Adipose-Derived Mesenchymal Stromal Cells Treated with Interleukin 1 Beta Produced Chondro-Protective Vesicles Able to Fast Penetrate in Cartilage
Abstract
1. Introduction
2. Materials and Methods
2.1. ASCs Isolation, Expansion and Cartilage Collection
2.2. EVs Isolation
2.3. EV-Embedded miRNA Expression
2.4. miRNA Data Normalization
2.5. miRNA Differential Expression Analysis
2.6. miRNA Target Prediction Analysis
2.7. Time-Lapse Microscopy for EVs Incorporation in Cartilage
2.8. Multimodal Microscopy Data Analysis
3. Results
3.1. IL-1β Preconditioning Modulated the Expression of a Small Amount of miRNAs
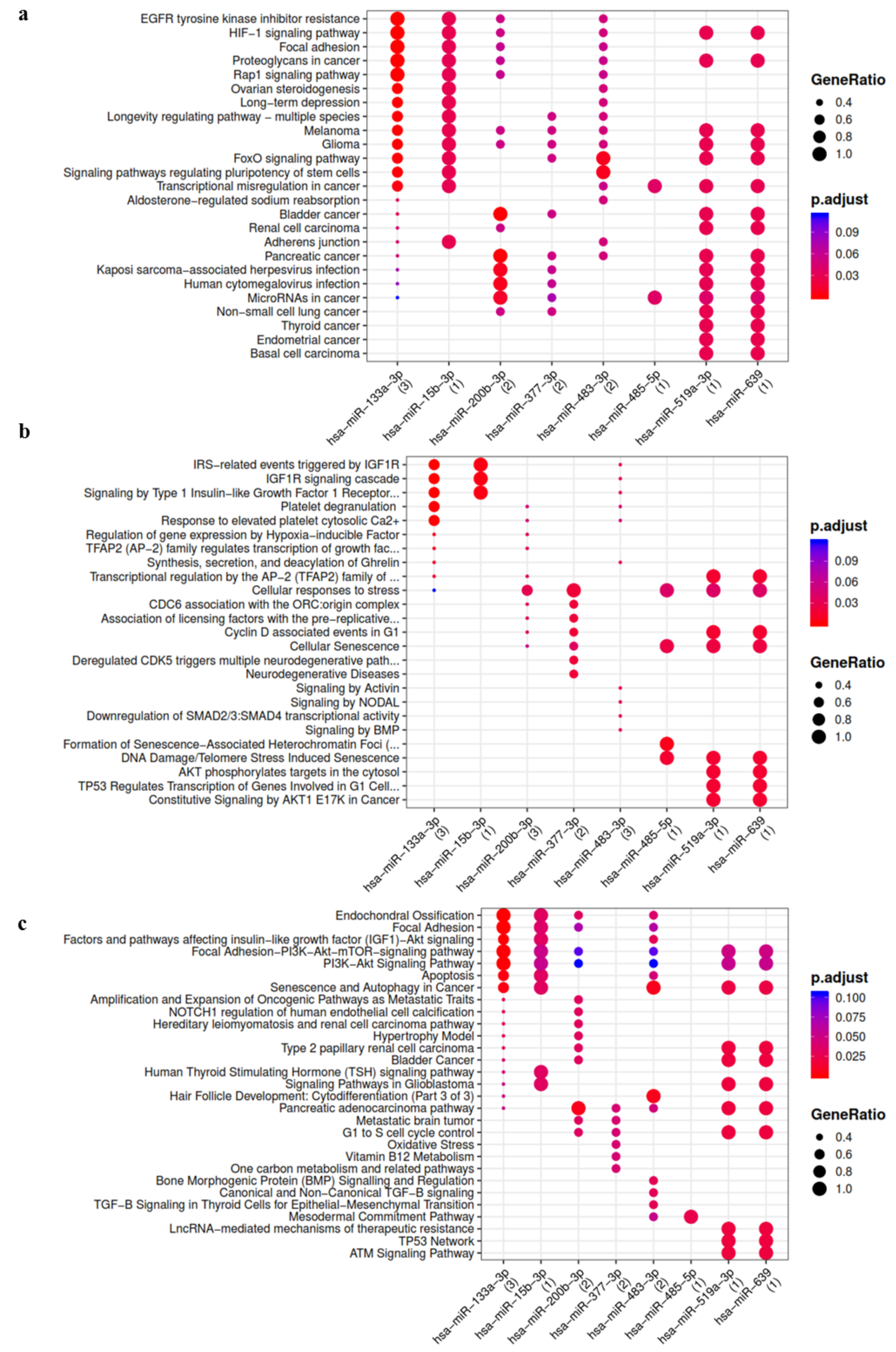
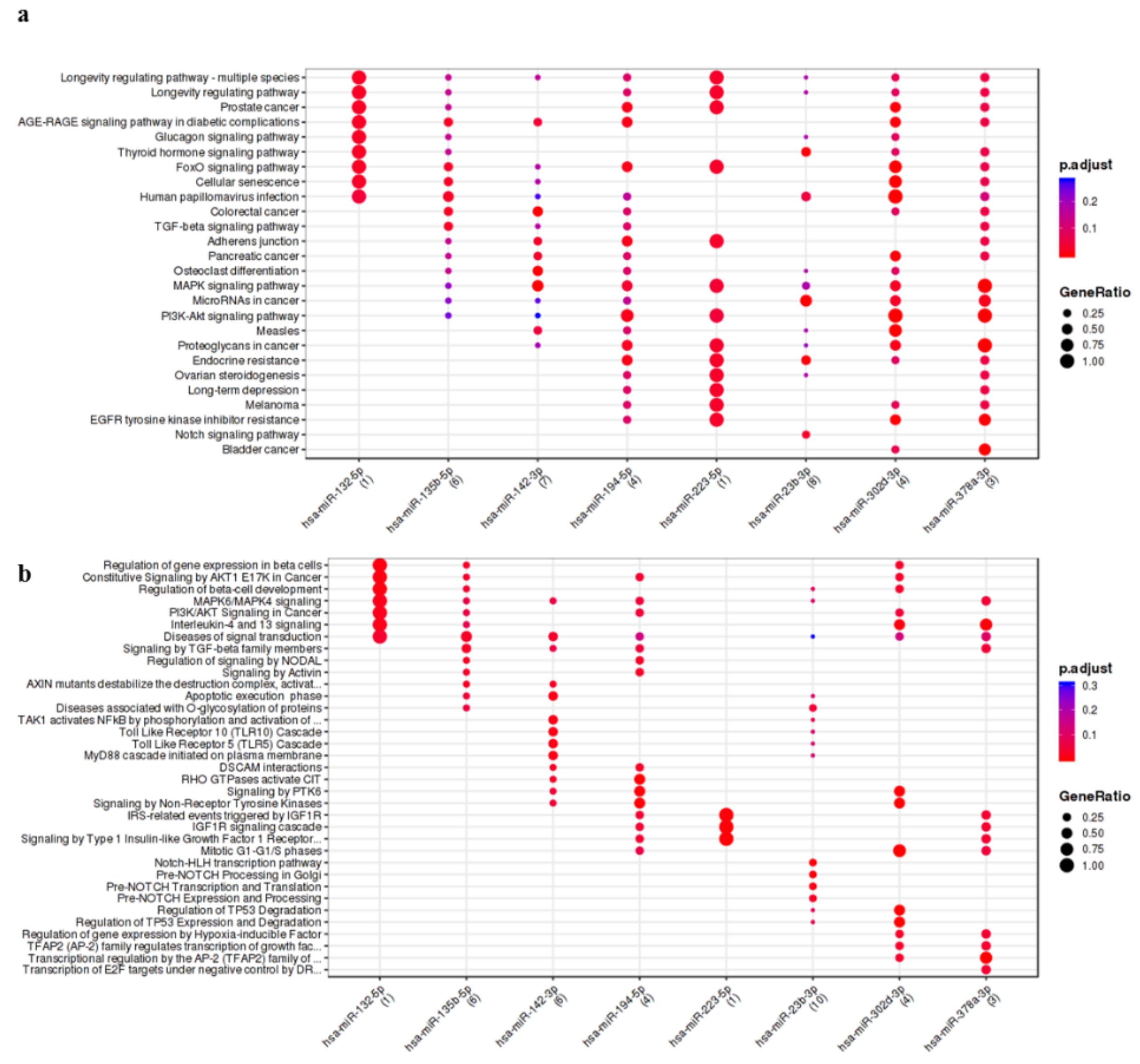
3.2. IL-1β Up-Regulated/Activated miRNAs Showed Interaction with Pathways and Process Relevant to OA
3.3. OA Focused Analysis Revealed Few miRNAs with a Key Role in the Wnt Pathway and Inflammatory Response
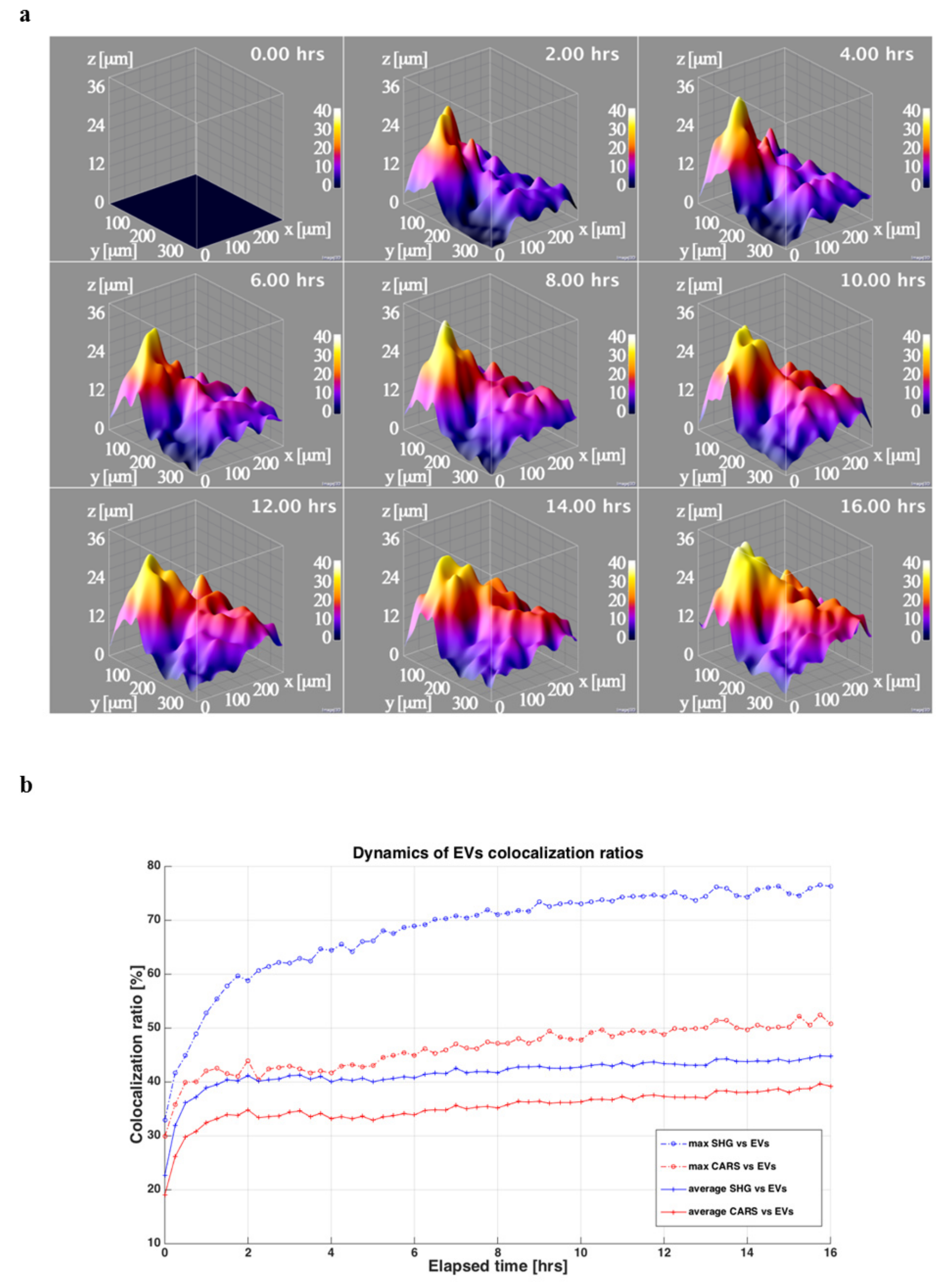
3.4. ASC-EVs Diffuse in Both Cells and Surrounding Matrix with Differential Kinetics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Lopa, S.; Colombini, A.; Moretti, M.; de Girolamo, L. Injective mesenchymal stem cell-based treatments for knee os-teoarthritis: From mechanisms of action to current clinical evidences. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2003–2020. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; Kouroupis, D.; Viganò, M.; Perucca-Orfei, C.; Kaplan, L.; Zagra, L.; de Girolamo, L.; Correa, D.; Colombini, A. Human Diseased Articular Cartilage Con-tains a Mesenchymal Stem Cell-Like Population of Chondroprogenitors with Strong Immunomodulatory Responses. J. Clin. Med. 2019, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1beta signaling in osteoarthritis—chondrocytes in focus. Cell Signal 2019, 53, 212–223. [Google Scholar] [CrossRef]
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Colombini, A.; Viganò, M.; de Girolamo, L. Secreted Factors and EV-miRNAs Orchestrate the Healing Capacity of Adipose Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 1582. [Google Scholar] [CrossRef]
