The Role of GH/IGF Axis in Dento-Alveolar Complex from Development to Aging and Therapeutics: A Narrative Review
Abstract
:1. Introduction
2. Search Strategy
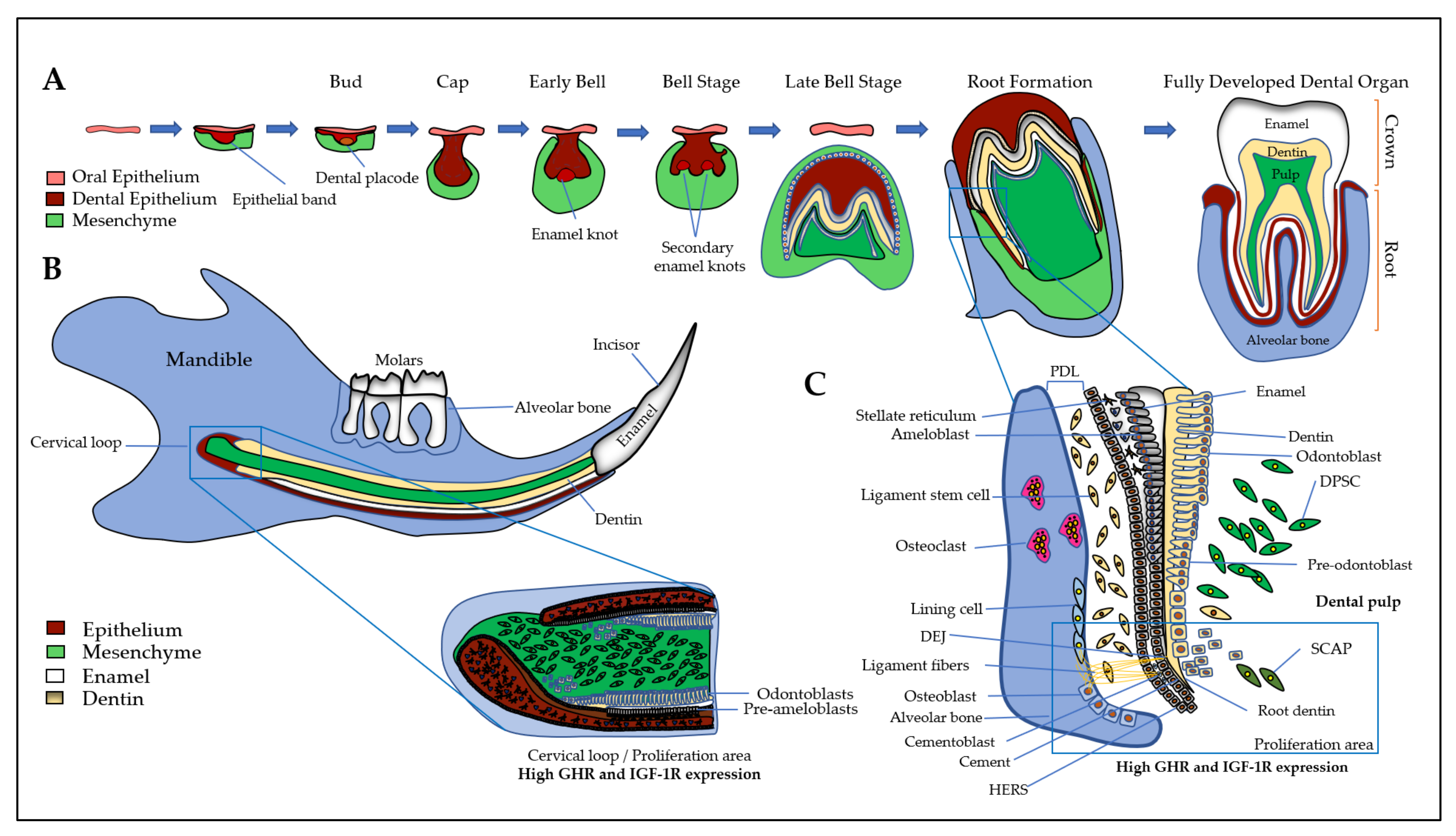
3. Expression and Action of GH/IGF Axis in the Dento-Alveolar Complex
3.1. Dental Epithelium and Enamel
3.2. Dental Mesenchyme and Dentin
3.3. Dental Pulp
3.4. Cement and Periodontal Ligament
3.5. Alveolar Bone
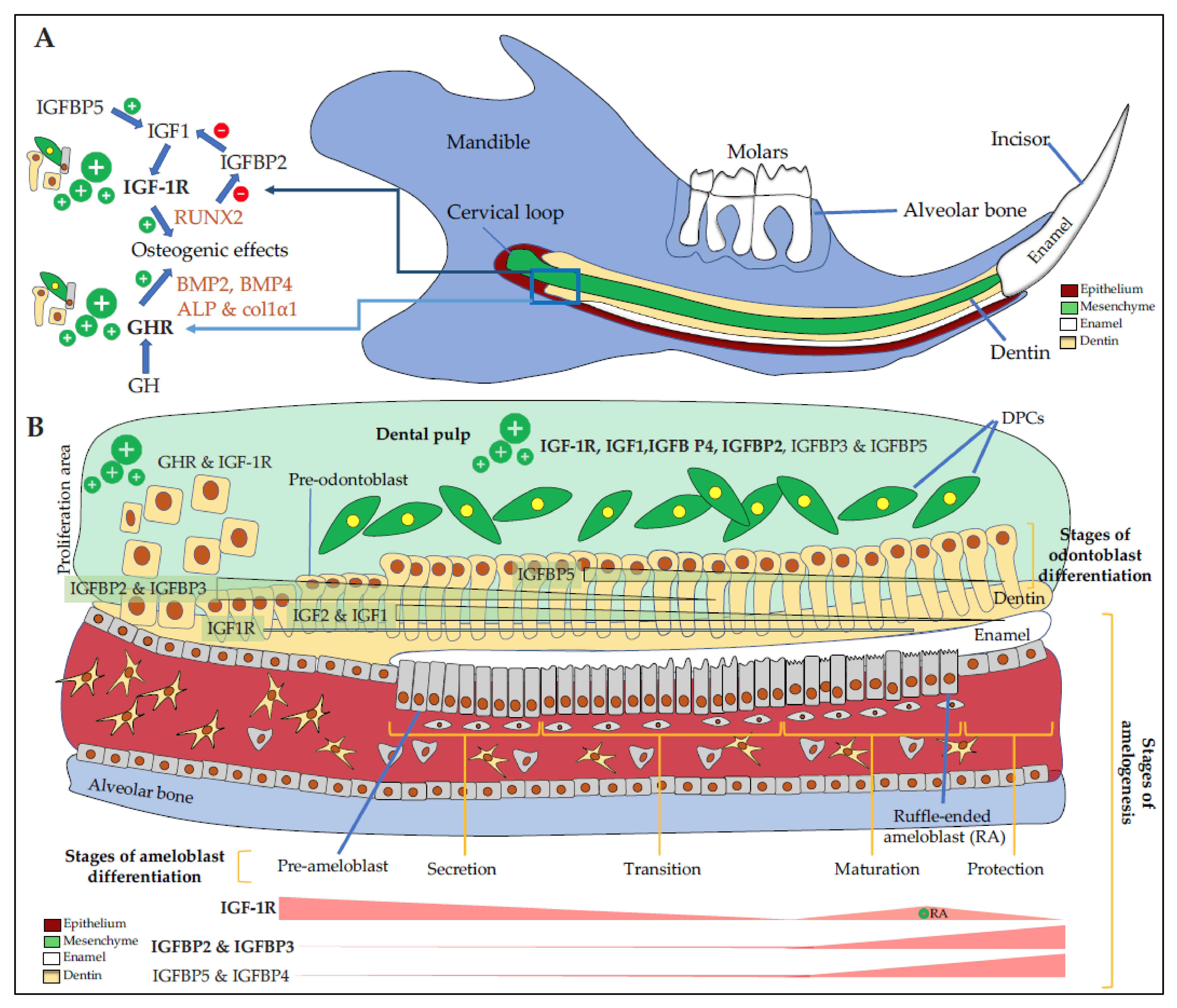
4. Mechanisms of Action of the GH/IGFs/IGFBPs Axis on Dental and Bone Cells
5. GH/IGF Axis Modulates the Morphology of the Dento-Alveolar Complex and Cranio-Facial Bones
6. Therapeutic Systemic Applications of GH or IGF1 for Craniofacial Tissue Defects
6.1. GH Systemic Therapy in Patients with Growth Defects
6.2. GH and IGF Systemic Treatments in Animal Models
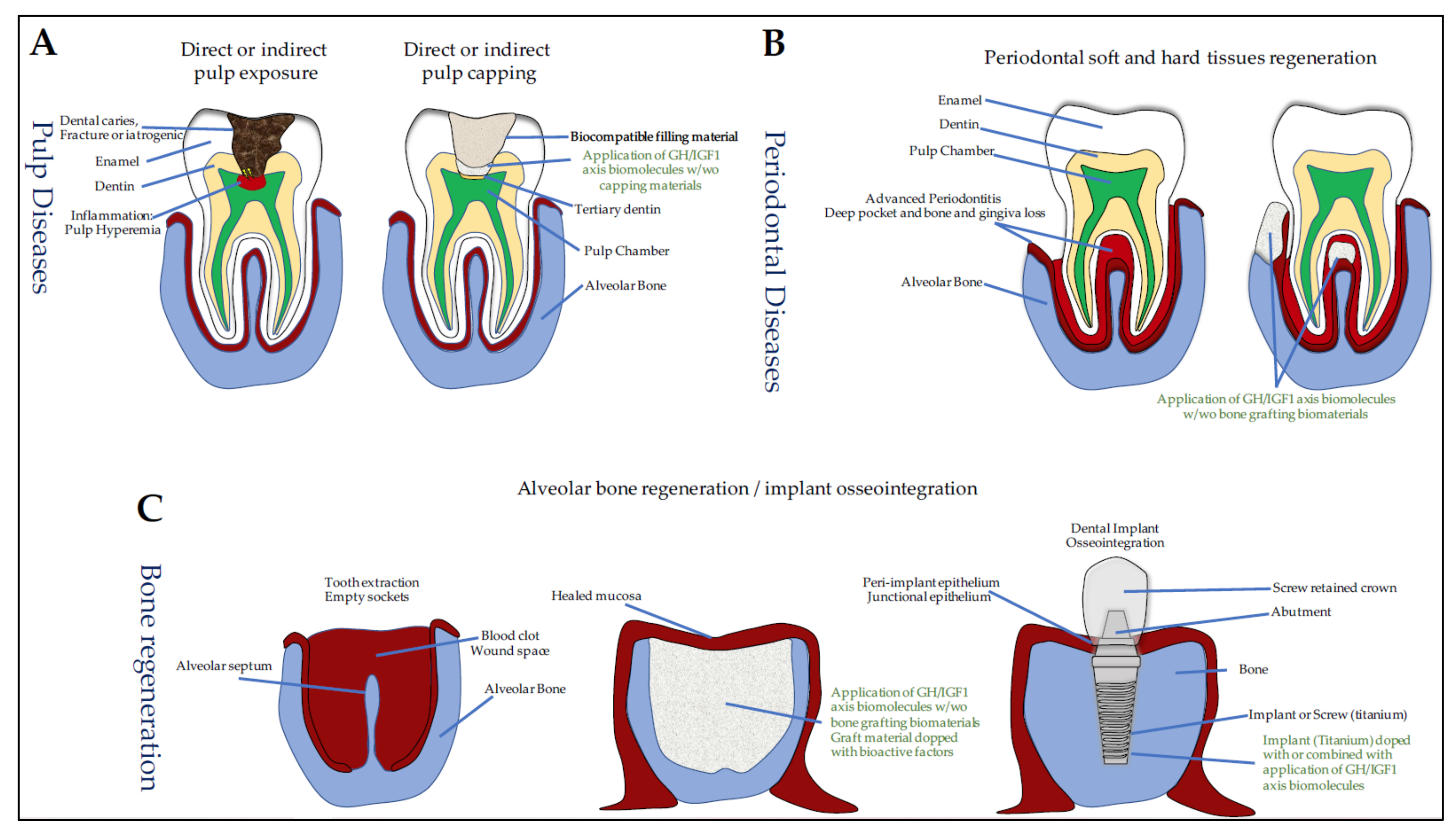
7. GH/IGF and Oral Tissue Engineering
7.1. Dentin Lesions, Reparative Dentinogenesis, and Pulp-Capping Materials
7.2. Dental Movements Induced by Orthodontic Treatments
7.2.1. GH/IGF Axis and Dental Movement
7.2.2. GH and Dental Movement
7.3. Periodontal Regeneration
7.3.1. IGF1 and Periodontal Regeneration
7.3.2. IGF1 and Sockets in Medication-Related Osteonecrosis of the Jaw (MRONJ)
7.4. Osseointegration of Oral Implants
7.4.1. IGF1 and IGFBP3 and Osseointegration
7.4.2. GH and Osseointegration
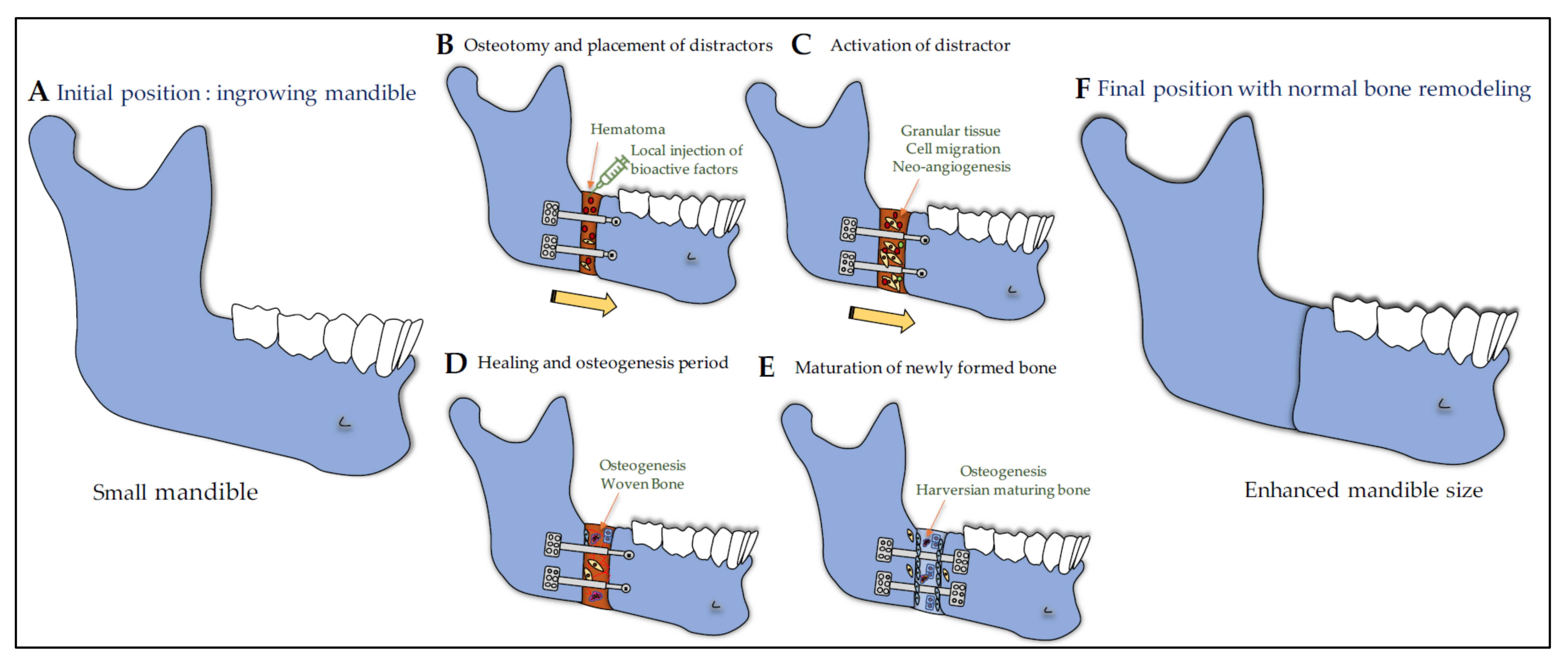
7.5. Craniofacial Bone Repair
7.5.1. GH/IGF Axis in Distraction Osteogenesis and Bone Healing
7.5.2. IGF1 and GH in Distraction Osteogenesis
7.5.3. IGF1 and Mandibular Bone Defect
7.5.4. GH and Calvaria Bone Defect
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Young, W.G. Growth Hormone and Insulin-like Growth Factor-I in Odontogenesis. Int. J. Dev. Biol. 1995, 39, 263–272. [Google Scholar] [PubMed]
- Bayram, S.; Basciftci, F.A.; Kurar, E. Relationship between P561T and C422F Polymorphisms in Growth Hormone Receptor Gene and Mandibular Prognathism. Angle Orthod. 2014, 84, 803–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, H.; Katz, J. The Emerging Role of the Insulin-like Growth Factors in Oral Biology. J. Dent. Res. 2004, 83, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Litsas, G. Growth Hormone Therapy and Craniofacial Bones: A Comprehensive Review. Oral Dis. 2013, 19, 559–567. [Google Scholar] [CrossRef]
- Al-Kharobi, H.; El-Gendy, R.; Devine, D.A.; Beattie, J. The Role of the Insulin-like Growth Factor (IGF) Axis in Osteogenic and Odontogenic Differentiation. Cell Mol. Life Sci. 2014, 71, 1469–1476. [Google Scholar] [CrossRef]
- Yakar, S.; Isaksson, O. Regulation of Skeletal Growth and Mineral Acquisition by the GH/IGF-1 Axis: Lessons from Mouse Models. Growth Horm. IGF Res. 2016, 28, 26–42. [Google Scholar] [CrossRef] [Green Version]
- Kasperk, C.; Wergedal, J.; Strong, D.; Farley, J.; Wangerin, K.; Gropp, H.; Ziegler, R.; Baylink, D.J. Human Bone Cell Phenotypes Differ Depending on Their Skeletal Site of Origin. J. Clin. Endocrinol. Metab. 1995, 80, 2511–2517. [Google Scholar] [CrossRef] [Green Version]
- Krekmanova, L.; Carlstedt-Duke, J.; Brönnegård, M.; Marcus, C.; Gröndahl, E.; Modéer, T.; Dahllöf, G. Dental Maturity in Children of Short Stature, with or without Growth Hormone Deficiency. Eur. J. Oral Sci. 1997, 105, 551–556. [Google Scholar] [CrossRef]
- Hikita, Y.; Yamaguchi, T.; Tomita, D.; Adel, M.; Nakawaki, T.; Katayama, K.; Maki, K.; Kimura, R. Growth Hormone Receptor Gene Is Related to Root Length and Tooth Length in Human Teeth. Angle Orthod. 2018, 88, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Shinde, G.R.; Mhaisekar, R.D.; Chaube, S.H.; Barad, A.N.; Bhadange, S.; Patel, H.J. Assessment of Correlation of Growth Hormone Receptor Gene with Tooth Dimensions: A CBCT and Genotyping Study. J. Pharm. Bioallied Sci. 2019, 11, S457–S462. [Google Scholar] [CrossRef]
- Nakawaki, T.; Yamaguchi, T.; Isa, M.; Kawaguchi, A.; Tomita, D.; Hikita, Y.; Suzuki-Tomoyasu, Y.; Adel, M.; Ishida, H.; Maki, K.; et al. Growth Hormone Receptor Gene Variant and Three-Dimensional Mandibular Morphology. Angle Orthod. 2017, 87, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Arid, J.; Oliveira, D.B.; Evangelista, S.S.; Vasconcelos, K.R.F.; Dutra, A.L.T.; de Oliveira, S.S.; de Queiroz, A.M.; Nelson-Filho, P.; Vieira, A.R.; Küchler, E.C. Oestrogen Receptor Alpha, Growth Hormone Receptor, and Developmental Defect of Enamel. Int. J. Paediatr. Dent. 2019, 29, 29–35. [Google Scholar] [CrossRef]
- Roumeau, S.; Thevenon, J.; Ouchchane, L.; Maqdasy, S.; Batisse-Lignier, M.; Duale, C.; Pham Dang, N.; Caron, P.; Tauveron, I.; Devoize, L. Assessment of Oro-Dental Manifestations in a Series of Acromegalic Patients, the AcroDent Study. Endocr. Connect 2020, 9, 824–833. [Google Scholar] [CrossRef]
- BaŞÇil, S.; Turhan İyİdİr, Ö.; Bayraktar, N.; ErtÖrer, M.E.; BaŞÇil TÜtÜncÜ, N. Severe Chronic Periodontitis Is Not Common in Acromegaly: Potential Protective Role of Gingival BMP-2. Turk. J. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental Enamel Formation and Implications for Oral Health and Disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef]
- Lignon, G.; de la Dure-Molla, M.; Dessombz, A.; Berdal, A.; Babajko, S. Enamel: A unique self-assembling in mineral world. Med. Sci. 2015, 31, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Hathaway-Schrader, J.D.; Novince, C.M. Maintaining Homeostatic Control of Periodontal Bone Tissue. Periodontol 2000 2021. [Google Scholar] [CrossRef]
