Physiological and Pathological Inflammation Induced by Antibodies and Pentraxins
Abstract
:1. Introduction
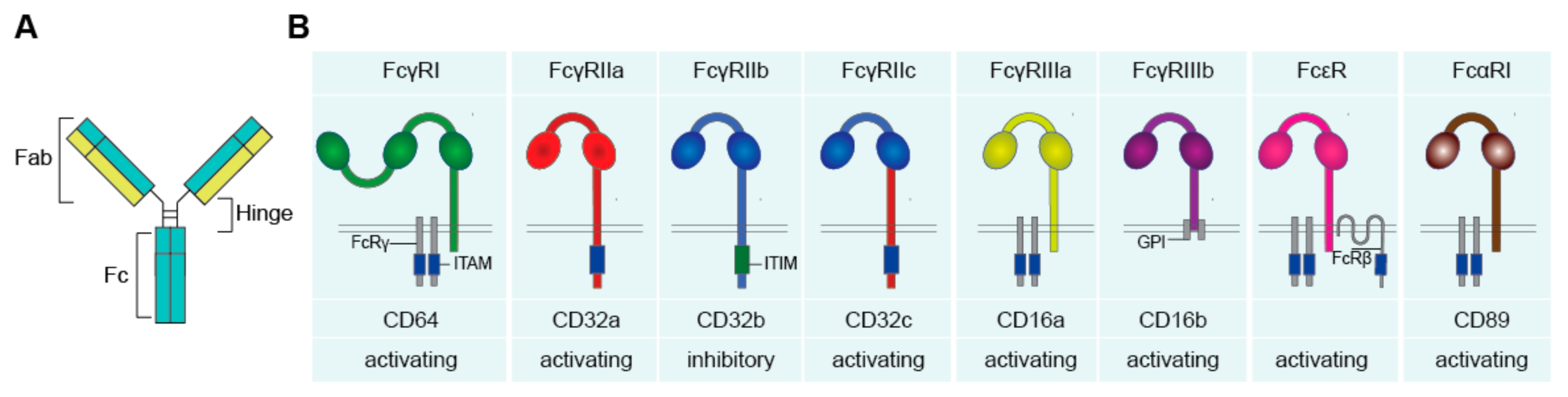
Antibodies and Fc Receptors
2. Physiological Immune Activation: Host Defense against Pathogens
2.1. Molecular Mechanism of Antibody-Induced Inflammation
2.1.1. Complex Formation
2.1.2. Co-Stimulation
2.1.3. Antibody-Intrinsic Differences
Isotype
Subclasses
Allotypes
Glycosylation
2.1.4. Location
2.2. Inflammation by Pentraxins
2.2.1. Pentraxin Family
2.2.2. Pentraxin-Induced Inflammation
3. Pathological Immune Activation
3.1. Pathogenic Antibodies
3.2. Autoantibodies
3.3. Cell-Intrinsic Overactivation
3.4. Aberrant Location/Concentration
4. Therapeutic Opportunities
4.1. Inducing Controlled ADI to Boost Host Defense
4.2. Interfering with Uncontrolled ADI in Chronic Inflammation
5. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Egmond, M.; Vidarsson, G.; Bakema, J.E. Cross-talk between pathogen recognizing Toll-like receptors and immunoglobulin Fc receptors in immunity. Immunol. Rev. 2015, 268, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Kieser, K.J.; Kagan, J.C. Multi-receptor detection of individual bacterial products by the innate immune system. Nat. Rev. Immunol. 2017, 17, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.H.; Gringhuis, S.I. Signalling through C-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Lu, L.L.; Suscovich, T.J.; Fortune, S.M.; Alter, G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2018, 18, 46–61. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [Green Version]
- Van Erp, E.A.; Luytjes, W.; Ferwerda, G.; van Kasteren, P.B. Fc-Mediated Antibody Effector Functions During Respiratory Syncytial Virus Infection and Disease. Front. Immunol. 2019, 10, 548. [Google Scholar] [CrossRef] [Green Version]
- Kapur, R.; Einarsdottir, H.K.; Vidarsson, G. IgG-effector functions: "the good, the bad and the ugly". Immunol. Lett. 2014, 160, 139–144. [Google Scholar] [CrossRef]
- De Taeye, S.W.; Rispens, T.; Vidarsson, G. The Ligands for Human IgG and Their Effector Functions. Antibodies 2019, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Nimmerjahn, F.; Ravetch, J.V. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Gordan, S.; Lux, A. FcgammaR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activities. Trends Immunol. 2015, 36, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Getahun, A.; Cambier, J.C. Of ITIMs, ITAMs, and ITAMis: Revisiting immunoglobulin Fc receptor signaling. Immunol. Rev. 2015, 268, 66–73. [Google Scholar] [CrossRef] [Green Version]
- van der Heijden, J.; Breunis, W.B.; Geissler, J.; de Boer, M.; van den Berg, T.K.; Kuijpers, T.W. Phenotypic Variation in IgG Receptors by Nonclassical FCGR2C Alleles. J. Immunol. 2012, 188, 1318. [Google Scholar] [CrossRef] [Green Version]
- Bruhns, P.; Jönsson, F. Mouse and human FcR effector functions. Immunol. Rev. 2015, 268, 25–51. [Google Scholar] [CrossRef] [Green Version]
- Pasquier, B.; Launay, P.; Kanamaru, Y.; Moura, I.C.; Pfirsch, S.; Ruffié, C.; Hénin, D.; Benhamou, M.; Pretolani, M.; Blank, U.; et al. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: Dual role of FcRgamma ITAM. Immunity 2005, 22, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, N.M.; Einarsdóttir, H.K.; Stemerding, A.M.; Vidarsson, G. The multiple facets of FcRn in immunity. Immunol. Rev. 2015, 268, 253–268. [Google Scholar] [CrossRef]
- Stapleton, N.M.; Andersen, J.T.; Stemerding, A.M.; Bjarnarson, S.P.; Verheul, R.C.; Gerritsen, J.; Zhao, Y.; Kleijer, M.; Sandlie, I.; de Haas, M.; et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat. Commun. 2011, 2, 599. [Google Scholar] [CrossRef] [Green Version]
- Vidarsson, G.; Stemerding, A.M.; Stapleton, N.M.; Spliethoff, S.E.; Janssen, H.; Rebers, F.E.; de Haas, M.; van de Winkel, J.G. FcRn: An IgG receptor on phagocytes with a novel role in phagocytosis. Blood 2006, 108, 3573–3579. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, D.A.; Isenberg, D.A. TRIM21 and the Function of Antibodies inside Cells. Trends Immunol. 2017, 38, 916–926. [Google Scholar] [CrossRef] [Green Version]