- Colombini, A.; Orfei, C.P.; Kouroupis, D.; Ragni, E.; De Luca, P.; Viganò, M.; Correa, D.; de Girolamo, L. Mesenchymal stem cells in the treatment of articular cartilage degeneration: New biological insights for an old-timer cell. Cytotherapy 2019, 21, 1179–1197. [Google Scholar] [CrossRef]
- Kouroupis, D.; Sanjurjo-Rodriguez, C.; Jones, E.; Correa, D. Mesenchymal Stem Cell Functionalization for Enhanced Therapeutic Applications. Tissue Eng. Part B Rev. 2019, 25, 55–77. [Google Scholar] [CrossRef]
- Cheng, A.; Choi, D.; Lora, M.; Shum-Tim, D.; Rak, J.; Colmegna, I. Human multipotent mesenchymal stromal cells cytokine prim-ing promotes RAB27B-regulated secretion of small extracellular vesicles with immunomodulatory cargo. Stem Cell Res. Ther. 2020, 11, 539. [Google Scholar] [CrossRef]
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Mondadori, C.; Viganò, M.; Colombini, A.; de Girolamo, L. Inflammatory priming enhances mesenchymal stromal cell secretome potential as a clinical product for regenerative medicine approaches through secreted factors and EV-miRNAs: The example of joint disease. Stem Cell Res. Ther. 2020, 11, 165. [Google Scholar] [CrossRef]
- Sondag, G.R.; Haqqi, T.M. The Role of MicroRNAs and Their Targets in Osteoarthritis. Curr. Rheumatol. Rep. 2016, 18, 1–15. [Google Scholar] [CrossRef]
- Beyer, C.; Zampetaki, A.; Lin, N.-Y.; Kleyer, A.; Perricone, C.; Iagnocco, A.; Distler, A.; Langley, S.R.; Gelse, K.; Sesselmann, S.; et al. Signature of circulating microRNAs in osteoarthritis. Ann. Rheum. Dis. 2014, 74, e18. [Google Scholar] [CrossRef]
- Nugent, M. MicroRNAs: Exploring new horizons in osteoarthritis. Osteoarthr. Cartil. 2016, 24, 573–580. [Google Scholar] [CrossRef]
- Mortati, L.; de Girolamo, L.; Perucca Orfei, C.; Viganò, M.; Brayda-Bruno, M.; Ragni, E.; Colombini, A. In Vitro Study of Extracellular Vesicles Migration in Cartilage-Derived Osteoarthritis Samples Using Real-Time Quantitative Multimodal Nonlinear Optics Imaging. Pharmaceutics 2020, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Stanco, D.; De Girolamo, L.; Sansone, V.; Moretti, M. Donor-matched mesenchymal stem cells from knee infrapatellar and subcutaneous adipose tissue of osteoarthritic donors display differential chondrogenic and osteogenic commitment. Eur. Cells Mater. 2014, 27, 298–311. [Google Scholar] [CrossRef]