- Nassif, A.; Senussi, I.; Meary, F.; Loiodice, S.; Hotton, D.; Robert, B.; Bensidhoum, M.; Berdal, A.; Babajko, S. Msx1 Role in Craniofacial Bone Morphogenesis. Bone 2014, 66, 96–104. [Google Scholar] [CrossRef]
- Yu, T.; Klein, O.D. Molecular and Cellular Mechanisms of Tooth Development, Homeostasis and Repair. Development 2020, 147. [Google Scholar] [CrossRef]
- Nuti, N.; Corallo, C.; Chan, B.M.F.; Ferrari, M.; Gerami-Naini, B. Multipotent Differentiation of Human Dental Pulp Stem Cells: A Literature Review. Stem Cell Rev. Rep. 2016, 12, 511–523. [Google Scholar] [CrossRef]
- Palma, P.J.; Martins, J.; Diogo, P.; Sequeira, D.; Ramos, J.C.; Diogenes, A.; Santos, J.M. Does Apical Papilla Survive and Develop in Apical Periodontitis Presence after Regenerative Endodontic Procedures? Appl. Sci. 2019, 9, 3942. [Google Scholar] [CrossRef] [Green Version]
- Aydin, S.; Şahin, F. Stem Cells Derived from Dental Tissues. Adv. Exp. Med. Biol. 2019, 1144, 123–132. [Google Scholar] [CrossRef]
- Diana, R.; Ardhani, R.; Kristanti, Y.; Santosa, P. Dental Pulp Stem Cells Response on the Nanotopography of Scaffold to Regenerate Dentin-Pulp Complex Tissue. Regen. Ther. 2020, 15, 243–250. [Google Scholar] [CrossRef]
- Kang, J.; Fan, W.; Deng, Q.; He, H.; Huang, F. Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy. Biomed. Res. Int. 2019, 2019, 6104738. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, A.; Harada, H.; Saito, M.; Taniguchi, A. Induction of Insulin-like Growth Factor 2 Expression in a Mesenchymal Cell Line Co-Cultured with an Ameloblast Cell Line. In Vitro Cell Dev. Biol. Anim. 2011, 47, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.; Taya, Y.; Shimazu, Y.; Aoba, T.; Soeno, Y. Molecular Signaling at the Fusion Stage of the Mouse Mandibular Arch: Involvement of Insulin-like Growth Factor Family. Int. J. Dev. Biol. 2013, 57, 399–406. [Google Scholar] [CrossRef]
- Joseph, B.K.; Savage, N.W.; Young, W.G.; Waters, M.J. Prenatal Expression of Growth Hormone Receptor/Binding Protein and Insulin-like Growth Factor-I (IGF-I) in the Enamel Organ. Role for Growth Hormone and IGF-I in Cellular Differentiation during Early Tooth Formation? Anat. Embryol. 1994, 189, 489–494. [Google Scholar] [CrossRef]
- Joseph, B.K.; Savage, N.W.; Daley, T.J.; Young, W.G. In Situ Hybridization Evidence for a Paracrine/Autocrine Role for Insulin-like Growth Factor-I in Tooth Development. Growth Factors 1996, 13, 11–17. [Google Scholar] [CrossRef]
- Joseph, B.K.; Savage, N.W.; Young, W.G.; Gupta, G.S.; Breier, B.H.; Waters, M.J. Expression and Regulation of Insulin-like Growth Factor-I in the Rat Incisor. Growth Factors 1993, 8, 267–275. [Google Scholar] [CrossRef]
- Joseph, B.K.; Harbrow, D.J.; Sugerman, P.B.; Smid, J.R.; Savage, N.W.; Young, W.G. Ameloblast Apoptosis and IGF-1 Receptor Expression in the Continuously Erupting Rat Incisor Model. Apoptosis 1999, 4, 441–447. [Google Scholar] [CrossRef]
- Yamamoto, T.; Oida, S.; Inage, T. Gene Expression and Localization of Insulin-like Growth Factors and Their Receptors throughout Amelogenesis in Rat Incisors. J. Histochem. Cytochem. 2006, 54, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Catón, J.; Bringas, P.; Zeichner-David, M. IGFs Increase Enamel Formation by Inducing Expression of Enamel Mineralizing Specific Genes. Arch Oral Biol. 2005, 50, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kero, D.; Cigic, L.; Medvedec Mikic, I.; Galic, T.; Cubela, M.; Vukojevic, K.; Saraga-Babic, M. Involvement of IGF-2, IGF-1R, IGF-2R and PTEN in Development of Human Tooth Germ-an Immunohistochemical Study. Organogenesis 2016, 12, 152–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smid, J.R.; Rowland, J.E.; Young, W.G.; Coschigano, K.T.; Kopchick, J.J.; Waters, M.J. Mouse Molar Dentin Size/Shape Is Dependent on Growth Hormone Status. J. Dent. Res. 2007, 86, 463–468. [Google Scholar] [CrossRef]
- Greene, S.L.; Mamaeva, O.; Crossman, D.K.; Lu, C.; MacDougall, M. Gene-Expression Analysis Identifies IGFBP2 Dysregulation in Dental Pulp Cells From Human Cleidocranial Dysplasia. Front Genet 2018, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Mu, J.; Fan, Z.; Lei, G.; Yan, M.; Wang, S.; Tang, C.; Wang, Z.; Yu, J.; Zhang, G. Insulin-like Growth Factor 1 Enhances the Proliferation and Osteogenic Differentiation of Human Periodontal Ligament Stem Cells via ERK and JNK MAPK Pathways. Histochem. Cell Biol. 2012, 137, 513–525. [Google Scholar] [CrossRef]
- Götz, W.; Heinen, M.; Lossdörfer, S.; Jäger, A. Immunohistochemical Localization of Components of the Insulin-like Growth Factor System in Human Permanent Teeth. Arch Oral Biol. 2006, 51, 387–395. [Google Scholar] [CrossRef]
- Aizawa, C.; Saito, K.; Ohshima, H. Regulation of IGF-I by IGFBP3 and IGFBP5 during Odontoblast Differentiation in Mice. J. Oral Biosci. 2019, 61, 157–162. [Google Scholar] [CrossRef]
- Caviedes-Bucheli, J.; Muñoz, H.R.; Rodríguez, C.E.; Lorenzana, T.C.; Moreno, G.C.; Lombana, N. Expression of Insulin-like Growth Factor-1 Receptor in Human Pulp Tissue. J. Endod. 2004, 30, 767–769. [Google Scholar] [CrossRef]
