Macrophages and Immune Responses in Uterine Fibroids
Abstract
1. Uterine Leiomyoma: A Typical Fibrotic Pathology
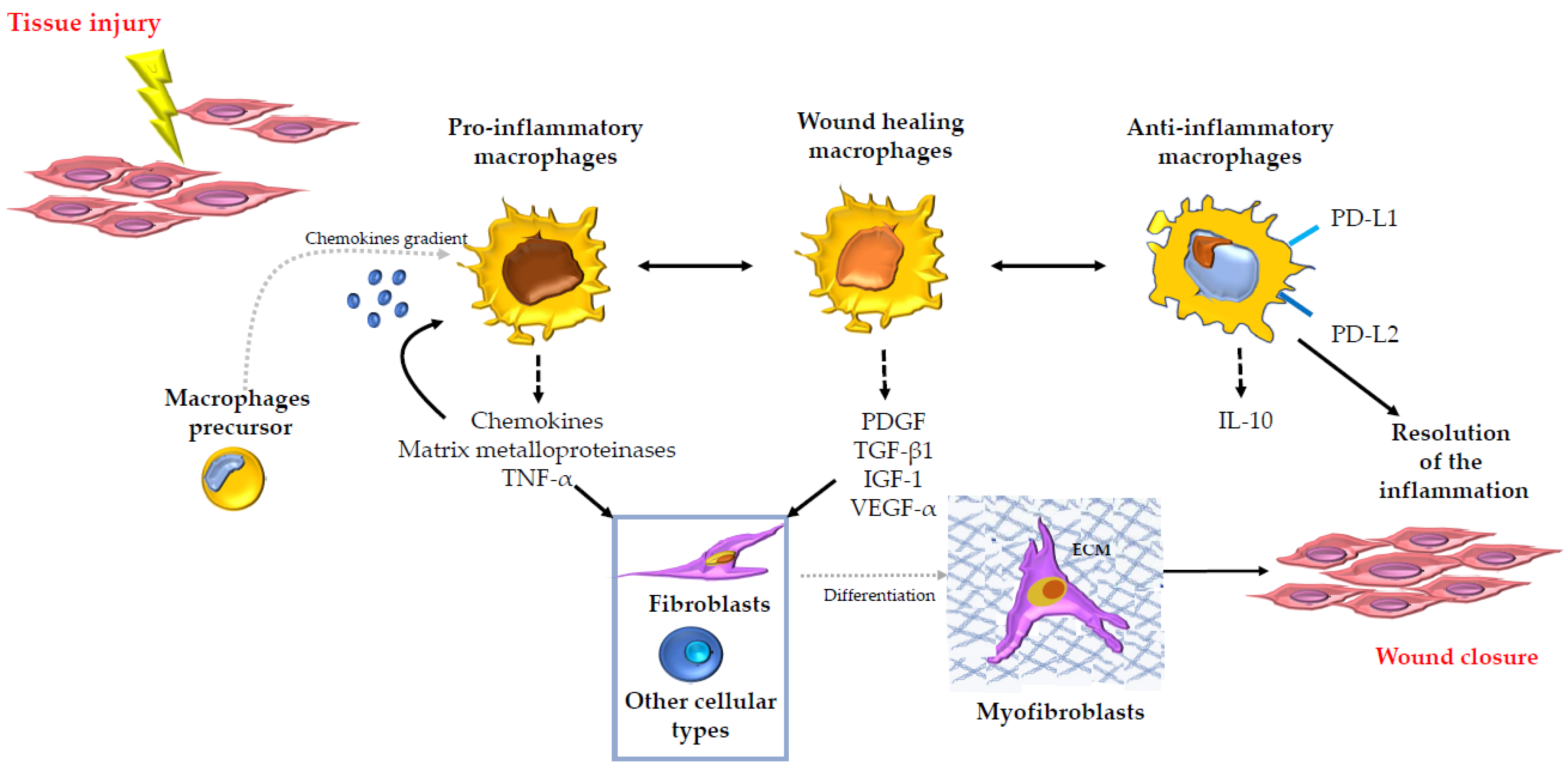
2. The Role of Macrophages in Tissue Repair and Fibrosis in Several Organs
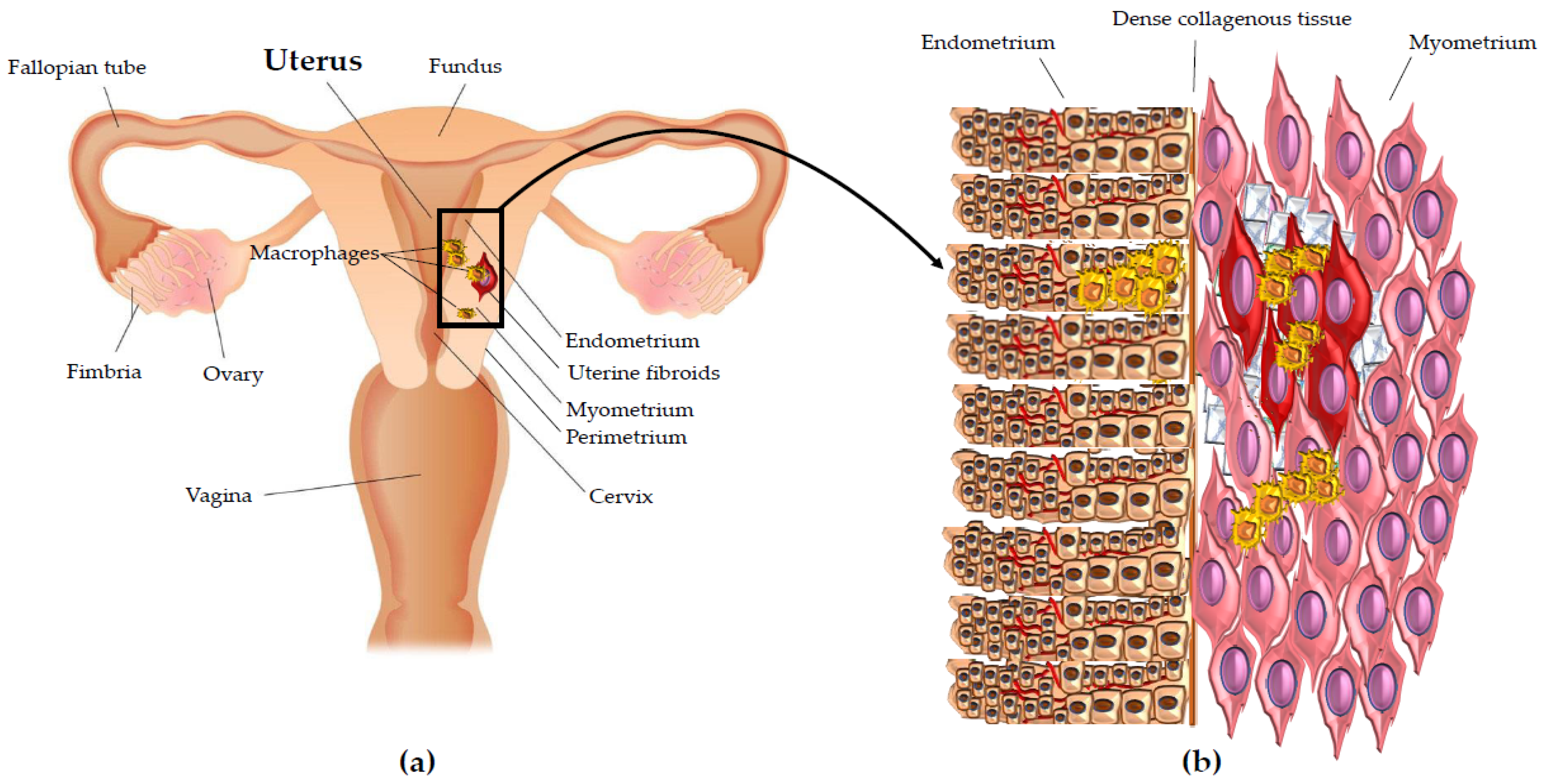
3. Macrophages in Uterine Fibroids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McEvoy, A.; Sabir, S. Physiology, Pregnancy Contractions; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Valente, R.; Malesani, M.G. Dizionario Medico; Larousse: Paris, France, 1984; p. 928. [Google Scholar]
- Gentile, F. Enciclopedia Italiana; Grolier: New Delhi, India, 1987; Volume 16, p. 271. [Google Scholar]
- Day Baird, D.; Dunson, D.B.; Hill, M.C.; Cousins, D.; Schectman, J.M. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am. J. Obstet. Gynecol. 2003, 188, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A. Uterine fibroids. Lancet 2001, 357, 293–298. [Google Scholar] [CrossRef]
- Buttram, V.C., Jr.; Reiter, R.C. Uterine leiomyomata: Etiology, symptomatology, and management. Fertil. Steril. 1981, 36, 433–445. [Google Scholar] [PubMed]
- Eldar-Geva, T.; Meagher, S.; Healy, D.L.; MacLachlan, V.; Breheny, S.; Wood, C. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil. Steril. 1998, 70, 687–691. [Google Scholar] [CrossRef]
- Evans, P.; Brunsell, S. Uterine fibroid tumors: Diagnosis and treatment. Am. Fam. Physician 2007, 75, 1503–1508. [Google Scholar]
- Marsh, E.E.; Bulun, S.E. Steroid hormones and leiomyomas. Obstet. Gynecol. Clin. N. Am. 2006, 33, 59–67. [Google Scholar] [CrossRef]
- Okolo, S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Protic, O.; Stortoni, P.; Grechi, G.; Lamanna, P.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil. Steril. 2013, 100, 178–193. [Google Scholar] [CrossRef]