- den Dunnen, J.; Vogelpoel, L.T.; Wypych, T.; Muller, F.J.; de Boer, L.; Kuijpers, T.W.; Zaat, S.A.; Kapsenberg, M.L.; de Jong, E.C. IgG opsonization of bacteria promotes Th17 responses via synergy between TLRs and FcgammaRIIa in human dendritic cells. Blood 2012, 120, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Newling, M.; Hoepel, W.; Vogelpoel, L.T.C.; Heineke, M.H.; van Burgsteden, J.A.; Taanman-Kueter, E.W.M.; Eggink, D.; Kuijpers, T.W.; Beaumont, T.; van Egmond, M.; et al. Fc gamma receptor IIa suppresses type I and III interferon production by human myeloid immune cells. Eur. J. Immunol. 2018, 48, 1796–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelpoel, L.T.; Baeten, D.L.; de Jong, E.C.; den Dunnen, J. Control of cytokine production by human fc gamma receptors: Implications for pathogen defense and autoimmunity. Front. Immunol. 2015, 6, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Mkaddem, S.; Benhamou, M.; Monteiro, R.C. Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools. Front. Immunol. 2019, 10, 811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, A.K. Human CD4+ T-Cells: A Role for Low-Affinity Fc Receptors. Front. Immunol. 2016, 7, 215. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jönsson, F. Expression, Role, and Regulation of Neutrophil Fcγ Receptors. Front. Immunol. 2019, 10, 1958. [Google Scholar] [CrossRef]
- Devaraj, S.; Du Clos Terry, W.; Jialal, I. Binding and Internalization of C-Reactive Protein by Fcgamma Receptors on Human Aortic Endothelial Cells Mediates Biological Effects. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1359–1363. [Google Scholar] [CrossRef] [Green Version]
- Cheeseman, H.M.; Carias, A.M.; Evans, A.B.; Olejniczak, N.J.; Ziprin, P.; King, D.F.L.; Hope, T.J.; Shattock, R.J. Expression Profile of Human Fc Receptors in Mucosal Tissue: Implications for Antibody-Dependent Cellular Effector Functions Targeting HIV-1 Transmission. PLoS ONE 2016, 11, e0154656. [Google Scholar] [CrossRef] [Green Version]
- Golebski, K.; Hoepel, W.; van Egmond, D.; de Groot, E.J.; Amatngalim, G.D.; Beekman, J.M.; Fokkens, W.J.; van Drunen, C.M.; den Dunnen, J. FcgammaRIII stimulation breaks the tolerance of human nasal epithelial cells to bacteria through cross-talk with TLR4. Mucosal Immunol. 2019, 12, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Hoepel, W.; Newling, M.; Vogelpoel, L.T.C.; Sritharan, L.; Hansen, I.S.; Kapsenberg, M.L.; Baeten, D.L.P.; Everts, B.; den Dunnen, J. FcgammaR-TLR Cross-Talk Enhances TNF Production by Human Monocyte-Derived DCs via IRF5-Dependent Gene Transcription and Glycolytic Reprogramming. Front. Immunol. 2019, 10, 739. [Google Scholar] [CrossRef]
- Vogelpoel, L.T.; Hansen, I.S.; Visser, M.W.; Nagelkerke, S.Q.; Kuijpers, T.W.; Kapsenberg, M.L.; de Jong, E.C.; den Dunnen, J. FcgammaRIIa cross-talk with TLRs, IL-1R, and IFNgammaR selectively modulates cytokine production in human myeloid cells. Immunobiology 2015, 220, 193–199. [Google Scholar] [CrossRef]
- Hansen, I.S.; Hoepel, W.; Zaat, S.A.J.; Baeten, D.L.P.; den Dunnen, J. Serum IgA Immune Complexes Promote Proinflammatory Cytokine Production by Human Macrophages, Monocytes, and Kupffer Cells through FcalphaRI-TLR Cross-Talk. J. Immunol. 2017, 199, 4124–4131. [Google Scholar] [CrossRef] [Green Version]
- Sokolove, J.; Zhao, X.; Chandra, P.E.; Robinson, W.H. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcgamma receptor. Arthritis Rheumatol. 2011, 63, 53–62. [Google Scholar] [CrossRef]
- Bakema, J.E.; Tuk, C.W.; van Vliet, S.J.; Bruijns, S.C.; Vos, J.B.; Letsiou, S.; Dijkstra, C.D.; van, K.Y.; Brenkman, A.B.; Van Egmond, M. Antibody-opsonized bacteria evoke an inflammatory dendritic cell phenotype and polyfunctional Th cells by cross-talk between TLRs and FcRs. J. Immunol. 2015, 194, 1856–1866. [Google Scholar] [CrossRef] [Green Version]
- Vogelpoel, L.T.; Hansen, I.S.; Rispens, T.; Muller, F.J.; van Capel, T.M.; Turina, M.C.; Vos, J.B.; Baeten, D.L.; Kapsenberg, M.L.; de Jong, E.C.; et al. Fc gamma receptor-TLR cross-talk elicits pro-inflammatory cytokine production by human M2 macrophages. Nat. Commun. 2014, 5, 5444. [Google Scholar] [CrossRef] [Green Version]
- van der Poel, M.; Hoepel, W.; Hamann, J.; Huitinga, I.; Dunnen, J.d. IgG Immune Complexes Break Immune Tolerance of Human Microglia. J. Immunol. 2020, ji2000130. [Google Scholar] [CrossRef]
- Ben Mkaddem, S.; Hayem, G.; Jönsson, F.; Rossato, E.; Boedec, E.; Boussetta, T.; El Benna, J.; Launay, P.; Goujon, J.-M.; Benhamou, M.; et al. Shifting FcγRIIA-ITAM from activation to inhibitory configuration ameliorates arthritis. J. Clin. Investig. 2014, 124, 3945–3959. [Google Scholar] [CrossRef] [Green Version]
- Breedveld, A.; van Egmond, M. IgA and FcαRI: Pathological Roles and Therapeutic Opportunities. Front. Immunol. 2019, 10, 553. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Barreda, D.R.; Worth, R.G.; Indik, Z.K.; Kim, M.K.; Chien, P.; Schreiber, A.D. Differential kinase requirements in human and mouse Fc-gamma receptor phagocytosis and endocytosis. J. Leukoc. Biol. 2006, 80, 1553–1562. [Google Scholar] [CrossRef]