- Caviedes-Bucheli, J.; Canales-Sánchez, P.; Castrillón-Sarria, N.; Jovel-Garcia, J.; Alvarez-Vásquez, J.; Rivero, C.; Azuero-Holguín, M.M.; Diaz, E.; Munoz, H.R. Expression of Insulin-like Growth Factor-1 and Proliferating Cell Nuclear Antigen in Human Pulp Cells of Teeth with Complete and Incomplete Root Development. Int. Endod. J. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Al-Khafaji, H.; Noer, P.R.; Alkharobi, H.; Alhodhodi, A.; Meade, J.; El-Gendy, R.; Oxvig, C.; Beattie, J. A Characteristic Signature of Insulin-like Growth Factor (IGF) Axis Expression during Osteogenic Differentiation of Human Dental Pulp Cells (HDPCs): Potential Co-Ordinated Regulation of IGF Action. Growth Horm IGF Res. 2018, 42–43, 14–21. [Google Scholar] [CrossRef]
- Götz, W.; Kunert, D.; Zhang, D.; Kawarizadeh, A.; Lossdörfer, S.; Jäger, A. Insulin-like Growth Factor System Components in the Periodontium during Tooth Root Resorption and Early Repair Processes in the Rat. Eur. J. Oral Sci. 2006, 114, 318–327. [Google Scholar] [CrossRef]
- Alkharobi, H.; Alhodhodi, A.; Hawsawi, Y.; Alkafaji, H.; Devine, D.; El-Gendy, R.; Beattie, J. IGFBP-2 and -3 Co-Ordinately Regulate IGF1 Induced Matrix Mineralisation of Differentiating Human Dental Pulp Cells. Stem Cell Res. 2016, 17, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Saito, K.; Ohshima, H. The Putative Role of Insulin-like Growth Factor (IGF)-Binding Protein 5 Independent of IGF in the Maintenance of Pulpal Homeostasis in Mice. Regen. Ther. 2019, 11, 217–224. [Google Scholar] [CrossRef]
- Brochado Martins, J.F.; Rodrigues, C.F.D.; Nunes, P.D.; Paulo, S.; Palma, P.J.; do Vale, F.F. Remodelling Compartment in Root Cementum. Folia Morphol. 2020. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Young, W.G.; Li, H.; Clayden, A.M.; Garcia-Aragon, J.; Waters, M.J. Expression of Growth Hormone Receptor by Immunocytochemistry in Rat Molar Root Formation and Alveolar Bone Remodeling. Calcif. Tissue Int. 1992, 50, 541–546. [Google Scholar] [CrossRef]
- Smid, J.R.; Rowland, J.E.; Young, W.G.; Daley, T.J.; Coschigano, K.T.; Kopchick, J.J.; Waters, M.J. Mouse Cellular Cementum Is Highly Dependent on Growth Hormone Status. J. Dent. Res. 2004, 83, 35–39. [Google Scholar] [CrossRef]
- Li, H.; Bartold, P.M.; Young, W.G.; Xiao, Y.; Waters, M.J. Growth Hormone Induces Bone Morphogenetic Proteins and Bone-Related Proteins in the Developing Rat Periodontium. J. Bone Miner Res. 2001, 16, 1068–1076. [Google Scholar] [CrossRef]
- Haase, H.R.; Ivanovski, S.; Waters, M.J.; Bartold, P.M. Growth Hormone Regulates Osteogenic Marker MRNA Expression in Human Periodontal Fibroblasts and Alveolar Bone-Derived Cells. J. Periodont. Res. 2003, 38, 366–374. [Google Scholar] [CrossRef]
- Li, X.; Yao, J.; Wu, J.; Du, X.; Jing, W.; Liu, L. Roles of PRF and IGF-1 in Promoting Alveolar Osteoblast Growth and Proliferation and Molecular Mechanism. Int. J. Clin. Exp. Pathol. 2018, 11, 3294–3301. [Google Scholar]
- Götz, W.; Lossdörfer, S.; Krüger, U.; Braumann, B.; Jäger, A. Immunohistochemical Localization of Insulin-like Growth Factor-II and Its Binding Protein-6 in Human Epithelial Cells of Malassez. Eur. J. Oral Sci. 2003, 111, 26–33. [Google Scholar] [CrossRef]
- Fujiwara, N.; Tabata, M.J.; Endoh, M.; Ishizeki, K.; Nawa, T. Insulin-like Growth Factor-I Stimulates Cell Proliferation in the Outer Layer of Hertwig’s Epithelial Root Sheath and Elongation of the Tooth Root in Mouse Molars in Vitro. Cell Tissue Res. 2005, 320, 69–75. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.; Jia, Z.; Wang, L.; Wang, J.; Yang, D.; Song, J.; Wang, S.; Fan, Z. Demethylation of IGFBP5 by Histone Demethylase KDM6B Promotes Mesenchymal Stem Cell-Mediated Periodontal Tissue Regeneration by Enhancing Osteogenic Differentiation and Anti-Inflammation Potentials. Stem Cells 2015, 33, 2523–2536. [Google Scholar] [CrossRef]
- Han, N.; Zhang, F.; Li, G.; Zhang, X.; Lin, X.; Yang, H.; Wang, L.; Cao, Y.; Du, J.; Fan, Z. Local Application of IGFBP5 Protein Enhanced Periodontal Tissue Regeneration via Increasing the Migration, Cell Proliferation and Osteo/Dentinogenic Differentiation of Mesenchymal Stem Cells in an Inflammatory Niche. Stem Cell Res. Ther. 2017, 8, 210. [Google Scholar] [CrossRef]
- Huang, H.; Wang, J.; Zhang, Y.; Zhu, G.; Li, Y.-P.; Ping, J.; Chen, W. Bone Resorption Deficiency Affects Tooth Root Development in RANKL Mutant Mice Due to Attenuated IGF-1 Signaling in Radicular Odontoblasts. Bone 2018, 114, 161–171. [Google Scholar] [CrossRef]
- Gama, A.; Vargas-Franco, J.W.; Sánchez Mesa, D.C.; Restrepo Bedoya, E.; Amiaud, J.; Babajko, S.; Berdal, A.; Acevedo, A.C.; Heymann, D.; Lézot, F.; et al. Origins of Alterations to Rankl Null Mutant Mouse Dental Root Development. Int. J. Mol. Sci. 2020, 21, 2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litsas, G. Growth Hormone and Craniofacial Tissues. An Update. Open Dent. J. 2015, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Crippa, G.E.; Beloti, M.M.; Cardoso, C.R.; Silva, J.S.; Rosa, A.L. Effect of Growth Hormone on in Vitro Osteogenesis and Gene Expression of Human Osteoblastic Cells Is Donor-Age-Dependent. J. Cell Biochem. 2008, 104, 369–376. [Google Scholar] [CrossRef]
- Chihara, K.; Sugimoto, T. The Action of GH/IGF-I/IGFBP in Osteoblasts and Osteoclasts. Horm Res. 1997, 48 (Suppl. 5), 45–49. [Google Scholar] [CrossRef]