- Ciarmela, P.; Islam, M.S.; Reis, F.M.; Gray, P.C.; Bloise, E.; Petraglia, F.; Vale, W.; Castellucci, M. Growth factors and myometrium: Biological effects in uterine fibroid and possible clinical implications. Hum. Reprod. Update 2011, 17, 772–790. [Google Scholar] [CrossRef]
- Lethaby, A.; Puscasiu, L.; Vollenhoven, B. Preoperative medical therapy before surgery for uterine fibroids. Cochrane Database Syst. Rev. 2017, 11, CD000547. [Google Scholar] [CrossRef]
- Angioni, S.; D’Alterio, M.N.; Daniilidis, A. Highlights on Medical Treatment of Uterine Fibroids. Curr. Pharm. Des. 2021. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.J.; Hoffman, D.I.; Comite, F.; Browneller, R.W.; Miller, J.D. Treatment of leiomyomata uteri with leuprolide acetate depot: A double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet. Gynecol. 1991, 77, 720–725. [Google Scholar]
- Schlaff, W.D.; Zerhouni, E.A.; Huth, J.A.; Chen, J.; Damewood, M.D.; Rock, J.A. A placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (leuprolide) in the treatment of uterine leiomyomata. Obstet. Gynecol 1989, 74, 856–862. [Google Scholar] [PubMed]
- Stovall, T.G.; Muneyyirci-Delale, O.; Summitt, R.L., Jr.; Scialli, A.R. GnRH agonist and iron versus placebo and iron in the anemic patient before surgery for leiomyomas: A randomized controlled trial. Leuprolide Acetate Study Group. Obstet. Gynecol. 1995, 86, 65–71. [Google Scholar] [CrossRef]
- Carbonell, J.L.; Acosta, R.; Perez, Y.; Garces, R.; Sanchez, C.; Tomasi, G. Treatment of Uterine Myoma with 2.5 or 5 mg Mifepristone Daily during 3 Months with 9 Months Posttreatment Followup: Randomized Clinical Trial. ISRN Obstret. Gynecol. 2013, 2013, 649030. [Google Scholar] [CrossRef]