- Ragni, E.; Colombini, A.; De Luca, P.; Libonati, F.; Viganò, M.; Orfei, C.P.; Zagra, L.; de Girolamo, L. miR-103a-3p and miR-22-5p Are Reliable Reference Genes in Extracellular Vesicles from Cartilage, Adipose Tissue, and Bone Marrow Cells. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- Ragni, E.; Orfei, C.P.; De Luca, P.; Lugano, G.; Viganò, M.; Colombini, A.; Valli, F.; Zacchetti, D.; Bollati, V.; de Girolamo, L. Interaction with hyaluronan matrix and miRNA cargo as contributors for in vitro potential of mesenchymal stem cell-derived extracellular vesicles in a model of human osteoar-thritic synoviocytes. Stem Cell Res. Ther. 2019, 10, 109. [Google Scholar] [CrossRef]
- D’haene, B.; Mestdagh, P.; Hellemans, J.; Vandesompele, J. miRNA expression profiling: From reference genes to global mean normalization. Methods Mol. Biol. 2012, 822, 261–272. [Google Scholar] [PubMed]
- Licursi, V.; Conte, F.; Fiscon, G.; Paci, P. Mienturnet: An interactive web tool for microRNA-target enrichment and network-based analysis. BMC Bioinf. 2019, 20, 545. [Google Scholar] [CrossRef]
- Mortati, L.; Divieto, C.; Sassi, M.P. CARS and SHG microscopy to follow collagen production in living human corneal fibro-blasts and mesenchymal stem cells in fibrin hydrogel 3D cultures. J. Raman Spectrosc. 2012, 43, 675–680. [Google Scholar] [CrossRef]
- Nazari-Shafti, T.Z.; Neuber, S.; Duran, A.G.; Exarchos, V.; Beez, C.M.; Meyborg, H.; Krüger, K.; Wolint, P.; Buschmann, J.; Böni, R.; et al. MiRNA Profiles of Extracellular Vesicles Secreted by Mesenchymal Stromal Cells—Can They Predict Potential Off-Target Effects? Biomolecules 2020, 10, 1353. [Google Scholar] [CrossRef]