- DiGirolamo, D.J.; Mukherjee, A.; Fulzele, K.; Gan, Y.; Cao, X.; Frank, S.J.; Clemens, T.L. Mode of Growth Hormone Action in Osteoblasts. J. Biol. Chem. 2007, 282, 31666–31674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, B.L. Bone Morphogenetic Proteins: Multifunctional Regulators of Vertebrate Development. Genes Dev. 1996, 10, 1580–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, M.; Rodan, G.A. Increased Activity of Insulin-like Growth Factor (IGF) in Osteoblastic Cells in the Presence of Growth Hormone (GH): Positive Correlation with the Presence of the GH-Induced IGF-Binding Protein BP-3. Endocrinology 1990, 127, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Bauss, F.; Lang, K.; Dony, C.; Kling, L. The Complex of Recombinant Human Insulin-like Growth Factor-I (RhIGF-I) and Its Binding Protein-5 (IGFBP-5) Induces Local Bone Formation in Murine Calvariae and in Rat Cortical Bone after Local or Systemic Administration. Growth Horm IGF Res. 2001, 11, 1–9. [Google Scholar] [CrossRef]
- Gustafson, J.A.; Park, S.S.; Cunningham, M.L. Calvarial Osteoblast Gene Expression in Patients with Craniosynostosis Leads to Novel Polygenic Mouse Model. PLoS ONE 2019, 14, e0221402. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Li, L.; Cai, W.; Jiang, B. The Potential Application of Concentrated Growth Factor in Regenerative Endodontics. Int. Endod. J. 2019, 52, 646–655. [Google Scholar] [CrossRef]
- Wang, S.; Mu, J.; Fan, Z.; Yu, Y.; Yan, M.; Lei, G.; Tang, C.; Wang, Z.; Zheng, Y.; Yu, J.; et al. Insulin-like Growth Factor 1 Can Promote the Osteogenic Differentiation and Osteogenesis of Stem Cells from Apical Papilla. Stem Cell Res. 2012, 8, 346–356. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Amar, S. IGF-1 Signaling Enhances Cell Survival in Periodontal Ligament Fibroblasts vs. Gingival Fibroblasts. J. Dent. Res. 2003, 82, 454–459. [Google Scholar] [CrossRef]
- Ma, S.; Liu, G.; Jin, L.; Pang, X.; Wang, Y.; Wang, Z.; Yu, Y.; Yu, J. IGF-1/IGF-1R/Hsa-Let-7c Axis Regulates the Committed Differentiation of Stem Cells from Apical Papilla. Sci. Rep. 2016, 6, 36922. [Google Scholar] [CrossRef]
- Bian, M.; Yu, Y.; Li, Y.; Zhou, Z.; Wu, X.; Ye, X.; Yu, J. Upregulating the Expression of LncRNA ANRIL Promotes Osteogenesis via the MiR-7-5p/IGF-1R Axis in the Inflamed Periodontal Ligament Stem Cells. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Gan, K.; Dong, G.-H.; Wang, N.; Zhu, J.-F. MiR-221-3p and MiR-222-3p Downregulation Promoted Osteogenic Differentiation of Bone Marrow Mesenchyme Stem Cells through IGF-1/ERK Pathway under High Glucose Condition. Diabetes Res. Clin. Pract. 2020, 167, 108121. [Google Scholar] [CrossRef]
- Bhattarai, G.; Lee, Y.-H.; Lee, M.-H.; Park, I.-S.; Yi, H.-K. Insulin-like Growth Factor Binding Protein-3 Affects Osteogenic Efficacy on Dental Implants in Rat Mandible. Mater. Sci. Eng. C Mater Biol. Appl. 2015, 55, 490–496. [Google Scholar] [CrossRef]
- Schleicher, I.; Parker, A.; Leavesley, D.; Crawford, R.; Upton, Z.; Xiao, Y. Surface Modification by Complexes of Vitronectin and Growth Factors for Serum-Free Culture of Human Osteoblasts. Tissue Eng. 2005, 11, 1688–1698. [Google Scholar] [CrossRef]
- Shi, S.; Robey, P.G.; Gronthos, S. Comparison of Human Dental Pulp and Bone Marrow Stromal Stem Cells by CDNA Microarray Analysis. Bone 2001, 29, 532–539. [Google Scholar] [CrossRef]
- Ramirez-Yañez, G.O.; Smid, J.R.; Young, W.G.; Waters, M.J. Influence of Growth Hormone on the Craniofacial Complex of Transgenic Mice. Eur. J. Orthod. 2005, 27, 494–500. [Google Scholar] [CrossRef]
- McAlarney, M.E.; Rizos, M.; Rocca, E.G.; Nicolay, O.F.; Efstratiadis, S. The Quantitative and Qualitative Analysis of the Craniofacial Skeleton of Mice Lacking the IGF-I Gene. Clin. Orthod. Res. 2001, 4, 206–219. [Google Scholar] [CrossRef]
- Marchant, C.; Anderson, P.; Schwarz, Q.; Wiszniak, S. Vessel-Derived Angiocrine IGF1 Promotes Meckel’s Cartilage Proliferation to Drive Jaw Growth during Embryogenesis. Development 2020, 147. [Google Scholar] [CrossRef]
- Masoud, M.; Masoud, I.; Kent, R.L.; Gowharji, N.; Cohen, L.E. Assessing Skeletal Maturity by Using Blood Spot Insulin-like Growth Factor I (IGF-I) Testing. Am. J. Orthod. Dentofacial Orthop. 2008, 134, 209–216. [Google Scholar] [CrossRef]
- Masoud, M.I.; Marghalani, H.Y.A.; Masoud, I.M.; Gowharji, N.F. Prospective Longitudinal Evaluation of the Relationship between Changes in Mandibular Length and Blood-Spot IGF-1 Measurements. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Masoud, M.I.; Marghalani, H.Y.A.; Bamashmous, M.; Alamoudi, N.M.; El Derwi, D.; Masoud, I.M.; Allareddy, V.; Gowharji, N.F. Predicting Changes in Mandibular Length and Total Anterior Facial Height Using IGF-1, Cervical Stage, Skeletal Classification, and Gender. Prog. Orthod. 2015, 16, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidopoulou, S.; Chatzigianni, A. Craniofacial Morphology and Dental Maturity in Children with Reduced Somatic Growth of Different Aetiology and the Effect of Growth Hormone Treatment. Prog. Orthod. 2017, 18, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wójcik, D.; Beń-Skowronek, I. Craniofacial Morphology in Children with Growth Hormone Deficiency and Turner Syndrome. Diagnostics 2020, 10, 88. [Google Scholar] [CrossRef] [Green Version]