- Chwalisz, K.; Larsen, L.; Mattia-Goldberg, C.; Edmonds, A.; Elger, W.; Winkel, C.A. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertil. Steril. 2007, 87, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Wiehle, R.D.; Goldberg, J.; Brodniewicz, T.; Jarus-Dziedzic, K.; Jabiry-Zieniewicz, Z. Effects of a new progesterone receptor modulator, CDB-4124, on fibroid size and uterine bleeding. Obstet. Gynecol. 2008, 3, 17–20. [Google Scholar]
- Levens, E.D.; Potlog-Nahari, C.; Armstrong, A.Y.; Wesley, R.; Premkumar, A.; Blithe, D.L.; Blocker, W.; Nieman, L.K. CDB-2914 for uterine leiomyomata treatment: A randomized controlled trial. Obstret. Gynecol. 2008, 111, 1129–1136. [Google Scholar] [CrossRef]
- Nieman, L.K.; Blocker, W.; Nansel, T.; Mahoney, S.; Reynolds, J.; Blithe, D.; Wesley, R.; Armstrong, A. Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: A randomized, double-blind, placebo-controlled, phase IIb study. Fertil. Steril. 2011, 95, 767–772.e2. [Google Scholar] [CrossRef]
- Donnez, J.; Tatarchuk, T.F.; Bouchard, P.; Puscasiu, L.; Zakharenko, N.F.; Ivanova, T.; Ugocsai, G.; Mara, M.; Jilla, M.P.; Bestel, E. Ulipristal acetate versus placebo for fibroid treatment before surgery. N. Engl. J. Med. 2012, 366, 409–420. [Google Scholar] [CrossRef]
- Donnez, J.; Tomaszewski, J.; Vázquez, F.; Bouchard, P.; Lemieszczuk, B.; Baró, F.; Nouri, K.; Selvaggi, L.; Sodowski, K.; Bestel, E. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N. Engl. J. Med. 2012, 366, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Blithe, D.L.; Nieman, L.K.; Blye, R.P.; Stratton, P.; Passaro, M. Development of the selective progesterone receptor modulator CDB-2914 for clinical indications. Steroids 2003, 68, 1013–1017. [Google Scholar] [CrossRef]
