Metabolic Signatures in Coronary Artery Disease: Results from the BioHEART-CT Study
Abstract
:1. Introduction
2. Methods
2.1. BioHEART-CT Study
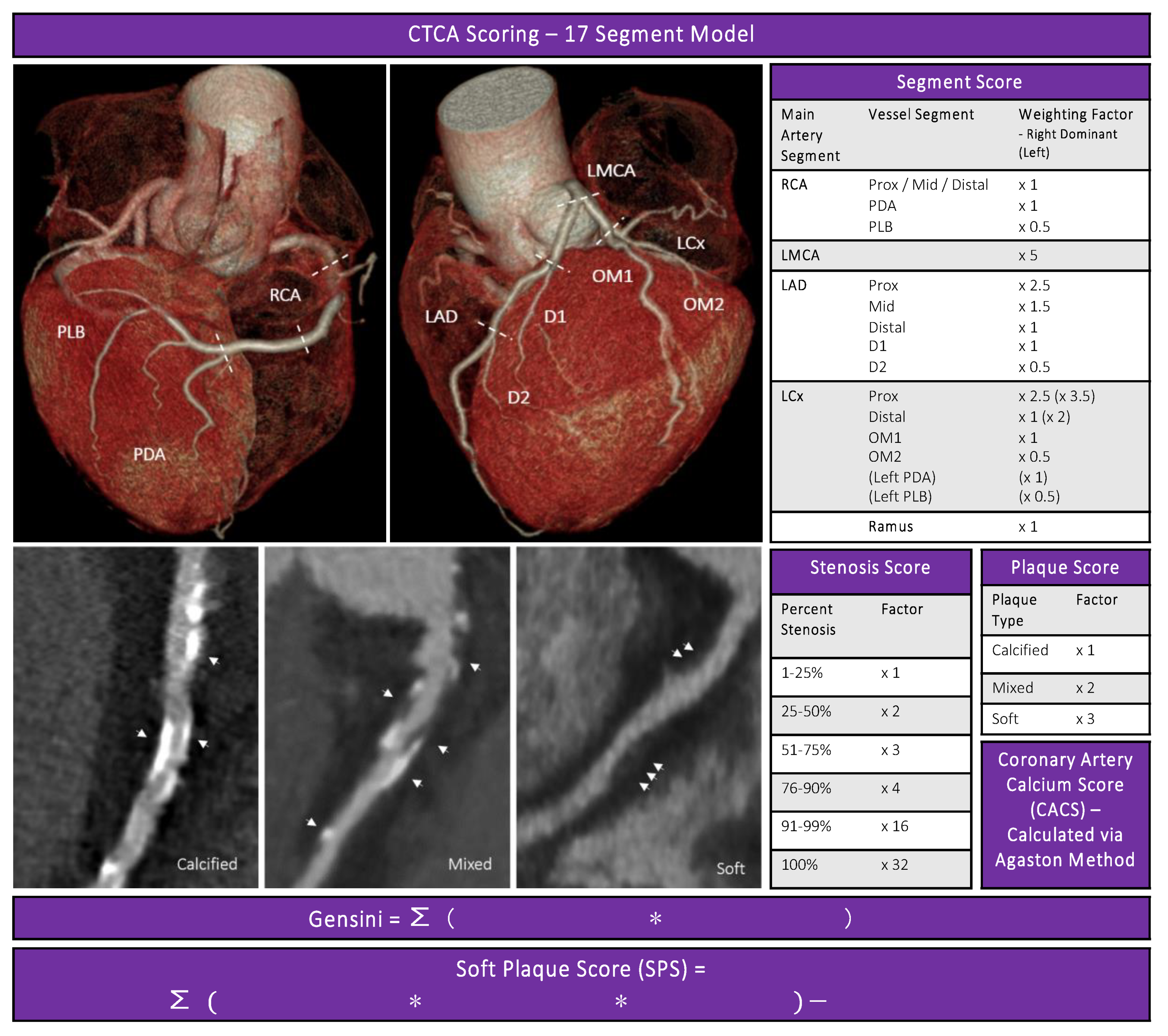
2.2. CTCA
2.3. CTCA Disease Severity Scores
2.4. Coronary Artery Calcium Score
2.5. Gensini Score
2.6. Soft Plaque Score (SPS)
2.7. Plasma Sample Processing and Extraction Procedure
2.8. Liquid Chromatography Mass Spectrometry
2.9. Data Processing and Normalisation
2.10. Candidate Metabolites
2.11. Total Metabolite Analysis
2.12. Metabolic Pathways
2.13. Statistical Analysis
3. Results
3.1. Candidate Metabolite Associations with CAD
3.2. Metabolomic Analyses
3.3. CAD Associations with Metabolic Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawber, T.R.; Moore, F.E.; Mann, G.V. Coronary heart disease in the Framingham study. Am. J. Public Health Nations Health 1957, 47, 4–24. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef] [Green Version]
- Vernon, S.T.; Coffey, S.; Bhindi, R.; Hoo, S.Y.S.; Nelson, G.I.; Ward, M.R.; Hansen, P.S.; Asrress, K.N.; Chow, C.K.; Celermajer, D.S.; et al. Increasing proportion of ST elevation myocardial infarction patients with coronary atherosclerosis poorly explained by standard modifiable risk factors. Eur. J. Prev. Cardiol. 2017, 24, 1824–1830. [Google Scholar] [CrossRef]
- Vernon, S.T.; Coffey, S.; D’Souza, M.; Chow, C.K.; Kilian, J.; Hyun, K.; Shaw, J.A.; Adams, M.; Roberts-Thomson, P.; Brieger, D.; et al. ST-Segment-Elevation Myocardial Infarction (STEMI) Patients Without Standard Modifiable Cardiovascular Risk Factors- How Common Are They, and What Are Their Outcomes? J. Am. Heart Assoc. 2019, 8, e013296. [Google Scholar] [CrossRef] [PubMed]
- Khot, U.N.; Khot, M.B.; Bajzer, C.T.; Sapp, S.K.; Ohman, E.M.; Brener, S.J.; Ellis, S.G.; Lincoff, A.M.; Topol, E.J. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA 2003, 290, 898–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; Coggeshall, M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [Green Version]
- Nelson, C.P.; Goel, A.; Butterworth, A.S.; Kanoni, S.; Webb, T.R.; Marouli, E.; Zeng, L.; Ntalla, I.; Lai, F.Y.; Hopewell, J.C.; et al. Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat. Genet. 2017, 49, 1385–1391. [Google Scholar] [CrossRef]