- Partyka, M.; Chałas, R.; Dunin-Wilczyńska, I.; Drohomyretska, M.; Klatka, M. Influence of Growth Hormone Therapy on Selected Dental and Skeletal System Parameters. Ann. Agric. Environ. Med. 2018, 25, 60–65. [Google Scholar] [CrossRef]
- Partyka, M.; Klatka, M.; Chałas, R. Tooth Decay Prevalence among Children with Somatotropin Hypopituitarism. Bull. Int. Assoc. Paleodont. 2015, 9, 67–72. [Google Scholar]
- Dündar, B.; Erçal, D.; Böber, E.; Berk, T.; Büyükgebiz, A. Amelogenesis Imperfecta with Growth Hormone Deficiency in a 12 Year-Old Boy. J. Pediatr Endocrinol. Metab. 2002, 15, 659–662. [Google Scholar] [CrossRef]
- Aren, G.; Ozdemir, D.; Firatli, S.; Uygur, C.; Sepet, E.; Firatli, E. Evaluation of Oral and Systemic Manifestations in an Amelogenesis Imperfecta Population. J. Dent. 2003, 31, 585–591. [Google Scholar] [CrossRef]
- Tobón-Arroyave, S.I.; Jiménez-Arbeláez, G.A.; Alvarado-Gómez, V.A.; Isaza-Guzmán, D.M.; Flórez-Moreno, G.A.; Pérez-Cano, M.I. Association Analysis between Rs6184 and Rs6180 Polymorphisms of Growth Hormone Receptor Gene Regarding Skeletal-Facial Profile in a Colombian Population. Eur. J. Orthod. 2018, 40, 378–386. [Google Scholar] [CrossRef]
- Marañón-Vásquez, G.A.; Vieira, A.R.; de Carvalho Ramos, A.G.; Dantas, B.; Romano, F.L.; Palma-Dibb, R.G.; Arid, J.; Carpio, K.; Nelson-Filho, P.; de Rossi, A.; et al. GHR and IGF2R Genes May Contribute to Normal Variations in Craniofacial Dimensions: Insights from an Admixed Population. Am. J. Orthod. Dentofac. Orthop. 2020, 158, 722–730.e16. [Google Scholar] [CrossRef]
- Dahllöf, G.; Forsberg, C.M.; Näsman, M.; Mattsson, T.; Modéer, T.; Borgström, B.; Bolme, P.; Ringdén, O. Craniofacial Growth in Bone Marrow Transplant Recipients Treated with Growth Hormone after Total Body Irradiation. Scand J. Dent. Res. 1991, 99, 44–47. [Google Scholar] [CrossRef]
- Forsberg, C.-M.; Krekmanova, L.; Dahllöf, G. The Effect of Growth Hormone Therapy on Mandibular and Cranial Base Development in Children Treated with Total Body Irradiation. Eur. J. Orthod. 2002, 24, 285–292. [Google Scholar] [CrossRef]
- Edler, R.J. Dental and Skeletal Ages in Hypopituitary Patients. J. Dent. Res. 1977, 56, 1145–1153. [Google Scholar] [CrossRef]
- Bevis, R.R.; Hayles, A.B.; Isaacson, R.J.; Sather, A.H. Facial Growth Response to Human Growth Hormone in Hypopituitary Dwarfs. Angle Orthod. 1977, 47, 193–205. [Google Scholar] [CrossRef]
- Symons, A.L.; Seymour, G.J. A Histological Study of the Effect of Growth Hormone on Odontogenesis in the Lewis Dwarf Rat. Arch. Oral Biol. 2000, 45, 123–131. [Google Scholar] [CrossRef]
- Young, W.G.; Li, H.; Xiao, Y.; Waters, M.J.; Bartold, P.M. Growth-Hormone-Stimulated Dentinogenesis in Lewis Dwarf Rat Molars. J. Dent. Res. 2001, 80, 1742–1747. [Google Scholar] [CrossRef]
- Iikubo, M.; Ikeda, H.; Kobayashi, A.; Kojima, I.; Hashimoto, K.; Sakamoto, M.; Sasano, T. Insulin-like Growth Factor-I Stimulates Acromegaly-like Specific Mandibular Enlargement in Rats. Horm. Metab. Res. 2004, 36, 696–701. [Google Scholar] [CrossRef]
- da Rosa, W.L.O.; Piva, E.; da Silva, A.F. Disclosing the Physiology of Pulp Tissue for Vital Pulp Therapy. Int. Endod. J. 2018, 51, 829–846. [Google Scholar] [CrossRef] [Green Version]
- Tomson, P.L.; Lumley, P.J.; Smith, A.J.; Cooper, P.R. Growth Factor Release from Dentine Matrix by Pulp-Capping Agents Promotes Pulp Tissue Repair-Associated Events. Int. Endod. J. 2017, 50, 281–292. [Google Scholar] [CrossRef]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef]
- McLachlan, J.L.; Smith, A.J.; Bujalska, I.J.; Cooper, P.R. Gene Expression Profiling of Pulpal Tissue Reveals the Molecular Complexity of Dental Caries. Biochim. Biophys. Acta 2005, 1741, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Masuda, Y.M.; Unno, A.; Hossain, M.; Okano, T.; Matsumoto, K. Isolation and Partial Sequencing of CDNA Clones from Rat Incisor after Nd:YAG Laser Irradiation in Root Canal. Arch Oral Biol. 2006, 51, 527–534. [Google Scholar] [CrossRef]
- Alkharobi, H.E.; Al-Khafaji, H.; Beattie, J.; Devine, D.A.; El-Gendy, R. Insulin-Like Growth Factor Axis Expression in Dental Pulp Cells Derived From Carious Teeth. Front Bioeng. Biotechnol. 2018, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Kalyva, M.; Papadimitriou, S.; Tziafas, D. Transdentinal Stimulation of Tertiary Dentine Formation and Intratubular Mineralization by Growth Factors. Int. Endod. J. 2010, 43, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Lovschall, H.; Fejerskov, O.; Flyvbjerg, A. Pulp-Capping with Recombinant Human Insulin-like Growth Factor I (RhIGF-I) in Rat Molars. Adv. Dent. Res. 2001, 15, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Lefranc, G.; Aftimos, G. Local Application of IGF1 on Dental Pulp Mechanically Exposed; in Vivo Study on Rabbit. Bull. Group Int. Rech Sci. Stomatol. Odontol. 2003, 45, 12–17. [Google Scholar] [PubMed]
- Ong, C.K.; Joseph, B.K.; Waters, M.J.; Symons, A.L. Growth Hormone Receptor and IGF-I Receptor Immunoreactivity during Orthodontic Tooth Movement in the Prednisolone-Treated Rat. Angle Orthod. 2001, 71, 486–493. [Google Scholar] [CrossRef]
- Termsuknirandorn, S.; Hosomichi, J.; Soma, K. Occlusal Stimuli Influence on the Expression of IGF-1 and the IGF-1 Receptor in the Rat Periodontal Ligament. Angle Orthod. 2008, 78, 610–616. [Google Scholar] [CrossRef]