- Rousseau, J.C.; Millet, M.; Croset, M.; Sornay-Rendu, E.; Borel, O.; Chapurlat, R. Association of circulating microRNAs with preva-lent and incident knee osteoarthritis in women: The OFELY study. Arthritis Res. Ther. 2020, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Skrzypa, M.; Szala, D.; Gablo, N.; Czech, J.; Pajak, J.; Kopanska, M.; Trzeciak, M.; Gargasz, K.; Snela, S.; Zawlik, I. miRNA-146a-5p is upregulated in serum and cartilage samples of patients with osteoarthritis. Pol. J. Surg. 2018, 91, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kopańska, M.; Szala, D.; Czech, J.; Gabło, N.; Gargasz, K.; Trzeciak, M.; Zawlik, I.; Snela, S. MiRNA expression in the cartilage of patients with osteoarthritis. J. Orthop. Surg. Res. 2017, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Nakasa, T.; Miyaki, S.; Ishikawa, M.; Deie, M.; Adachi, N.; Yasunaga, Y.; Asahara, H.; Ochi, M. Expression of MicroRNA-146a in osteoarthritis cartilage. Arthritis Rheum. 2009, 60, 1035–1041. [Google Scholar] [CrossRef]
- Jones, S.W.; Watkins, G.; Le Good, N.; Roberts, S.; Murphy, C.L.; Brockbank, S.M.V.; Needham, M.R.C.; Read, S.J.; Newham, P. The identification of differentially expressed mi-croRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage 2009, 17, 464–472. [Google Scholar] [CrossRef]
- Wu, C.; Tian, B.; Qu, X.; Liu, F.; Tang, T.; Qin, A.; Zhu, Z.; Dai, K. MicroRNAs play a role in chondrogenesis and osteoarthritis (Review). Int. J. Mol. Med. 2014, 34, 13–23. [Google Scholar] [CrossRef]
- Li, X.; Gibson, G.; Kim, J.S.; Kroin, J.; Xu, S.; Van Wijnen, A.J.; Im, H.J. MicroRNA-146a is linked to pain-related pathophysiology of osteoarthritis. Gene 2011, 480, 34–41. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Dai, L.; Yu, D.; Chen, Q.; Zhang, X.; Dai, K. miR-146a, an IL-1β responsive miRNA, induces vascular endothelial growth factor and chondrocyte apoptosis by targeting Smad4. Arthritis Res. Ther. 2012, 14, R75. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, J.; Jing, W.; Yan, S.; Wang, X.; Xiao, C.; Ma, B. Role of miR-146a in human chondrocyte apoptosis in response to mechan-ical pressure injury in vitro. Int. J. Mol. Med. 2014, 34, 451–463. [Google Scholar] [CrossRef]