- Fernández-Friera, L.; Fuster, V.; López-Melgar, B.; Oliva, B.; García-Ruiz, J.M.; Mendiguren, J.; Bueno, H.; Pocock, S.; Ibáñez, B.; Fernández-Ortiz, A.; et al. Normal LDL-Cholesterol Levels Are Associated With Subclinical Atherosclerosis in the Absence of Risk Factors. J. Am. Coll. Cardiol. 2017, 70, 2979–2991. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.G.; Blaha, M.J.; Krumholz, H.M.; Budoff, M.J.; Blankstein, R.; Sibley, C.T.; Agatston, A.; Blumenthal, R.S.; Nasir, K. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: The Multi-Ethnic Study of Atherosclerosis. Eur. Heart J. 2014, 35, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Kott, K.A.; Vernon, S.T.; Hansen, T.; Yu, C.; Bubb, K.J.; Coffey, S.; Sullivan, D.; Yang, J.; O’Sullivan, J.; Chow, C.; et al. Biobanking for discovery of novel cardiovascular biomarkers using imaging-quantified disease burden: Protocol for the longitudinal, prospective, BioHEART-CT cohort study. BMJ Open 2019, 9, e028649. [Google Scholar] [CrossRef] [Green Version]
- Vernon, S.T.; Hansen, T.; Kott, K.A.; Yang, J.Y.; O’Sullivan, J.F.; Figtree, G.A. Utilizing state-of-the-art “omics” technology and bioinformatics to identify new biological mechanisms and biomarkers for coronary artery disease. Microcirculation 2019, 26, e12488. [Google Scholar] [CrossRef] [PubMed]
- Erjavec, G.N.; Konjevod, M.; Perkovic, M.; Strac, D.S.; Tudor, L.; Barbas, C.; Grune, T.; Zarkovic, N.; Pivac, N. Short overview on metabolomic approach and redox changes in psychiatric disorders. Redox Biol. 2018, 14, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef]
- Ruiz-Canela, M.; Hruby, A.; Clish, C.B.; Liang, L.; Martínez-González, M.A.; Hu, F.B. Comprehensive Metabolomic Profiling and Incident Cardiovascular Disease: A Systematic Review. J. Am. Heart Assoc. 2017, 6, e005705. [Google Scholar] [CrossRef] [Green Version]
- Ottosson, F.; Ericson, U.; Almgren, P.; Smith, E.; Brunkwall, L.; Hellstrand, S.; Nilsson, P.M.; Orho-Melander, M.; Fernandez, C.; Melander, O. Dimethylguanidino Valerate: A Lifestyle-Related Metabolite Associated With Future Coronary Artery Disease and Cardiovascular Mortality. J. Am. Heart Assoc. 2019, 8, e012846. [Google Scholar] [CrossRef]
- Harden, S.; Bull, R.; Bury, R.; Castellano, E.; Clayton, B.; Hamilton, M.; Morgan-Hughes, G.; O’Regan, D.; Padley, S.; Roditi, G.; et al. The safe practice of CT coronary angiography in adult patients in UK imaging departments. Clin. Radiol. 2016, 71, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Abbara, S.; Blanke, P.; Maroules, C.D.; Cheezum, M.; Choi, A.D.; Han, B.K.; Marwan, M.; Naoum, C.; Norgaard, B.L.; Rubinshtein, R.; et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J. Cardiovasc. Comput. Tomogr. 2016, 10, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Gensini, G.G. A more meaningful scoring system for determining the severity of coronary heart disease. Am. J. Cardiol. 