- Pereira, L.J.; Macari, S.; Coimbra, C.C.; Pereira, T.D.S.F.; Barrioni, B.R.; Gomez, R.S.; Silva, T.A.; Paiva, S.M. Aerobic and Resistance Training Improve Alveolar Bone Quality and Interferes with Bone-Remodeling during Orthodontic Tooth Movement in Mice. Bone 2020, 138, 115496. [Google Scholar] [CrossRef]
- Li, T.; Yan, Z.; He, S.; Zhou, C.; Wang, H.; Yin, X.; Zou, S.; Duan, P. Intermittent Parathyroid Hormone Improves Orthodontic Retention via Insulin-like Growth Factor-1. Oral Dis. 2021, 27, 290–300. [Google Scholar] [CrossRef]
- Tengku, B.S.; Joseph, B.K.; Harbrow, D.; Taverne, A.A.; Symons, A.L. Effect of a Static Magnetic Field on Orthodontic Tooth Movement in the Rat. Eur. J. Orthod. 2000, 22, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.S.; Maciel, J.V.B.; Knop, L.A.H.; Machado, M.Â.N.; Grégio, A.M.T.; Camargo, E.S. Effect of Growth Hormone in Experimental Tooth Movement. Braz. Dent. J. 2013, 24, 503–507. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Liu, Z.; Kuang, W.; He, H. Receptor Activator of Nuclear Factor-Kappa Ligand, OPG, and IGF-I Expression during Orthodontically Induced Inflammatory Root Resorption in the Recombinant Human Growth Hormone-Treated Rats. Angle Orthod. 2015, 85, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Dereka, X.E.; Markopoulou, C.E.; Vrotsos, I.A. Role of Growth Factors on Periodontal Repair. Growth Factors 2006, 24, 260–267. [Google Scholar] [CrossRef]
- Giannobile, W.V.; Hernandez, R.A.; Finkelman, R.D.; Ryan, S.; Kiritsy, C.P.; D’Andrea, M.; Lynch, S.E. Comparative Effects of Platelet-Derived Growth Factor-BB and Insulin-like Growth Factor-I, Individually and in Combination, on Periodontal Regeneration in Macaca Fascicularis. J. Periodontal Res. 1996, 31, 301–312. [Google Scholar] [CrossRef]
- Lynch, S.E.; Williams, R.C.; Polson, A.M.; Howell, T.H.; Reddy, M.S.; Zappa, U.E.; Antoniades, H.N. A Combination of Platelet-Derived and Insulin-like Growth Factors Enhances Periodontal Regeneration. J. Clin. Periodontol. 1989, 16, 545–548. [Google Scholar] [CrossRef]
- Lynch, S.E.; de Castilla, G.R.; Williams, R.C.; Kiritsy, C.P.; Howell, T.H.; Reddy, M.S.; Antoniades, H.N. The Effects of Short-Term Application of a Combination of Platelet-Derived and Insulin-like Growth Factors on Periodontal Wound Healing. J. Periodontol. 1991, 62, 458–467. [Google Scholar] [CrossRef]
- Félix Lanao, R.P.; Hoekstra, J.W.M.; Wolke, J.G.C.; Leeuwenburgh, S.C.G.; Plachokova, A.S.; Boerman, O.C.; van den Beucken, J.J.J.P.; Jansen, J.A. Porous Calcium Phosphate Cement for Alveolar Bone Regeneration. J. Tissue Eng. Regen. Med. 2014, 8, 473–482. [Google Scholar] [CrossRef]
- De Abreu, F.A.M.; Ferreira, C.L.; Silva, G.A.B.; de Paulo, C.O.; Miziara, M.N.; Silveira, F.F.; Alves, J.B. Effect of PDGF-BB, IGF-I Growth Factors and Their Combination Carried by Liposomes in Tooth Socket Healing. Braz. Dent. J. 2013, 24, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Soares, F.P.; Hayashi, F.; Yorioka, C.W.; Pannuti, C.M.; Gioso, M.A.; de Lima, L.A.P.A.; Romito, G.A.; Pustiglioni, F.E. Repair of Class II Furcation Defects after a Reparative Tissue Graft Obtained from Extraction Sockets Treated with Growth Factors: A Histologic and Histometric Study in Dogs. J. Periodontol. 2005, 76, 1681–1689. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, Y.; Wu, H.; Deng, Z.; Wang, Q.; Zhou, W.; Liu, Q.; Dong, G.; Li, K.; Wu, Z.; et al. Enhancement of Periodontal Tissue Regeneration by Locally Controlled Delivery of Insulin-like Growth Factor-I from Dextran-Co-Gelatin Microspheres. J. Control Release 2006, 114, 209–222. [Google Scholar] [CrossRef]
- Zairi, A.; Lambrianidis, T.; Pantelidou, O.; Papadimitriou, S.; Tziafas, D. Periradicular Tissue Responses to Biologically Active Molecules or MTA When Applied in Furcal Perforation of Dogs’ Teeth. Int. J. Dent. 2012, 2012, 257832. [Google Scholar] [CrossRef]
- Kumasaka, A.; Iikubo, M.; Nishioka, T.; Kojima, I.; Shoji, N.; Sakamoto, M.; Sasano, T. Insulin-Like Growth Factor I Inhibits Alveolar Bone Loss Following Tooth Extraction in Rats. Clin. Implant Dent. Relat. Res. 2015, 17, 1174–1179. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, L.-P.; Du, F.-L.; Liu, W.-J.; Ren, G.-L. Effects of Insulin-like Growth Factor I on Alveolar Bone Remodeling in Diabetic Rats. J. Periodontal Res. 2013, 48, 144–150. [Google Scholar] [CrossRef]
- Howell, T.H.; Fiorellini, J.P.; Paquette, D.W.; Offenbacher, S.; Giannobile, W.V.; Lynch, S.E. A Phase I/II Clinical Trial to Evaluate a Combination of Recombinant Human Platelet-Derived Growth Factor-BB and Recombinant Human Insulin-like Growth Factor-I in Patients with Periodontal Disease. J. Periodontol. 1997, 68, 1186–1193. [Google Scholar] [CrossRef]
- Devi, R.; Dixit, J. Clinical Evaluation of Insulin like Growth Factor-I and Vascular Endothelial Growth Factor with Alloplastic Bone Graft Material in the Management of Human Two Wall Intra-Osseous Defects. J. Clin. Diagn. Res. 2016, 10, ZC41–ZC46. [Google Scholar] [CrossRef]
- Watts, N.B.; Harris, S.T.; McClung, M.R.; Bilezikian, J.P.; Greenspan, S.L.; Luckey, M.M. Bisphosphonates and Osteonecrosis of the Jaw. Ann. Intern. Med. 2006, 145, 791–792. [Google Scholar] [CrossRef]