- Hepburn, A.L.; Mason, J.C.; Wang, S.; Shepherd, C.J.; Florey, O.; Haskard, D.O.; Davies, K.A. Both Fcgamma and complement receptors mediate transfer of immune complexes from erythrocytes to human macrophages under physiological flow conditions in vitro. Clin. Exp. Immunol. 2006, 146, 133–145. [Google Scholar] [CrossRef]
- Hansen, I.S.; Krabbendam, L.; Bernink, J.H.; Loayza-Puch, F.; Hoepel, W.; van Burgsteden, J.A.; Kuijper, E.C.; Buskens, C.J.; Bemelman, W.A.; Zaat, S.A.J.; et al. FcalphaRI co-stimulation converts human intestinal CD103(+) dendritic cells into pro-inflammatory cells through glycolytic reprogramming. Nat. Commun. 2018, 9, 863. [Google Scholar] [CrossRef] [Green Version]
- Pfeifle, R.; Rothe, T.; Ipseiz, N.; Scherer, H.U.; Culemann, S.; Harre, U.; Ackermann, J.A.; Seefried, M.; Kleyer, A.; Uderhardt, S.; et al. Regulation of autoantibody activity by the IL-23-T(H)17 axis determines the onset of autoimmune disease. Nat. Immunol. 2017, 18, 104–113. [Google Scholar] [CrossRef]
- Steffen, U.; Koeleman, C.A.; Sokolova, M.V.; Bang, H.; Kleyer, A.; Rech, J.; Unterweger, H.; Schicht, M.; Garreis, F.; Hahn, J.; et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat. Commun. 2020, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Gazendam, R.P.; van de Geer, A.; Roos, D.; van den Berg, T.K.; Kuijpers, T.W. How neutrophils kill fungi. Immunol. Rev. 2016, 273, 299–311. [Google Scholar] [CrossRef]
- Li, X.; Leonardi, I.; Semon, A.; Doron, I.; Gao, I.H.; Putzel, G.G.; Kim, Y.; Kabata, H.; Artis, D.; Fiers, W.D.; et al. Response to Fungal Dysbiosis by Gut-Resident CX3CR1(+) Mononuclear Phagocytes Aggravates Allergic Airway Disease. Cell Host Microbe 2018, 24, 847–856.e844. [Google Scholar] [CrossRef] [Green Version]
- Khoyratty, T.E.; Udalova, I.A. Diverse mechanisms of IRF5 action in inflammatory responses. Int J. Biochem. Cell Biol. 2018, 99, 38–42. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Myasoedova, V.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 2018, 223, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Krausgruber, T.; Blazek, K.; Smallie, T.; Alzabin, S.; Lockstone, H.; Sahgal, N.; Hussell, T.; Feldmann, M.; Udalova, I.A. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 2011, 12, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Blazek, K.; Byrne, A.J.; Perocheau, D.P.; Udalova, I.A. IRF5 is a specific marker of inflammatory macrophages in vivo. Mediat. Inflamm. 2013, 2013, 245804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; De, S.; Nelson, V.; Chopra, S.; LaPan, M.; Kampta, K.; Sun, S.; He, M.; Thompson, C.D.; Li, D.; et al. Inhibition of IRF5 hyper-activation protects from lupus onset and severity. J. Clin. Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Pandey, S.P.; Barnes, B.J.; Turner, J.R.; Abraham, C. T Cell-Intrinsic IRF5 Regulates T Cell Signaling, Migration, and Differentiation and Promotes Intestinal Inflammation. Cell Rep. 2020, 31, 107820. [Google Scholar] [CrossRef]
- Krausgruber, T.; Saliba, D.; Ryzhakov, G.; Lanfrancotti, A.; Blazek, K.; Udalova, I.A. IRF5 is required for late-phase TNF secretion by human dendritic cells. Blood 2010, 115, 4421–4430. [Google Scholar] [CrossRef]
- Tsokos, G.C.; Lo, M.S.; Costa Reis, P.; Sullivan, K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2016, 12, 716–730. [Google Scholar] [CrossRef]
- Ryzhakov, G.; Eames, H.L.; Udalova, I.A. Activation and function of interferon regulatory factor 5. J. Interferon Cytokine Res. 2015, 35, 71–78. [Google Scholar] [CrossRef]
- Hedl, M.; Yan, J.; Abraham, C. IRF5 and IRF5 Disease-Risk Variants Increase Glycolysis and Human M1 Macrophage Polarization by Regulating Proximal Signaling and Akt2 Activation. Cell Rep. 2016, 16, 2442–2455. [Google Scholar] [CrossRef] [Green Version]
- Hedl, M.; Yan, J.; Witt, H.; Abraham, C. IRF5 Is Required for Bacterial Clearance in Human M1-Polarized Macrophages, and IRF5 Immune-Mediated Disease Risk Variants Modulate This Outcome. J. Immunol. 2019, 202, 920–930. [Google Scholar] [CrossRef] [Green Version]
- Balkhi, M.Y.; Fitzgerald, K.A.; Pitha, P.M. Functional regulation of MyD88-activated interferon regulatory factor 5 by K63-linked polyubiquitination. Mol. Cell Biol. 2008, 28, 7296–7308. [Google Scholar] [CrossRef] [Green Version]