1983, 51, 606. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Leipsic, J.; Abbara, S.; Achenbach, S.; Cury, R.; Earls, J.P.; Mancini, G.J.; Nieman, K.; Pontone, G.; Raff, G.L. SCCT guidelines for the interpretation and reporting of coronary CT angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J. Cardiovasc. Comput. Tomogr. 2014, 8, 342–358. [Google Scholar] [CrossRef]
- Ahmadi, N.; Nabavi, V.; Hajsadeghi, F.; Flores, F.; French, W.J.; Mao, S.S.; Shavelle, D.; Ebrahimi, R.; Budoff, M. Mortality Incidence of Patients With Non-Obstructive Coronary Artery Disease Diagnosed by Computed Tomography Angiography. Am. J. Cardiol. 2011, 107, 10–16. [Google Scholar] [CrossRef]
- Voros, S.; Rinehart, S.; Qian, Z.; Joshi, P.; Vazquez, G.; Fischer, C.; Belur, P.; Hulten, E.; Villines, T.C. Coronary atherosclerosis imaging by coronary CT angiography: Current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc. Imaging 2011, 4, 537–548. [Google Scholar] [CrossRef] [Green Version]
- Voros, S.; Rinehart, S.; Qian, Z.; Vazquez, G.; Anderson, H.; Murrieta, L.; Wilmer, C.; Carlson, H.; Taylor, K.; Ballard, W.; et al. Prospective Validation of Standardized, 3-Dimensional, Quantitative Coronary Computed Tomographic Plaque Measurements Using Radiofrequency Backscatter Intravascular Ultrasound as Reference Standard in Intermediate Coronary Arterial Lesions: Results From the ATLANTA (Assessment of Tissue Characteristics, Lesion Morphology, and Hemodynamics by Angiography With Fractional Flow Reserve, Intravascular Ultrasound and Virtual Histology, and Noninvasive Computed Tomography in Atherosclerotic Plaques) I Study. JACC Cardiovasc. Interv. 2011, 4, 198–208. [Google Scholar] [PubMed] [Green Version]
- Lehman, S.J.; Schlett, C.L.; Bamberg, F.; Lee, H.; Donnelly, P.; Shturman, L.; Kriegel, M.F.; Brady, T.J.; Hoffmann, U. Assessment of Coronary Plaque Progression in Coronary Computed Tomography Angiography Using a Semiquantitative Score. JACC Cardiovasc. Imaging 2009, 2, 1262–1270. [Google Scholar] [CrossRef] [Green Version]
- Kimberly, W.T.; O’Sullivan, J.F.; Nath, A.K.; Keyes, M.; Shi, X.; Larson, M.G.; Yang, Q.; Long, M.T.; Vasan, R.; Peterson, R.T.; et al. Metabolite profiling identifies anandamide as a biomarker of nonalcoholic steatohepatitis. JCI Insight 2017, 2, e92989. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.F.; Morningstar, J.E.; Yang, Q.; Zheng, B.; Gao, Y.; Jeanfavre, S.; Scott, J.; Fernandez, C.; Zheng, H.; O’Connor, S.; et al. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J. Clin. Investig. 2017, 127, 4394–4402. [Google Scholar] [CrossRef] [PubMed]