- Stelcer, E.; Kulcenty, K.; Rucinski, M.; Jopek, K.; Richter, M.; Trzeciak, T.; Suchorska, W.M. The Role of MicroRNAs in Early Chondrogenesis of Human Induced Pluripotent Stem Cells (hiPSCs). Int. J. Mol. Sci. 2019, 20, 4371. [Google Scholar] [CrossRef]
- Stanczyk, J.; Pedrioli, D.M.L.; Brentano, F.; Sanchez-Pernaute, O.; Kolling, C.; Gay, R.E.; Detmar, M.; Gay, S.; Kyburz, D. Altered expression of MicroRNA in syno-vial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum 2008, 58, 1001–1009. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.-J.; Cimino, A.; Costinean, S.; Dumitru, C.D.; Adair, B.; Fabbri, M.; Alder, H.; Liu, C.G.; Calin, G.A.; et al. Modulation of miR-155 and miR-125b Levels following Lipopolysaccharide/TNF-α Stimulation and Their Possible Roles in Regulating the Response to Endotoxin Shock. J. Immunol. 2007, 179, 5082–5089. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Malizos, K.N.; Oikonomou, P.; Tsezou, A. Integrative MicroRNA and Proteomic Approaches Identify Novel Osteoarthritis Genes and Their Collaborative Metabolic and Inflammatory Networks. PLoS ONE 2008, 3, e3740. [Google Scholar] [CrossRef]
- McAlinden, A.; Varghese, N.; Wirthlin, L.; Chang, L.-W. Differentially Expressed MicroRNAs in Chondrocytes from Distinct Regions of Developing Human Cartilage. PLoS ONE 2013, 8, e75012. [Google Scholar] [CrossRef]
- Qi, Y.; Ma, N.; Yan, F.; Yu, Z.; Wu, G.; Qiao, Y.; Han, D.; Xiang, Y.; Li, F.; Wang, W.; et al. The expression of intronic miRNAs, miR-483 and miR-483*, and their host gene, Igf2, in murine osteoarthritis cartilage. Int. J. Biol. Macromol. 2013, 61, 43–49. [Google Scholar] [CrossRef]
- Díaz-Prado, S.; Cicione, C.; Muiños-López, E.; Hermida-Gómez, T.; Oreiro, N.; Fernández-López, C.; Blanco, F.J. Characterization of microRNA expres-sion profiles in normal and osteoarthritic human chondrocytes. BMC Musculoskelet Disord. 2012, 13, 144. [Google Scholar] [CrossRef]
- Akhtar, N.; Rasheed, Z.; Ramamurthy, S.; Anbazhagan, A.N.; Voss, F.R.; Haqqi, T.M. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010, 62, 1361–1371. [Google Scholar] [CrossRef]