- Corbin, A.L.; Gomez-Vazquez, M.; Berthold, D.L.; Attar, M.; Arnold, I.C.; Powrie, F.M.; Sansom, S.N.; Udalova, I.A. IRF5 guides monocytes toward an inflammatory CD11c+ macrophage phenotype and promotes intestinal inflammation. Sci. Immunol. 2020, 5, eaax6085. [Google Scholar] [CrossRef]
- Heinz, L.X.; Lee, J.; Kapoor, U.; Kartnig, F.; Sedlyarov, V.; Papakostas, K.; César-Razquin, A.; Essletzbichler, P.; Goldmann, U.; Stefanovic, A.; et al. TASL is the SLC15A4-associated adaptor for IRF5 activation by TLR7–9. Nature 2020, 581, 316–322. [Google Scholar] [CrossRef]
- Irani, V.; Guy, A.J.; Andrew, D.; Beeson, J.G.; Ramsland, P.A.; Richards, J.S. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol. Immunol. 2015, 67, 171–182. [Google Scholar] [CrossRef]
- Knol, E.F. Requirements for effective IgE cross-linking on mast cells and basophils. Mol. Nutr. Food Res. 2006, 50, 620–624. [Google Scholar] [CrossRef]
- Suurmond, J.; Stoop, J.N.; Rivellese, F.; Bakker, A.M.; Huizinga, T.W.J.; Toes, R.E.M. Activation of human basophils by combined toll-like receptor- and FcεRI-triggering can promote Th2 skewing of naive T helper cells. Eur. J. Immunol. 2014, 44, 386–396. [Google Scholar] [CrossRef]
- Guilliams, M.; Bruhns, P.; Saeys, Y.; Hammad, H.; Lambrecht, B.N. The function of Fcgamma receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 2014, 14, 94–108. [Google Scholar] [CrossRef]
- Kubagawa, H.; Oka, S.; Kubagawa, Y.; Torii, I.; Takayama, E.; Kang, D.-W.; Gartland, G.L.; Bertoli, L.F.; Mori, H.; Takatsu, H.; et al. Identity of the elusive IgM Fc receptor (FcmuR) in humans. J. Exp. Med. 2009, 206, 2779–2793. [Google Scholar] [CrossRef] [Green Version]
- Nyamboya, R.A.; Sutton, B.J.; Calvert, R.A. Mapping of the binding site for FcμR in human IgM-Fc. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140266. [Google Scholar] [CrossRef]
- Chen, K.; Magri, G.; Grasset, E.K.; Cerutti, A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat. Rev. Immunol. 2020. [Google Scholar] [CrossRef]
- Shan, M.; Carrillo, J.; Yeste, A.; Gutzeit, C.; Segura-Garzón, D.; Walland, A.C.; Pybus, M.; Grasset, E.K.; Yeiser, J.R.; Matthews, D.B.; et al. Secreted IgD Amplifies Humoral T Helper 2 Cell Responses by Binding Basophils via Galectin-9 and CD44. Immunity 2018, 49, 709–724. [Google Scholar] [CrossRef] [Green Version]
- Hoepel, W.; Allahverdiyeva, S.; Harbiye, H.; de Taeye, S.W.; van der Ham, A.J.; de Boer, L.; Zaat, S.A.J.; van Weeghel, M.; Baeten, D.L.P.; Houtkooper, R.H.; et al. IgG Subclasses Shape Cytokine Responses by Human Myeloid Immune Cells through Differential Metabolic Reprogramming. J. Immunol. 2020, 205, 3400–3407. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, J.; Lu, H. Antibody glycosylation: Impact on antibody drug characteristics and quality control. Appl. Microbiol. Biotechnol. 2020, 104, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- de Taeye, S.W.; Bentlage, A.E.H.; Mebius, M.M.; Meesters, J.I.; Lissenberg-Thunnissen, S.; Falck, D.; Sénard, T.; Salehi, N.; Wuhrer, M.; Schuurman, J.; et al. FcγR Binding and ADCC Activity of Human IgG Allotypes. Front. Immunol. 2020, 11, 740. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.D.; de Graaf, E.L.; Sonneveld, M.E.; Plomp, H.R.; Nouta, J.; Hoepel, W.; Chen, H.-J.; Linty, F.; Visser, R.; Brinkhaus, M.; et al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science 2021, 371, eabc8378. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 2009, 8, 226–234. [Google Scholar] [CrossRef]
- Dekkers, G.; Rispens, T.; Vidarsson, G. Novel Concepts of Altered Immunoglobulin G Galactosylation in Autoimmune Diseases. Front. Immunol. 2018, 9, 553. [Google Scholar] [CrossRef] [Green Version]
- Biermann, M.H.C.; Griffante, G.; Podolska, M.J.; Boeltz, S.; Stürmer, J.; Muñoz, L.E.; Bilyy, R.; Herrmann, M. Sweet but dangerous—The role of immunoglobulin G glycosylation in autoimmunity and inflammation. Lupus 2016, 25, 934–942. [Google Scholar] [CrossRef]
- Jennewein, M.F.; Alter, G. The Immunoregulatory Roles of Antibody Glycosylation. Trends Immunol. 2017, 38, 358–372. [Google Scholar] [CrossRef]
- Bondt, A.; Nicolardi, S.; Jansen, B.C.; Kuijper, T.M.; Hazes, J.M.W.; van der Burgt, Y.E.M.; Wuhrer, M.; Dolhain, R.J.E.M. IgA N- and O-glycosylation profiling reveals no association with the pregnancy-related improvement in rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 160. [Google Scholar] [CrossRef] [Green Version]