- Koay, Y.C.; Stanton, K.; Kienzle, V.; Li, M.; Yang, J.; Celermajer, D.S.; O’Sullivan, J.F. Effect of chronic exercise in healthy young male adults: A metabolomic analysis. Cardiovasc. Res. 2020, 117, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Koay, Y.C.; Wali, J.A.; Luk, A.W.S.; Macia, L.; Cogger, V.C.; Pulpitel, T.J.; Wahl, D.; Solon-Biet, S.M.; Holmes, A.; Simpson, S.J.; et al. Ingestion of resistant starch by mice markedly increases microbiome-derived metabolites. FASEB J. 2019, 33, 8033–8042. [Google Scholar] [CrossRef]
- O’Sullivan, J.F.; Morningstar, J.E.; Zheng, B.; Jeanfavre, S.; Scott, J.; Yang, Q.; Fernandez, C.; Vasan, R.S.; Long, M.T.; Melander, O.; et al. DMGV is a Novel Marker of Liver Fat and Predicts Future Development of Type 2 Diabetes. Circulation 2016, 134, A13814-A. [Google Scholar]
- Patsch, C.; Challet-Meylan, L.; Thoma, E.C.; Urich, E.; Heckel, T.; O’Sullivan, J.F.; Grainger, S.J.; Kapp, F.G.; Sun, L.; Christensen, K.; et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 2015, 17, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Tang, O.; Vernon, S.T.; Kott, K.A.; Koay, Y.C.; Park, J.; James, D.; Speed, T.P.; Yang, P.; O’Sullivan, J.F.; et al. hRUV: Hierarchical approach to removal of unwanted variation for large-scale metabolomics data. bioRxiv 2020. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Kashani, H.; Zeraati, H.; Mohammad, K.; Goodarzynejad, H.; Mahmoudi, M.; Sadeghian, S.; Boroumand, M. Analyzing Gensini Score as a Semi-Continuous Outcome. J. Tehran Heart Cent. 2016, 11, 55–61. [Google Scholar]
- Gudmundsson, E.F.; Gudnason, V.; Sigurdsson, S.; Launer, L.J.; Harris, T.B.; Aspelund, T. Coronary artery calcium distributions in older persons in the AGES-Reykjavik study. Eur. J. Epidemiol. 2012, 27, 673–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pletcher, M.J.; Tice, J.A.; Pignone, M.; McCulloch, C.; Callister, T.Q.; Browner, W.S. What does my patient’s coronary artery calcium score mean? Combining information from the coronary artery calcium score with information from conventional risk factors to estimate coronary heart disease risk. BMC Med. 2004, 2, 31. [Google Scholar] [CrossRef] [Green Version]
- Pletcher, M.J.; Tice, J.A.; Pignone, M.; Browner, W.S. Using the coronary artery calcium score to predict coronary heart disease events: A systematic review and meta-analysis. Arch. Intern. Med. 2004, 164, 1285–1292. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2020. Available online: https://www.r-project.org/ (accessed on 20 November 2020).