- Wotton, C.J.; Green, J.; Brown, A.; Armstrong, M.E.G.; Floud, S.; Beral, V.; Reeves, G.K. Million Women Study collaborators Use of Oral Bisphosphonates and Risk of Hospital Admission with Osteonecrosis of the Jaw: Large Prospective Cohort Study in UK Women. Bone 2019, 124, 69–74. [Google Scholar] [CrossRef]
- Ogata, K.; Matsumura, M.; Moriyama, M.; Katagiri, W.; Hibi, H.; Nakamura, S. Cytokine Mixtures Mimicking Secretomes From Mesenchymal Stem Cells Improve Medication-Related Osteonecrosis of the Jaw in a Rat Model. JBMR Plus 2018, 2, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Wennerberg, A. On Osseointegration in Relation to Implant Surfaces. Clin. Implant Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Lynch, S.E.; Buser, D.; Hernandez, R.A.; Weber, H.P.; Stich, H.; Fox, C.H.; Williams, R.C. Effects of the Platelet-Derived Growth Factor/Insulin-like Growth Factor-I Combination on Bone Regeneration around Titanium Dental Implants. Results of a Pilot Study in Beagle Dogs. J. Periodontol. 1991, 62, 710–716. [Google Scholar] [CrossRef]
- Stefani, C.M.; Machado, M.A.; Sallum, E.A.; Sallum, A.W.; Toledo, S.; Nociti, F.H. Platelet-Derived Growth Factor/Insulin-like Growth Factor-1 Combination and Bone Regeneration around Implants Placed into Extraction Sockets: A Histometric Study in Dogs. Implant Dent. 2000, 9, 126–131. [Google Scholar] [CrossRef]
- Nociti Júnior, F.H.; Stefani, C.M.; Machado, M.A.; Sallum, E.A.; Toledo, S.; Sallum, A.W. Histometric Evaluation of Bone Regeneration around Immediate Implants Partially in Contact with Bone: A Pilot Study in Dogs. Implant Dent. 2000, 9, 321–328. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Furuhashi, A.; Yamaza, T.; Tsukiyama, Y.; Koyano, K. Promotive Effect of Insulin-like Growth Factor-1 for Epithelial Sealing to Titanium Implants. J. Biomed. Mater. Res. A 2013, 101, 2896–2904. [Google Scholar] [CrossRef] [PubMed]
- Abduljabbar, T.; Kellesarian, S.V.; Vohra, F.; Akram, Z.; Kotsakis, G.A.; Yunker, M.; Romanos, G.E.; Javed, F. Effect of Growth Hormone Supplementation on Osseointegration: A Systematic Review and Meta-Analyses. Implant Dent. 2017, 26, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Morberg, P.H.; Isaksson, O.G.; Johansson, C.B.; Sandstedt, J.; Törnell, J. Improved Long-Term Bone-Implant Integration. Experiments in Transgenic Mice Overexpressing Bovine Growth Hormone. Acta Orthop. Scand 1997, 68, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Moreno, G.; Cutando, A.; Arana, C.; Worf, C.V.; Guardia, J.; Muñoz, F.; Lopez-Peña, M.; Stephenson, J. The Effects of Growth Hormone on the Initial Bone Formation around Implants. Int. J. Oral Maxillofac. Implants 2009, 24, 1068–1073. [Google Scholar] [PubMed]
- Calvo-Guirado, J.L.; Mate-Sanchez, J.; Delgado-Ruiz, R.; Ramirez-Fernández, M.P.; Cutando-Soriano, A.; Peña, M. Effects of Growth Hormone on Initial Bone Formation around Dental Implants: A Dog Study. Clin. Oral Implants Res. 2011, 22, 587–593. [Google Scholar] [CrossRef]
- Muñoz, F.; López-Peña, M.; Miño, N.; Gómez-Moreno, G.; Guardia, J.; Cutando, A. Topical Application of Melatonin and Growth Hormone Accelerates Bone Healing around Dental Implants in Dogs. Clin. Implant Dent. Relat. Res. 2012, 14, 226–235. [Google Scholar] [CrossRef]
- Hossam Eldein, A.M.; Elghamrawy, S.H.; Osman, S.M.; Elhak, A.R. Histological Evaluation of the Effect of Using Growth Hormone around Immediate Dental Implants in Fresh Extraction Sockets: An Experimental Study. Implant Dent. 2011, 20, 47–55. [Google Scholar] [CrossRef]
- Brody-Camp, S.; Winters, R. Craniofacial Distraction Osteogenesis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Dryl, D.; Grabowska, S.Z.; Citko, A.; Pałka, J.; Antonowicz, B.; Rogowski, F. Insulin-like Growth Factor-I (IGF-I) in Serum and Bone Tissue during Rat Mandible Fracture Healing. Rocz Akad Med. Bialymst 2001, 46, 290–299. [Google Scholar]
- Camati, P.R.; Giovanini, A.F.; de Miranda Peixoto, H.E.; Schuanka, C.M.; Giacomel, M.C.; de Araújo, M.R.; Zielak, J.C.; Scariot, R.; Deliberador, T.M. Immunoexpression of IGF1, IGF2, and Osteopontin in Craniofacial Bone Repair Associated with Autogenous Grafting in Rat Models Treated with Alendronate Sodium. Clin. Oral Investig. 2017, 21, 1895–1903. [Google Scholar] [CrossRef]
- Farhadieh, R.D.; Dickinson, R.; Yu, Y.; Gianoutsos, M.P.; Walsh, W.R. The Role of Transforming Growth Factor-Beta, Insulin-like Growth Factor I, and Basic Fibroblast Growth Factor in Distraction Osteogenesis of the Mandible. J. Craniofac. Surg. 1999, 10, 80–86. [Google Scholar] [CrossRef]
- Steinbrech, D.S.; Mehrara, B.J.; Rowe, N.M.; Dudziak, M.E.; Saadeh, P.B.; Gittes, G.K.; Longaker, M.T. Gene Expression of Insulin-like Growth Factors I and II in Rat Membranous Osteotomy Healing. Ann. Plast Surg. 1999, 42, 481–487. [Google Scholar] [CrossRef]
- Tavakoli, K.; Yu, Y.; Shahidi, S.; Bonar, F.; Walsh, W.R.; Poole, M.D. Expression of Growth Factors in the Mandibular Distraction Zone: A Sheep Study. Br. J. Plast Surg. 1999, 52, 434–439. [Google Scholar] [CrossRef]
- Yates, K.E.; Troulis, M.J.; Kaban, L.B.; Glowacki, J. IGF-I, TGF-Beta, and BMP-4 Are Expressed during Distraction Osteogenesis of the Pig Mandible. Int. J. Oral Maxillofac. Surg. 2002, 31, 173–178. [Google Scholar] [CrossRef]