- Hoepel, W.; Chen, H.-J.; Allahverdiyeva, S.; Manz, X.; Aman, J.; Bonta, P.; Brouwer, P.; de Taeye, S.; Caniels, T.; van der Straten, K.; et al. Anti-SARS-CoV-2 IgG from severely ill COVID-19 patients promotes macrophage hyper-inflammatory responses. Sci. Transl. Med. 2021, eabf8654. [Google Scholar] [CrossRef]
- Hoepel, W.; Golebski, K.; van Drunen, C.M.; den Dunnen, J. Active control of mucosal tolerance and inflammation by human IgA and IgG antibodies. J. Allergy Clin. Immunol. 2020, 146, 273–275. [Google Scholar] [CrossRef]
- den Dunnen, J.; Mes, L.; Hoepel, W.; Smolders, J. Multiple sclerosis: Why we should focus on both sides of the (auto)antibody. Neural Regen Res. 2021, 16, 2422–2424. [Google Scholar] [CrossRef]
- Du Clos, T.W. Pentraxins: Structure, function, and role in inflammation. Isrn Inflamm. 2013, 2013, 379040. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.; Singh, P.P.; Bottazzi, B.; Garlanda, C.; Mantovani, A. Pattern recognition by pentraxins. Adv. Exp. Med. Biol. 2009, 653, 98–116. [Google Scholar] [CrossRef] [Green Version]
- Daigo, K.; Inforzato, A.; Barajon, I.; Garlanda, C.; Bottazzi, B.; Meri, S.; Mantovani, A. Pentraxins in the activation and regulation of innate immunity. Immunol. Rev. 2016, 274, 202–217. [Google Scholar] [CrossRef] [Green Version]
- Mold, C.; Du Clos, T.W. C-reactive protein increases cytokine responses to Streptococcus pneumoniae through interactions with Fc gamma receptors. J. Immunol. 2006, 176, 7598–7604. [Google Scholar] [CrossRef] [Green Version]
- Volanakis, J.E.; Kaplan, M.H. Specificity of C-Reactive Protein for Choline Phosphate Residues of Pneumococcal C-Polysaccharide. Proc. Soc. Exp. Biol. Med. 1971, 136, 612–614. [Google Scholar] [CrossRef]
- Bodman-Smith, K.B.; Melendez, A.J.; Campbell, I.; Harrison, P.T.; Allen, J.M.; Raynes, J.G. C-reactive protein-mediated phagocytosis and phospholipase D signalling through the high-affinity receptor for immunoglobulin G (FcgammaRI). Immunology 2002, 107, 252–260. [Google Scholar] [CrossRef]
- Culley, F.J.; Harris, R.A.; Kaye, P.M.; McAdam, K.P.; Raynes, J.G. C-reactive protein binds to a novel ligand on Leishmania donovani and increases uptake into human macrophages. J. Immunol. 1996, 156, 4691–4696. [Google Scholar]
- Peisajovich, A.; Marnell, L.; Mold, C.; Du Clos, T.W. C-reactive protein at the interface between innate immunity and inflammation. Expert Rev. Clin. Immunol. 2008, 4, 379–390. [Google Scholar] [CrossRef]
- Rhodes, B.; Fürnrohr, B.G.; Vyse, T.J. C-reactive protein in rheumatology: Biology and genetics. Nat. Rev. Rheumatol. 2011, 7, 282–289. [Google Scholar] [CrossRef]
- Hart, S.P.; Alexander, K.M.; MacCall, S.M.; Dransfield, I. C-reactive protein does not opsonize early apoptotic human neutrophils, but binds only membrane-permeable late apoptotic cells and has no effect on their phagocytosis by macrophages. J. Inflamm. 2005, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Volanakis, J.E.; Wirtz, K.W. Interaction of C-reactive protein with artificial phosphatidylcholine bilayers. Nature 1979, 281, 155–157. [Google Scholar] [CrossRef]
- Li, Y.P.; Mold, C.; Du Clos, T.W. Sublytic complement attack exposes C-reactive protein binding sites on cell membranes. J. Immunol. 1994, 152, 2995–3005. [Google Scholar]
- Du Clos, T.W.; Marnell, L.; Zlock, L.R.; Burlingame, R.W. Analysis of the binding of C-reactive protein to chromatin subunits. J. Immunol. 1991, 146, 1220–1225. [Google Scholar] [PubMed]
- Du Clos, T.W.; Zlock, L.T.; Rubin, R.L. Analysis of the binding of C-reactive protein to histones and chromatin. J. Immunol. 1988, 141, 4266–4270. [Google Scholar] [PubMed]
- Newling, M.; Sritharan, L.; van der Ham, A.J.; Hoepel, W.; Fiechter, R.H.; de Boer, L.; Zaat, S.A.J.; Bisoendial, R.J.; Baeten, D.L.P.; Everts, B.; et al. C-Reactive Protein Promotes Inflammation through FcgammaR-Induced Glycolytic Reprogramming of Human Macrophages. J. Immunol. 2019, 203, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Diskin, C.; Pålsson-McDermott, E.M. Metabolic Modulation in Macrophage Effector Function. Front. Immunol. 2018, 9, 270. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, E.; Cuevas, V.D.; Fernández-Arroyo, S.; Riera-Borrull, M.; Orta-Zavalza, E.; Joven, J.; Rial, E.; Corbi, A.L.; Escribese, M.M. Reshaping of Human Macrophage Polarization through Modulation of Glucose Catabolic Pathways. J. Immunol. 2015, 195, 2442–2451. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Tang, D.; Lu, C.; Yang, J.; Lu, Y.; Wang, Y.; Jia, L.; Wang, J.; Ru, W.; Lu, Y.; et al. Irf5 deficiency in myeloid cells prevents necrotizing enterocolitis by inhibiting M1 macrophage polarization. Mucosal Immunol. 2019, 12, 888–896. [Google Scholar] [CrossRef]