- Bordoni, L.; Samulak, J.J.; Sawicka, A.K.; Pelikant-Malecka, I.; Radulska, A.; Lewicki, L.; Kalinowski, L.; Gabbianelli, R.; Olek, R.A. Trimethylamine N-oxide and the reverse cholesterol transport in cardiovascular disease: A cross-sectional study. Sci. Rep. 2020, 10, 18675. [Google Scholar] [CrossRef]
- Yao, M.-E.; Liao, P.-D.; Zhao, X.-J.; Wang, L. Trimethylamine-N-oxide has prognostic value in coronary heart disease: A meta-analysis and dose-response analysis. BMC Cardiovasc. Disord. 2020, 20, 7–9. [Google Scholar] [CrossRef]
- Kanitsoraphan, C.; Rattanawong, P.; Charoensri, S.; Senthong, V. Trimethylamine N-Oxide and Risk of Cardiovascular Disease and Mortality. Curr. Nutr. Rep. 2018, 7, 207–213. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.A.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplin, B.; Wang, Z.; Slaviero, A.; Tomlinson, J.; Dowsett, L.; Delahaye, M.; Salama, A.; Wheeler, D.C.; Leiper, J.; International Consortium for Blood Pressure Genome-Wide Association Studies. Alanine-Glyoxylate Aminotransferase-2 Metabolizes Endogenous Methylarginines, Regulates NO, and Controls Blood Pressure. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2892–2900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wali, J.A.; Koay, Y.C.; Chami, J.; Wood, C.; Corcilius, L.; Payne, R.J.; Rodionov, R.N.; Birkenfeld, A.L.; Samocha-Bonet, D.; Simpson, S.J.; et al. Nutritional and metabolic regulation of the metabolite dimethylguanidino valeric acid: An early marker of cardiometabolic disease. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E509–E518. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, R.N.; Murry, D.J.; Vaulman, S.F.; Stevens, J.W.; Lentz, S.R. Human alanine-glyoxylate aminotransferase 2 lowers asymmetric dimethylarginine and protects from inhibition of nitric oxide production. J. Biol. Chem. 2010, 285, 5385–5391. [Google Scholar] [CrossRef] [Green Version]
- Ganna, A.; Salihovic, S.; Sundström, J.; Broeckling, C.D.; Hedman, A.K.; Magnusson, P.K.E.; Pedersen, N.L.; Larsson, A.; Siegbahn, A.; Zilmer, M.; et al. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLoS Genet. 2014, 10, e1004801. [Google Scholar] [CrossRef]
- Lewis, G.D.; Wei, R.; Liu, E.; Yang, E.; Shi, X.; Martinovic, M.; Farrell, L.; Asnani, A.; Cyrille, M.; Ramanathan, A.; et al. Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J.Clin. Investig. 2008, 118, 3503–3512. [Google Scholar] [CrossRef]
