. J. Virol. 2006, 80, 10989–10999. [Google Scholar] [CrossRef] [Green Version]
- Grassmé, H.; Riehle, A.; Wilker, B.; Gulbins, E. Rhinoviruses Infect Human Epithelial Cells via Ceramide-enriched Membrane Platforms. J. Biol. Chem. 2005, 280, 26256–26262. [Google Scholar] [CrossRef] [Green Version]
- Avota, E.; Gulbins, E.; Schneider-Schaulies, S. DC-SIGN Mediated Sphingomyelinase-Activation and Ceramide Generation Is Essential for Enhancement of Viral Uptake in Dendritic Cells. PLoS Pathog. 2011, 7, e1001290. [Google Scholar] [CrossRef]
- Miller, E.H.; Obernosterer, G.; Raaben, M.; Herbert, A.S.; Deffieu, M.S.; Krishnan, A.; Ndungo, E.; Sandesara, R.G.; E Carette, J.; I Kuehne, A.; et al. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J. 2012, 31, 1947–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grammatikos, G.; Dietz, J.; Ferreiros, N.; Koch, A.; Dultz, G.; Bon, D.; Karakasiliotis, I.; Lutz, T.; Knecht, G.; Gute, P.; et al. Persistence of HCV in Acutely-Infected Patients Depletes C24-Ceramide and Upregulates Sphingosine and Sphinganine Serum Levels. Int. J. Mol. Sci. 2016, 17, 922. [Google Scholar] [CrossRef] [Green Version]
- A Karasneh, G.; Shukla, D. Herpes simplex virus infects most cell types in vitro: Clues to its success. Virol. J. 2011, 8, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nicola, S.; Colombo, M. The eradication of HCV. Minerva Gastroenterol. Dietol. 2016, 62, 63–75. [Google Scholar] [PubMed]
- Agelidis, A.M.; Shukla, D. Cell entry mechanisms of HSV: What we have learned in recent years. Future Virol. 2015, 10, 1145–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weed, D.J.; Nicola, A.V. Herpes simplex virus Membrane Fusion. Met. Norm. Cancer Cells 2017, 223, 29–47. [Google Scholar] [CrossRef] [Green Version]
- Fantini, J.; Di Scala, C.; Chahinian, H.; Yahi, N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents 2020, 55, 105960. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Chahinian, H.; Yahi, N. Leveraging coronavirus binding to gangliosides for innovative vaccine and therapeutic strategies against COVID-19. Biochem. Biophys. Res. Commun. 2021, 538, 132–136. [Google Scholar] [CrossRef]
- Sorice, M.; Misasi, R.; Riitano, G.; Manganelli, V.; Martellucci, S.; Longo, A.; Garofalo, T.; Mattei, V. Targeting Lipid Rafts as a Strategy Against Coronavirus. Front. Cell Dev. Biol. 2021, 8, 618296. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e9. [Google Scholar] [CrossRef] [PubMed]
- Meacci, E.; Garcia-Gil, M.; Pierucci, F. SARS-CoV-2 Infection: A Role for S1P/S1P Receptor Signaling in the Nervous System? Int. J. Mol. Sci. 2020, 21, 6773. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, F.; Zhao, S.; Han, J.; Chen, F. Role of the SphK-S1P-S1PRs pathway in invasion of the nervous system by SARS-CoV-2 infection. Clin. Exp. Pharmacol. Physiol. 2021, 48, 637–650. [Google Scholar] [CrossRef]
- Tasat, D.R.; Yakisich, J.S. Rationale for the use of sphingosine analogues in COVID-19 patients. Clin. Med. 2021, 21, e84–e87. [Google Scholar] [CrossRef]
- Billich, A.; Bornancin, F.; Dévay, P.; Mechtcheriakova, D.; Urtz, N.; Baumruker, T. Phosphorylation of the Immunomodulatory Drug FTY720 by Sphingosine Kinases. J. Biol. Chem. 2003, 278, 47408–47415. [Google Scholar] [CrossRef] [Green Version]