- Castro-Dopico, T.; Dennison, T.W.; Ferdinand, J.R.; Mathews, R.J.; Fleming, A.; Clift, D.; Stewart, B.J.; Jing, C.; Strongili, K.; Labzin, L.I.; et al. Anti-commensal IgG Drives Intestinal Inflammation and Type 17 Immunity in Ulcerative Colitis. Immunity 2019, 50, 1099–1114.e1010. [Google Scholar] [CrossRef] [Green Version]
- Newling, M.; Fiechter, R.H.; Sritharan, L.; Hoepel, W.; van Burgsteden, J.A.; Hak, A.E.; van Vollenhoven, R.F.; van de Sande, M.G.H.; Baeten, D.L.P.; den Dunnen, J. Dysregulated Fcγ receptor IIa-induced cytokine production in dendritic cells of lupus nephritis patients. Clin. Exp. Immunol. 2020, 199, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, R.T.; Lynch, J.B.; del Rio, C. Mild or Moderate Covid-19. N. Engl. J. Med. 2020, 383, 1757–1766. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Dekkers, G.; Treffers, L.; Plomp, R.; Bentlage, A.E.H.; de Boer, M.; Koeleman, C.A.M.; Lissenberg-Thunnissen, S.N.; Visser, R.; Brouwer, M.; Mok, J.Y.; et al. Decoding the Human Immunoglobulin G-Glycan Repertoire Reveals a Spectrum of Fc-Receptor- and Complement-Mediated-Effector Activities. Front. Immunol. 2017, 8, 877. [Google Scholar] [CrossRef]
- Bye, A.P.; Hoepel, W.; Mitchell, J.L.; Jégouic, S.; Loureiro, S.; Sage, T.; de Taeye, S.; van Gils, M.; Kriek, N.; Cooper, N.; et al. Aberrant glycosylation of anti-SARS-CoV-2 IgG is a pro-thrombotic stimulus for platelets. bioRxiv 2021. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Q.; Lin, Q.; Fang, J.; Wang, H.; Kwok, H.; Tang, H.; Nishiura, K.; Peng, J.; Tan, Z.; et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 2019, 4, e123158. [Google Scholar] [CrossRef]
- Su, Z.; Xie, Q.; Wang, Y.; Li, Y. Abberant Immunoglobulin G Glycosylation in Rheumatoid Arthritis by LTQ-ESI-MS. Int. J. Mol. Sci. 2020, 21, 2045. [Google Scholar] [CrossRef] [Green Version]
- Harre, U.; Lang, S.C.; Pfeifle, R.; Rombouts, Y.; Frühbeißer, S.; Amara, K.; Bang, H.; Lux, A.; Koeleman, C.A.; Baum, W.; et al. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat. Commun. 2015, 6, 6651. [Google Scholar] [CrossRef]
- Wuhrer, M.; Selman, M.H.J.; McDonnell, L.A.; Kümpfel, T.; Derfuss, T.; Khademi, M.; Olsson, T.; Hohlfeld, R.; Meinl, E.; Krumbholz, M. Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J. Neuroinflamm. 2015, 12, 235. [Google Scholar] [CrossRef] [Green Version]
- Toong, C.; Adelstein, S.; Phan, T.G. Clearing the complexity: Immune complexes and their treatment in lupus nephritis. Int. J. Nephrol. Renovasc. Dis. 2011, 4, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef] [Green Version]
- Klareskog, L.; Ronnelid, J.; Lundberg, K.; Padyukov, L.; Alfredsson, L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu. Rev. Immunol. 2008, 26, 651–675. [Google Scholar] [CrossRef] [Green Version]
- El Bannoudi, H.; Ioan-Facsinay, A.; Toes, R.E. Bridging autoantibodies and arthritis: The role of fc receptors. Curr. Top. Microbiol. Immunol. 2014, 382, 303–319. [Google Scholar] [CrossRef]
- Means, T.K.; Latz, E.; Hayashi, F.; Murali, M.R.; Golenbock, D.T.; Luster, A.D. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Investig. 2005, 115, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Devarapu, S.K.; Anders, H.-J. Toll-like receptors in lupus nephritis. J. Biomed. Sci. 2018, 25, 35. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Park, S.H.; Kim, H.Y.; Kwok, S.K. A Plasmacytoid Dendritic Cells-Type I Interferon Axis Is Critically Implicated in the Pathogenesis of Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2015, 16, 14158–14170. [Google Scholar] [CrossRef]
- Kim, W.-U.; Sreih, A.; Bucala, R. Toll-like receptors in systemic lupus erythematosus; prospects for therapeutic intervention. Autoimmun. Rev. 2009, 8, 204–208. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.S.; Hemmer, B.; Cepok, S. The role of antibodies in multiple sclerosis. Biochim. Biophys. Acta 2011, 1812, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Mavragani, C.P.; Tzioufas, A.G.; Moutsopoulos, H.M. Sjögren’s syndrome: Autoantibodies to cellular antigens. Clinical and molecular aspects. Int. Arch. Allergy Immunol. 2000, 123, 46–57. [Google Scholar] [CrossRef]
- Pan, M.; Liu, X.; Zheng, J. The pathogenic role of autoantibodies in pemphigus vulgaris. Clin. Exp. Dermatol. 2011, 36, 703–707. [Google Scholar] [CrossRef]
- Melief, J.; Koning, N.; Schuurman, K.G.; Van De Garde, M.D.; Smolders, J.; Hoek, R.M.; Van Eijk, M.; Hamann, J.; Huitinga, I. Phenotyping primary human microglia: Tight regulation of LPS responsiveness. Glia 2012, 60, 1506–1517. [Google Scholar] [CrossRef]