- Leopold, J.A.; Loscalzo, J. Emerging Role of Precision Medicine in Cardiovascular Disease. Circ. Res. 2018, 122, 1302–1315. [Google Scholar] [CrossRef]
- Figtree, G.A.; Vernon, S.T.; Nicholls, S.J. Taking the next steps to implement polygenic risk scoring for improved risk stratification and primary prevention of coronary artery disease. Eur. J. Prev. Cardiol. 2020. [Google Scholar] [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar]


| Whole Cohort n = 1002 | CAD Present n = 656) | No CAD n = 346 | Calcified Plaque Present n = 593 | No Calcified Plaque n = 409 | Soft Plaque Present n = 540 | No Soft Plaque n = 462 | Obstructive CAD n = 181 | No CAD n = 346 | |
|---|---|---|---|---|---|---|---|---|---|
| Age-years, median (IQR) | 62 (53–70) | 65 (58–72) | 53 (46–60) | 66 (59–72) | 54 (46–62) | 66 (59–72) | 56 (47–64) | 60 (63–75) | 53 (46–60) |
| Female, n (%) | 447 (44.6) | 297 (45.3) | 150 (43.4) | 266 (44.9) | 181 (44.3) | 249 (46.1) | 198 (42.9) | 83 (45.9) | 150 (43.4) |
| BMI-kg/m2, mean (SD) | 26.9 (4.8) | 27.1 (4.8) | 26.5 (4.9) | 27.1 (23.8–29.8) | 26.6 (4.8) | 27.0 (4.7) | 26.8 (5.0) | 26.9 (4.7) | 26.5 (4.9) |
| Hypertension, n (%) | 390 (38.9) | 296 (45.1) | 94 (27.2) | 278 (46.9) | 112 (27.4) | 244 (45.2) | 146 (31.6) | 95 (52.5) | 94 (27.2) |
| Diabetes Mellitus, n (%) | 87 (8.7) | 66 (10.1) | 21 (6.1) | 63 (10.6) | 24 (5.9) | 55 (10.2) | 32 (6.9) | 22 (12.2) | 21 (6.1) |
| Significant Smoking History, n (%) | 207 (20.7) | 164 (25) | 43 (12.4) | 155 (26.1) | 52 (12.7) | 143 (26.5) | 64 (13.9) | 60 (33.1) | 43 (12.4) |
| Hypercholesterolaemia, n (%) | 600 (59.9) | 436 (66.5) | 164 (47.4) | 408 (68.8) | 192 (46.9) | 360 (66.7) | 240 (51.9) | 122 (67.4) | 164 (47.4) |
| SMURFs: | |||||||||
| 0, n (%) | 223 (22.3) | 104 (15.9) | 119 (34.4) | 81 (13.7) | 142 (34.7) | 82 (15.2) | 141 (30.5) | 22 (12.2) | 119 (34.4) |
| 1, n (%) | 419 (42.8) | 266 (40.5) | 153 (44.2) | 240 (40.5) | 179 (43.8) | 223 (41.3) | 196 (42.4) | 65 (35.9) | 153 (44.2) |
| 2, n (%) | 266 (26.5) | 205 (31.3) | 61 (17.6) | 194 (32.7) | 72 (17.6) | 165 (30.6) | 101 (21.9) | 62 (34.3) | 61 (17.6) |
| 3, n (%) | 80 (8.0) | 67 (10.2) | 13 (3.8) | 64 (10.8) | 16 (3.9) | 57 (10.6) | 23 (5.0) | 29 (16.0) | 13 (3.8) |