- Obinata, H.; Hla, T. Sphingosine 1-phosphate and inflammation. Int. Immunol. 2019, 31, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R.; Hachem, R.; Wingard, J.R. Update on Epidemiology of and Preventive Strategies for Invasive Fungal Infections in Cancer Patients. Clin. Infect. Dis. 2014, 59, S352–S355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeid, L.M.; Okamoto, Y.; Mao, C. Yeast sphingolipids: Metabolism and biology. Biochim. et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2002, 1585, 163–171. [Google Scholar] [CrossRef]
- McEvoy, K.; Normile, T.G.; Del Poeta, M. Antifungal Drug Development: Targeting the Fungal Sphingolipid Pathway. J. Fungi 2020, 6, 142. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Travassos, L.R.; Miranda, K.R.; Franzen, A.J.; Rozental, S.; De Souza, W.; Alviano, C.S.; Barreto-Bergter, E. Human Antibodies against a Purified Glucosylceramide from Cryptococcus neoformans Inhibit Cell Budding and Fungal Growth. Infect. Immun. 2000, 68, 7049–7060. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Jeffries, M.W.; Georgopapadakou, N.H. Inhibition of Inositol Phosphorylceramide Synthase by Aureobasidin A in Candida and AspergillusSpecies. Antimicrob. Agents Chemother. 2000, 44, 651–653. [Google Scholar] [CrossRef] [Green Version]
- Levery, S.B.; Momany, M.; Lindsey, R.; Toledo, M.S.; A Shayman, J.; Fuller, M.; Brooks, K.; Doong, R.L.; Straus, A.H.; Takahashi, H.K. Disruption of the glucosylceramide biosynthetic pathway inAspergillus nidulansandAspergillus fumigatusby inhibitors of UDP-Glc:ceramide glucosyltransferase strongly affects spore germination, cell cycle, and hyphal growth. FEBS Lett. 2002, 525, 59–64. [Google Scholar] [CrossRef] [Green Version]

| Microbes | Species | References |
|---|---|---|
| bacteria | Acinetobacter baumannii | [21] |
| Brevibacterium epidermidis | [25] | |
| Burkholderia cepacia | [21] | |
| Corynebacterium bovis | [18] | |
| Corynebacterium striatum | [18] | |
| Corynebacterium jeikium | [18] | |
| Escherichia coli | [18,26,27] | |
| Haemophilus influenzae | [21] | |
| Micrococcus luteus | [25] | |
| Moraxella catarrhalis | [21] | |
| Neisseria gonorrhoeae | [28] | |
| Propionibacterium acnes | [25] | |
| Pseudomonas aeruginosa | [21,22,25,27,29,30,31,32] | |
| Staphylococcus aureus | [18,23,25,27,29,33,34] | |
| Staphylococcus epidermidis | [35] | |
| Streptococcus mitis | [18] | |
| Streptococcus pyogens | [25] | |
| Streptococcus sanguinis | [18] | |
| viruses | Hepatitis C virus | [19] |
| Herpes simplex virus type 1 | [36] | |
| SARS-CoV-2 | [37] | |
| fungi | Candida albicans | [25] |
| Epidermatophyton floccosum | [33] | |
| Trichophyton mentagrophytes | [33] | |
| Trichophyton tonsurans | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Liu, Y.; Gulbins, E.; Grassmé, H. The Anti-Infectious Role of Sphingosine in Microbial Diseases. Cells 2021, 10, 1105. https://doi.org/10.3390/cells10051105
Wu Y, Liu Y, Gulbins E, Grassmé H. The Anti-Infectious Role of Sphingosine in Microbial Diseases. Cells. 2021; 10(5):1105. https://doi.org/10.3390/cells10051105
Chicago/Turabian StyleWu, Yuqing, Yongjie Liu, Erich Gulbins, and Heike Grassmé. 2021. "The Anti-Infectious Role of Sphingosine in Microbial Diseases" Cells 10, no. 5: 1105. https://doi.org/10.3390/cells10051105
APA StyleWu, Y., Liu, Y., Gulbins, E., & Grassmé, H. (2021). The Anti-Infectious Role of Sphingosine in Microbial Diseases. Cells, 10(5), 1105. https://doi.org/10.3390/cells10051105