- Kramer, J.M. Early events in Sjögren’s Syndrome pathogenesis: The importance of innate immunity in disease initiation. Cytokine 2014, 67, 92–101. [Google Scholar] [CrossRef]
- Mehra, S.; Walker, J.; Patterson, K.; Fritzler, M.J. Autoantibodies in systemic sclerosis. Autoimmun. Rev. 2013, 12, 340–354. [Google Scholar] [CrossRef]
- Tsokos, G.C. Systemic Lupus Erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, T.; Sato, G.R.; Tamura, T. Regulation and role of the transcription factor IRF5 in innate immune responses and systemic lupus erythematosus. Int. Immunol. 2018, 30, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito-Zerón, P.; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjögren syndrome. Nat. Rev. Dis. Primers 2016, 2, 16047. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, B.; Ma, B.; Nie, S. Association between IRF5 polymorphisms and autoimmune diseases: A meta-analysis. Genet. Mol. Res. 2014, 13, 4473–4485. [Google Scholar] [CrossRef] [PubMed]
- Rochereau, N.; Roblin, X.; Michaud, E.; Gayet, R.; Chanut, B.; Jospin, F.; Corthésy, B.; Paul, S. NOD2 deficiency increases retrograde transport of secretory IgA complexes in Crohn’s disease. Nat. Commun. 2021, 12, 261. [Google Scholar] [CrossRef]
- Chang, M.K.; Binder, C.J.; Torzewski, M.; Witztum, J.L. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proc. Natl. Acad. Sci. USA 2002, 99, 13043–13048. [Google Scholar] [CrossRef] [Green Version]
- Pilely, K.; Fumagalli, S.; Rosbjerg, A.; Genster, N.; Skjoedt, M.O.; Perego, C.; Ferrante, A.M.R.; De Simoni, M.G.; Garred, P. C-Reactive Protein Binds to Cholesterol Crystals and Co-Localizes with the Terminal Complement Complex in Human Atherosclerotic Plaques. Front. Immunol. 2017, 8, 1040. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Rose, L.; Buring, J.E.; Cook, N.R. Comparison of C-Reactive Protein and Low-Density Lipoprotein Cholesterol Levels in the Prediction of First Cardiovascular Events. N. Engl. J. Med. 2002, 347, 1557–1565. [Google Scholar] [CrossRef] [Green Version]
- Oliviero, F.; Lo Nigro, A.; Bernardi, D.; Giunco, S.; Baldo, G.; Scanu, A.; Sfriso, P.; Ramonda, R.; Plebani, M.; Punzi, L. A comparative study of serum and synovial fluid lipoprotein levels in patients with various arthritides. Clin. Chim. Acta 2012, 413, 303–307. [Google Scholar] [CrossRef]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [Green Version]
- Khoury, D.S.; Aogo, R.; Randriafanomezantsoa-Radohery, G.; McCaw, J.M.; Simpson, J.A.; McCarthy, J.S.; Haque, A.; Cromer, D.; Davenport, M.P. Within-host modeling of blood-stage malaria. Immunol. Rev. 2018, 285, 168–193. [Google Scholar] [CrossRef]
- Ventura, J.D. Human Immunodeficiency Virus 1 (HIV-1): Viral Latency, the Reservoir, and the Cure. Yale J. Biol. Med. 2020, 93, 549–560. [Google Scholar]
- Chan, A.C.; Carter, P.J. Therapeutic antibodies for autoimmunity and inflammation. Nat. Rev. Immunol. 2010, 10, 301–316. [Google Scholar] [CrossRef]
- Westhrin, M.; Kovcic, V.; Zhang, Z.; Moen, S.H.; Nedal, T.M.V.; Bondt, A.; Holst, S.; Misund, K.; Buene, G.; Sundan, A.; et al. Monoclonal immunoglobulins promote bone loss in multiple myeloma. Blood 2020, 136, 2656–2666. [Google Scholar] [CrossRef]
- Castro-Dopico, T.; Clatworthy, M.R. Mucosal IgG in inflammatory bowel disease—A question of (sub)class? Gut Microbes 2020, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hussain, K.; Hargreaves, C.E.; Rowley, T.F.; Sopp, J.M.; Latham, K.V.; Bhatta, P.; Sherington, J.; Cutler, R.M.; Humphreys, D.P.; Glennie, M.J.; et al. Impact of Human FcγR Gene Polymorphisms on IgG-Triggered Cytokine Release: Critical Importance of Cell Assay Format. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Beretta, L.; Santaniello, A.; van Riel, P.L.C.M.; Coenen, M.J.H.; Scorza, R. Survival dimensionality reduction (SDR): Development and clinical application of an innovative approach to detect epistasis in presence of right-censored data. BMC Bioinform. 2010, 11, 416. [Google Scholar] [CrossRef] [Green Version]
- Almuttaqi, H.; Udalova, I.A. Advances and challenges in targeting IRF5, a key regulator of inflammation. FEBS J. 2019, 286, 1624–1637. [Google Scholar] [CrossRef]
- Banga, J.; Srinivasan, D.; Sun, C.-C.; Thompson, C.D.; Milletti, F.; Huang, K.-S.; Hamilton, S.; Song, S.; Hoffman, A.F.; Qin, Y.G.; et al. Inhibition of IRF5 cellular activity with cell-penetrating peptides that target homodimerization. Sci. Adv. 2020, 6, eaay1057. [Google Scholar] [CrossRef]