| 4, n (%) | 14 (1.4) | 14 (2.1) | 0 (0) | 14 (2.4) | 0 (0) | 13 (2.4) | 1 (0.2) | 3 (1.7) | 0 (0) |
| Significant Family History CAD, n (%) | 205 (20.5) | 135 (20.6) | 70 (20.2) | 118 (19.9) | 87 (21.3) | 111 (20.6) | 94 (20.3) | 37 (20.4) | 70 (20.2) |
| Oral anticoagulant, n (%) | 88 (8.8) | 69 (10.5) | 19 (5.5) | 62 (10.5) | 26 (6.4) | 55 (10.2) | 33 (7.1) | 19 (10.5) | 19 (5.5) |
| Antiplatelet, n (%) | 175 (17.5) | 128 (19.5) | 47 (13.6) | 120 (20.2) | 55 (13.4) | 108 (20.0) | 67 (14.5) | 44 (24.3) | 47 (13.6) |
| Statin, n (%) | 330 (32.9) | 268 (40.9) | 62 (17.9) | 259 (43.7) | 71 (17.4) | 227 (42.0) | 103 (22.3) | 85 (47.0) | 62 (17.9) |
| Beta Blocker, n (%) | 142 (14.2) | 108 (16.5) | 34 (9.8) | 100 (16.9) | 42 (10.3) | 86 (15.9) | 56 (12.1) | 40 (22.1) | 34 (9.8) |
| ACEi or ARB, n (%) | 316 (31.5) | 247 (37.3) | 69 (19.9) | 235 (39.6) | 81 (19.8) | 206 (38.1) | 110 (23.8) | 80 (44.2) | 69 (19.9) |
| Calcium Channel Blocker, n (%) | 102 (10.2) | 84 (12.8) | 18 (5.2) | 79 (13.3) | 23 (5.6) | 70 (13.0) | 32 (6.9) | 25 (13.8) | 18 (5.2) |
| Diuretics, n (%) | 72 (7.2) | 58 (8.8) | 14 (4.0) | 51 (8.6) | 21 (5.1) | 50 (9.3) | 22 (4.8) | 18 (9.9) | 14 (4.0) |
| CACS, median (IQR) | 9.9 (0–146.0) | 76 (11.1–314.5) | 0 (0-0) | 99.3 (23.5–371.4) | 0 (0–0) | 98.6 (18.3–371.9) | 0 (0–0) | 405.3 (124.7–967.7) | 0 (0-0) |
| Gensini, median (IQR) | 3.5 (0–11.5) | 8.5 (4–17.4) | 0 (0-0) | 9.5 (4.5–18.5) | 0 (0-0) | 10.0 (5.0–19.0) | 0 (0–0) | 23.5 (17.5–34.8) | 0 (0-0) |
| SPS, median (IQR) | 2.0 (0–9.5) | 6.5 (2.5–14.0) | 0 (0-0) | 6.5 (2.0–14.8) | 0 (0-0) | 8.5 (4.5–16.9) | 0 (0–0) | 20.5 (12.0–28.0) | 0 (0-0) |
| No CAD (Gensini = 0), n (%) | 346 (34.5) | 0 | 346 (100) | 0 (0) | 346 (84.6) | 0 (0) | 116 (25.1) | 0 (0) | 346 (100) |
| Univariate Binary Regressions | Multivariable Binary Regressions | Univariate Linear Regressions | Multivariable Linear Regressions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | ß Coeff | 95% CI | p Value | ß Coeff | 95% CI | p Value | |
| DMGV | ||||||||||||
| Gensini | 1.41 | 1.12 1.79 | 0.004 | 0.93 | 0.69 1.26 | 0.641 | 0.3 | 0.14 0.45 | <0.001 | 0.17 | 0.02 0.32 | 0.026 |
| CACS | 1.59 | 1.26 2.01 | 0 | 1.11 | 0.83 1.5 | 0.482 | 0.49 | 0.11 0.87 | 0.011 | 0.32 | −0.03 0.67 | 0.074 |
| SPS | 1.4 | 1.11 1.75 | 0.004 | 1.05 | 0.8 1.38 | 0.717 | 0.15 | −0.02 0.32 | 0.089 | 0.06 | −0.12 0.23 | 0.523 |