- Park, S.J.; Cheon, E.J.; Lee, M.H.; Kim, H.A. MicroRNA-127-5p regulates matrix metalloproteinase 13 expression and inter-leukin-1beta-induced catabolic effects in human chondrocytes. Arthritis Rheum. 2013, 65, 3141–3152. [Google Scholar] [CrossRef]
- Ham, O.; Lee, C.Y.; Song, B.W.; Lee, S.Y.; Kim, R.; Park, J.H.; Lee, J.; Seo, H.-H.; Lee, C.Y.; Chung, Y.-A.; et al. Upregulation of miR-23b enhances the autologous therapeutic poten-tial for degenerative arthritis by targeting PRKACB in synovial fluid-derived mesenchymal stem cells from patients. Mol. Cells 2014, 37, 449–456. [Google Scholar] [CrossRef]
- Li, Y.-H.; Tavallaee, G.; Tokar, T.; Nakamura, A.; Sundararajan, K.; Weston, A.; Sharma, A.; Mahomed, N.; Gandhi, R.; Jurisica, I.; et al. Identification of synovial fluid microRNA signature in knee osteoarthritis: Differentiating early- and late-stage knee osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1577–1586. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Xing, L.; Tian, F. Wnt signaling: A promising target for osteoarthritis therapy. Cell Commun. Signal. 2019, 17, 1–14. [Google Scholar] [CrossRef]
- Fan, Z.; Yang, H.; Bau, B.; Soder, S.; Aigner, T. Role of mitogen-activated protein kinases and NFkappaB on IL-1beta-induced effects on collagen type II, MMP-1 and 13 mRNA expression in normal articular human chondrocytes. Rheumatol. Int. 2006, 26, 900–903. [Google Scholar] [CrossRef]
- Zhong, L.; Schivo, S.; Huang, X.; Leijten, J.; Karperien, M.; Post, J.N. Nitric Oxide Mediates Crosstalk between Interleukin 1beta and WNT Signaling in Primary Human Chondrocytes by Reducing DKK1 and FRZB Expression. Int. J. Mol. Sci. 2017, 18, 2491. [Google Scholar] [CrossRef]
- Kim, H.A.; Cho, M.-L.; Choi, H.Y.; Yoon, C.S.; Jhun, J.Y.; Oh, H.J.; Kim, H.-Y. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006, 54, 2152–2163. [Google Scholar] [CrossRef]
- Ying, W.; Yuan, F.; He, P.; Ji, P. Inhibition of Notch1 protects against IL-1beta-induced inflammation and cartilage de-struction in temporomandibular chondrocytes. Mol. Med. Rep. 2017, 15, 4391–4397. [Google Scholar] [CrossRef]