- Thompson, C.D.; Matta, B.; Barnes, B.J. Therapeutic Targeting of IRFs: Pathway-Dependence or Structure-Based? Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Duffau, P.; Menn-Josephy, H.; Cuda, C.M.; Dominguez, S.; Aprahamian, T.R.; Watkins, A.A.; Yasuda, K.; Monach, P.; Lafyatis, R.; Rice, L.M.; et al. Promotion of Inflammatory Arthritis by Interferon Regulatory Factor 5 in a Mouse Model. Arthritis Rheumatol. 2015, 67, 3146–3157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, D.; Yang, L.; Bi, X.; Stone, R.C.; Patel, P.; Barnes, B.J. Irf5-deficient mice are protected from pristane-induced lupus via increased Th2 cytokines and altered IgG class switching. Eur. J. Immunol. 2012, 42, 1477–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, A.J.; Weiss, M.; Mathie, S.A.; Walker, S.A.; Eames, H.L.; Saliba, D.; Lloyd, C.M.; Udalova, I.A. A critical role for IRF5 in regulating allergic airway inflammation. Mucosal Immunol. 2017, 10, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.P.; Yan, J.; Turner, J.R.; Abraham, C. Reducing IRF5 expression attenuates colitis in mice, but impairs the clearance of intestinal pathogens. Mucosal Immunol. 2019, 12, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, H.; Koh, J.; Ko, J.S.; Yoon, B.R.; Jeon, Y.K.; Cho, Y.M.; Kim, T.H.; Suh, Y.S.; Lee, H.J.; et al. Cytosolic Pellino-1-Mediated K63-Linked Ubiquitination of IRF5 in M1 Macrophages Regulates Glucose Intolerance in Obesity. Cell Rep. 2017, 20, 832–845. [Google Scholar] [CrossRef] [Green Version]
- Lazzari, E.; Korczeniewska, J.; Ní Gabhann, J.; Smith, S.; Barnes, B.J.; Jefferies, C.A. TRIpartite Motif 21 (TRIM21) Differentially Regulates the Stability of Interferon Regulatory Factor 5 (IRF5) Isoforms. PLoS ONE 2014, 9, e103609. [Google Scholar] [CrossRef]
- McKeage, K.; Lyseng-Williamson, K.A. Fostamatinib in chronic immune thrombocytopenia: A profile of its use in the USA. Drugs Ther. Perspect 2018, 34, 451–456. [Google Scholar] [CrossRef]
- McAdoo, S.; Tam, F.W.K. Role of the Spleen Tyrosine Kinase Pathway in Driving Inflammation in IgA Nephropathy. Semin. Nephrol. 2018, 38, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.K.-W.; McAdoo, S.P.; Tam, F.W.K. Targeting the tyrosine kinase signalling pathways for treatment of immune-mediated glomerulonephritis: From bench to bedside and beyond. Nephrol. Dial. Transplant. 2017, 32, i129–i138. [Google Scholar] [CrossRef]
- Monach, P.A.; Hueber, W.; Kessler, B.; Tomooka, B.H.; BenBarak, M.; Simmons, B.P.; Wright, J.; Thornhill, T.S.; Monestier, M.; Ploegh, H.; et al. A broad screen for targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2009, 106, 15867. [Google Scholar] [CrossRef] [Green Version]
- Kristjansdottir, G.; Sandling, J.K.; Bonetti, A.; Roos, I.M.; Milani, L.; Wang, C.; Gustafsdottir, S.M.; Sigurdsson, S.; Lundmark, A.; Tienari, P.J.; et al. Interferon regulatory factor 5 (IRF5) gene variants are associated with multiple sclerosis in three distinct populations. J. Med. Genet. 2008, 45, 362–369. [Google Scholar] [CrossRef]
- Vandenbroeck, K.; Alloza, I.; Swaminathan, B.; Antigüedad, A.; Otaegui, D.; Olascoaga, J.; Barcina, M.G.; de las Heras, V.; Bartolomé, M.; Fernández-Arquero, M.; et al. Validation of IRF5 as multiple sclerosis risk gene: Putative role in interferon beta therapy and human herpes virus-6 infection. Genes Immun. 2011, 12, 40–45. [Google Scholar] [CrossRef]
- Holtman, I.R.; Skola, D.; Glass, C.K. Transcriptional control of microglia phenotypes in health and disease. J. Clin. Investig. 2017, 127, 3220–3229. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geyer, C.E.; Mes, L.; Newling, M.; den Dunnen, J.; Hoepel, W. Physiological and Pathological Inflammation Induced by Antibodies and Pentraxins. Cells 2021, 10, 1175. https://doi.org/10.3390/cells10051175
Geyer CE, Mes L, Newling M, den Dunnen J, Hoepel W. Physiological and Pathological Inflammation Induced by Antibodies and Pentraxins. Cells. 2021; 10(5):1175. https://doi.org/10.3390/cells10051175
Chicago/Turabian StyleGeyer, Chiara Elisabeth, Lynn Mes, Melissa Newling, Jeroen den Dunnen, and Willianne Hoepel. 2021. "Physiological and Pathological Inflammation Induced by Antibodies and Pentraxins" Cells 10, no. 5: 1175. https://doi.org/10.3390/cells10051175
APA StyleGeyer, C. E., Mes, L., Newling, M., den Dunnen, J., & Hoepel, W. (2021). Physiological and Pathological Inflammation Induced by Antibodies and Pentraxins. Cells, 10(5), 1175. https://doi.org/10.3390/cells10051175