| Obstructive | 2.33 | 1.59 3.43 | <0.0001 | 1.65 | 0.92 2.96 | 0.094 | ||||||
| TMAO | ||||||||||||
| Gensini | 0.85 | 0.62 1.16 | 0.305 | 0.83 | 0.57 1.22 | 0.35 | −0.16 | −0.39 0.07 | 0.185 | −0.21 | −0.42 −0.01 | 0.044 |
| CACS | 0.98 | 0.72 1.32 | 0.885 | 1.03 | 0.71 1.5 | 0.88 | −0.46 | −0.99 0.08 | 0.093 | −0.58 | −1.05 −0.1 | 0.017 |
| SPS | 0.72 | 0.53 0.97 | 0.029 | 0.65 | 0.46 0.92 | 0.016 | −0.05 | −0.29 0.19 | 0.707 | −0.09 | −0.32 0.15 | 0.475 |
| Obstructive | 0.79 | 0.51 1.22 | 0.292 | 0.84 | 0.44 1.59 | 0.60 | ||||||
| Phenylala-nine | ||||||||||||
| Gensini | 0.99 | 0.65 1.48 | 0.943 | 0.85 | 0.51 1.44 | 0.556 | 0.33 | 0.04 0.62 | 0.025 | 0.26 | 0.01 0.52 | 0.046 |
| CACS | 1.12 | 0.75 1.67 | 0.57 | 0.97 | 0.59 1.62 | 0.921 | 0.88 | 0.23 1.53 | 0.008 | 0.77 | 0.19 1.35 | 0.01 |
| SPS | 0.88 | 0.6 1.3 | 0.526 | 0.73 | 0.46 1.15 | 0.176 | 0.29 | −0.01 0.58 | 0.058 | 0.24 | −0.05 0.53 | 0.106 |
| Obstructive | 1.84 | 1.01 3.33 | 0.046 | 1.22 | 0.51 2.91 | 0.66 | ||||||
| Glutamate | ||||||||||||
| Gensini | 1.15 | 0.84 1.57 | 0.398 | 0.96 | 0.65 1.41 | 0.819 | 0.14 | −0.09 0.36 | 0.234 | 0.1 | −0.1 0.3 | 0.333 |
| CACS | 1.21 | 0.89 1.65 | 0.214 | 1.07 | 0.73 1.57 | 0.736 | 0.05 | −0.48 0.58 | 0.863 | 0.05 | −0.43 0.53 | 0.833 |
| SPS | 1.48 | 1.09 2.01 | 0.011 | 1.42 | 1.00 2.03 | 0.053 | 0.12 | −0.12 0.36 | 0.326 | 0.08 | −0.16 0.31 | 0.529 |
| Obstructive | 1.25 | 0.78 2.01 | 0.361 | 1.30 | 0.64 2.66 | 0.472 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernon, S.T.; Tang, O.; Kim, T.; Chan, A.S.; Kott, K.A.; Park, J.; Hansen, T.; Koay, Y.C.; Grieve, S.M.; O’Sullivan, J.F.; et al. Metabolic Signatures in Coronary Artery Disease: Results from the BioHEART-CT Study. Cells 2021, 10, 980. https://doi.org/10.3390/cells10050980
Vernon ST, Tang O, Kim T, Chan AS, Kott KA, Park J, Hansen T, Koay YC, Grieve SM, O’Sullivan JF, et al. Metabolic Signatures in Coronary Artery Disease: Results from the BioHEART-CT Study. Cells. 2021; 10(5):980. https://doi.org/10.3390/cells10050980
Chicago/Turabian StyleVernon, Stephen T., Owen Tang, Taiyun Kim, Adam S. Chan, Katharine A. Kott, John Park, Thomas Hansen, Yen C. Koay, Stuart M. Grieve, John F. O’Sullivan, and et al. 2021. "Metabolic Signatures in Coronary Artery Disease: Results from the BioHEART-CT Study" Cells 10, no. 5: 980. https://doi.org/10.3390/cells10050980