- Kim, I.S.; Cho, T.H.; Lee, Z.H.; Hwang, S.J. Bone Regeneration by Transplantation of Human Mesenchymal Stromal Cells in a Rabbit Mandibular Distraction Osteogenesis Model. Tissue Eng. Part A 2013, 19, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Weiss, S.; Baumgart, R.; Jochum, M.; Strasburger, C.J.; Bidlingmaier, M. Systemic Regulation of Distraction Osteogenesis: A Cascade of Biochemical Factors. J. Bone Miner Res. 2002, 17, 1280–1289. [Google Scholar] [CrossRef]
- Theyse, L.F.H.; Oosterlaken-Dijksterhuis, M.A.; van Doorn, J.; Terlou, M.; Mol, J.A.; Voorhout, G.; Hazewinkel, H.A.W. Expression of Osteotropic Growth Factors and Growth Hormone Receptor in a Canine Distraction Osteogenesis Model. J. Bone Miner Metab. 2006, 24, 266–273. [Google Scholar] [CrossRef]
- Stewart, K.J.; Weyand, B.; van’t Hof, R.J.; White, S.A.; Lvoff, G.O.; Maffulli, N.; Poole, M.D. A Quantitative Analysis of the Effect of Insulin-like Growth Factor-1 Infusion during Mandibular Distraction Osteogenesis in Rabbits. Br. J. Plast Surg. 1999, 52, 343–350. [Google Scholar] [CrossRef]
- Cho, B.C.; Moon, J.H.; Chung, H.Y.; Park, J.W.; Kweon, I.C.; Kim, I.S. The Bone Regenerative Effect of Growth Hormone on Consolidation in Mandibular Distraction Osteogenesis of a Dog Model. J. Craniofac. Surg. 2003, 14, 417–425. [Google Scholar] [CrossRef]
- Cho, B.C.; Kim, J.Y.; Lee, J.H.; Chung, H.Y.; Park, J.W.; Roh, K.H.; Kim, G.U.; Kwon, I.C.; Jang, K.H.; Lee, D.-S.; et al. The Bone Regenerative Effect of Chitosan Microsphere-Encapsulated Growth Hormone on Bony Consolidation in Mandibular Distraction Osteogenesis in a Dog Model. J. Craniofac. Surg. 2004, 15, 299–311, discussion 312–313. [Google Scholar] [CrossRef]
- Deppe, H.; Stemberger, A.; Hillemanns, M. Effects of Osteopromotive and Anti-Infective Membranes on Bone Regeneration: An Experimental Study in Rat Mandibular Defects. Int. J. Oral Maxillofac. Implants 2003, 18, 369–376. [Google Scholar]
- Srouji, S.; Rachmiel, A.; Blumenfeld, I.; Livne, E. Mandibular Defect Repair by TGF-Beta and IGF-1 Released from a Biodegradable Osteoconductive Hydrogel. J. Craniomaxillofac. Surg. 2005, 33, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Cacciafesta, V.; Dalstra, M.; Bosch, C.; Melsen, B.; Andreassen, T.T. Growth Hormone Treatment Promotes Guided Bone Regeneration in Rat Calvarial Defects. Eur. J. Orthod. 2001, 23, 733–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonietto, L.; Vasquez, A.F.; Dos Santos, L.A.; Weber, J.B. Histological and Structural Evaluation of Growth Hormone and PLGA Incorporation in Macroporous Scaffold of α-Tricalcium Phosphate Cement. J. Biomater. Appl. 2019, 33, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.H.; Giovanini, A.F.; Zielak, J.C.; Scariot, R.; Gonzaga, C.C.; Storrer, C.L.M.; Khajotia, S.S.; Esteban Florez, F.L.; Deliberador, T.M. Growth Hormone Effects on Healing Efficacy, Bone Resorption and Renal Morphology of Rats: Histological and Histometric Study in Rat Calvaria. Heliyon 2020, 6, e05226. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-S.; Nam, Y.-S.; Kang, J.-H.; Yang, D.-W.; Kim, D.-Y.; Lee, S.-Y.; Ko, H.-M.; Kim, M.-S.; Kim, S.-H. Regulatory Role of Insulin-like Growth Factor-Binding Proteins in Odontogenic Mineralization in Rats. J. Mol. Histol. 2021, 52, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.A. Current Ideas on the Biology of IGFBP-6: More than an IGF-II Inhibitor? Growth Horm. IGF Res. 2016, 30–31, 81–86. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Park, J.; Shin, J.-H.; Chang, M.-S. Insulin-like Growth Factor Binding Protein-6 Released from Human Mesenchymal Stem Cells Confers Neuronal Protection through IGF-1R-Mediated Signaling. Int. J. Mol. Med. 2017, 40, 1860–1868. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xing, H.; Qi, F.; Liu, H.; Gao, L.; Wang, X. Local Delivery of Insulin/IGF-1 for Bone Regeneration: Carriers, Strategies, and Effects. Nanotheranostics 2020, 4, 242–255. [Google Scholar] [CrossRef]
- Isaac, J.; Nassif, A.; Asselin, A.; Taïhi, I.; Fohrer-Ting, H.; Klein, C.; Gogly, B.; Berdal, A.; Robert, B.; Fournier, B.P. Involvement of Neural Crest and Paraxial Mesoderm in Oral Mucosal Development and Healing. Biomaterials 2018, 172, 41–53. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koffi, K.A.; Doublier, S.; Ricort, J.-M.; Babajko, S.; Nassif, A.; Isaac, J. The Role of GH/IGF Axis in Dento-Alveolar Complex from Development to Aging and Therapeutics: A Narrative Review. Cells 2021, 10, 1181. https://doi.org/10.3390/cells10051181
Koffi KA, Doublier S, Ricort J-M, Babajko S, Nassif A, Isaac J. The Role of GH/IGF Axis in Dento-Alveolar Complex from Development to Aging and Therapeutics: A Narrative Review. Cells. 2021; 10(5):1181. https://doi.org/10.3390/cells10051181
Chicago/Turabian StyleKoffi, Kouassi Armel, Sophie Doublier, Jean-Marc Ricort, Sylvie Babajko, Ali Nassif, and Juliane Isaac. 2021. "The Role of GH/IGF Axis in Dento-Alveolar Complex from Development to Aging and Therapeutics: A Narrative Review" Cells 10, no. 5: 1181. https://doi.org/10.3390/cells10051181
APA StyleKoffi, K. A., Doublier, S., Ricort, J.-M., Babajko, S., Nassif, A., & Isaac, J. (2021). The Role of GH/IGF Axis in Dento-Alveolar Complex from Development to Aging and Therapeutics: A Narrative Review. Cells, 10(5), 1181. https://doi.org/10.3390/cells10051181