- Ha, Y.J.; Choi, Y.S.; Kang, E.H.; Shin, K.; Kim, T.K.; Song, Y.W.; Lee, Y.J. SOCS1 suppresses IL-1beta-induced C/EBPbeta expression via transcriptional regulation in human chondrocytes. Exp. Mol. Med. 2016, 48, e241. [Google Scholar] [CrossRef]


| Up-Regulated | Down-Regulated | ||||
|---|---|---|---|---|---|
| miRNA | Fc | p-Value | miRNA | Fc | p-Value |
| miR-125a-3p | 2.2 | 0.04 | miR-191-3p | 0.4 | 0.02 |
| miR-134-5p | 5.7 | 0.007 | miR-500a-5p | 0.5 | 0.05 |
| miR-222-5p | 3.0 | 0.02 | miR-656-3p | 0.3 | 0.01 |
| miR-146a-5p | 33.2 | 0.004 | miR-1265 | 0.5 | 0.04 |
| miR-155-5p | 4.6 | 0.02 | |||
| miR-196b-5p | 2.4 | 0.003 | |||
| miR-520c-3p | 32.2 | 0.05 | |||
| Activated | Silenced |
|---|---|
| let-7f-2-3p | hsa-let-7f-1-3p |
| let-7i-3p | miR-132-5p |
| miR-100-3p | miR-122-3p |
| miR-10a-3p | miR-130b-5p |
| miR-127-5p | miR-135b-5p |
| miR-1282 | miR-142-3p |
| miR-1290 | miR-187-3p |
| miR-1304-5p | miR-194-5p |
| miR-1324 | miR-223-5p |
| miR-133a | miR-23b-3p |
| miR-15b-3p | miR-302d-3p |
| miR-181c-3p | miR-378a-3p |
| miR-200b-3p | miR-518d-3p |
| miR-29b-2-5p | miR-566 |
| miR-326 | miR-589-3p |
| miR-361-3p | miR-623 |
| miR-376b | miR-628-3p |
| miR-377-3p | |
| miR-380-3p | |
| miR-431-5p | |
| miR-449b-5p | |
| miR-483-3p | |
| miR-485-5p | |
| miR-489 | |
| miR-511 | |
| miR-517a-3p; miR-517b-3p | |
| miR-519a-3p | |
| miR-520b | |
| miR-523-3p | |
| miR-541-3p | |
| miR-550a-5p | |
| miR-551b-5p | |
| miR-604 | |
| miR-639 | |
| miR-646 | |
| miR-7-2-3p | |
| miR-92a-1-5p |
| miRNA | Target Genes | miRNA Properties | |
|---|---|---|---|
| Up-regulated | miR-146a-5p | SMAD4, VEGF, Bcl2 | TGFβ signaling inhibition, modulate chondrocytes apoptosis/autophagy and inflammatory functions |
| miR-155-5p | CEBPB, CTNNB1, SMAD1, TCF7L2 | Chondroprotective (suppress MMP-1 and MMP-3 production), Wnt signaling inhibition, modulate inflammatory functions | |
| miR-520c-3p | DKK1, RELA, SEMA3C | Wnt signaling activation, modulate inflammatory functions and chondrogenesis | |
| Activated | miR-127-5p | MMP13 | Chondroprotective (suppress MMP-13 production) |
| miR-326 | SMO | Chondroprotective | |
| miR-377-3p | CART1 | Cartilage homeostasis | |
| miR-449b-5p | JAG1, NOTCH1, SIRT1 | Chondroprotective (NOTHC signaling inhibition), senescence dysregulation | |
| miR-483-3p | ACAN | Cartilage homeostasis | |
| miR-519a-3p | PRKAA1 | Senescence and autophagy dysregulation | |
| miR-520b | DKK, RELA | Wnt signaling activation, modulate inflammatory functions | |
| Silenced | miR-135b-5p | RUNX2, SMAD5 | Wnt signaling inhibition, inhibition of osteogenic differentiation |
| miR-23b-3p | HES1, NOTCH1, PRKACB, CRTAP | Chondroprotective (NOTHC signaling inhibition), cartilage homeostasis | |
| miR-302d-3p | DKK1, RELA | Wnt signaling activation, modulate inflammatory functions | |
| miR-378a-3p | CASP9 | Marker of late stage OA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombini, A.; Ragni, E.; Mortati, L.; Libonati, F.; Perucca Orfei, C.; Viganò, M.; Brayda-Bruno, M.; de Girolamo, L. Adipose-Derived Mesenchymal Stromal Cells Treated with Interleukin 1 Beta Produced Chondro-Protective Vesicles Able to Fast Penetrate in Cartilage. Cells 2021, 10, 1180. https://doi.org/10.3390/cells10051180
Colombini A, Ragni E, Mortati L, Libonati F, Perucca Orfei C, Viganò M, Brayda-Bruno M, de Girolamo L. Adipose-Derived Mesenchymal Stromal Cells Treated with Interleukin 1 Beta Produced Chondro-Protective Vesicles Able to Fast Penetrate in Cartilage. Cells. 2021; 10(5):1180. https://doi.org/10.3390/cells10051180
Chicago/Turabian StyleColombini, Alessandra, Enrico Ragni, Leonardo Mortati, Francesca Libonati, Carlotta Perucca Orfei, Marco Viganò, Marco Brayda-Bruno, and Laura de Girolamo. 2021. "Adipose-Derived Mesenchymal Stromal Cells Treated with Interleukin 1 Beta Produced Chondro-Protective Vesicles Able to Fast Penetrate in Cartilage" Cells 10, no. 5: 1180. https://doi.org/10.3390/cells10051180
APA StyleColombini, A., Ragni, E., Mortati, L., Libonati, F., Perucca Orfei, C., Viganò, M., Brayda-Bruno, M., & de Girolamo, L. (2021). Adipose-Derived Mesenchymal Stromal Cells Treated with Interleukin 1 Beta Produced Chondro-Protective Vesicles Able to Fast Penetrate in Cartilage. Cells, 10(5), 1180. https://doi.org/10.3390/cells10051180