- Attardi, B.J.; Burgenson, J.; Hild, S.A.; Reel, J.R.; Blye, R.P. CDB-4124 and its putative monodemethylated metabolite, CDB-4453, are potent antiprogestins with reduced antiglucocorticoid activity: In vitro comparison to mifepristone and CDB-2914. Mol. Cell. Endocrinol. 2002, 188, 111–123. [Google Scholar] [CrossRef]
- Del Forno, S.; Degli Esposti, E.; Salucci, P.; Leonardi, D.; Iodice, R.; Arena, A.; Raimondo, D.; Paradisi, R.; Seracchioli, R. Liver function, tolerability and satisfaction during treatment with ulipristal acetate in women with fibroids: A single center experience. Gynecol. Endocrinol. 2020, 36, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Ciarmela, P.; Carrarelli, P.; Islam, M.S.; Janjusevic, M.; Zupi, E.; Tosti, C.; Castellucci, M.; Petraglia, F. Ulipristal acetate modulates the expression and functions of activin a in leiomyoma cells. Reprod. Sci. 2014, 21, 1120–1125. [Google Scholar] [CrossRef]
- Frasca, C.; Arena, A.; Degli Esposti, E.; Raimondo, D.; Del Forno, S.; Moro, E.; Zanello, M.; Mabrouk, M.; Seracchioli, R. First Impressions Can Be Deceiving: Surgical Outcomes of Laparoscopic Myomectomy in Patients Pretreated with Ulipristal Acetate. J. Minim. Invasive Gynecol. 2020, 27, 633–638. [Google Scholar] [CrossRef]
- Friedman, A.J.; Rein, M.S.; Harrison-Atlas, D.; Garfield, J.M.; Doubilet, P.M. A randomized, placebo-controlled, double-blind study evaluating leuprolide acetate depot treatment before myomectomy. Fertil. Steril. 1989, 52, 728–733. [Google Scholar] [CrossRef]
- Islam, M.S.; Protic, O.; Giannubilo, S.R.; Toti, P.; Tranquilli, A.L.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Uterine leiomyoma: Available medical treatments and new possible therapeutic options. J. Clin. Endocrinol. Metab. 2013, 98, 921–934. [Google Scholar] [CrossRef]
- Wallach, E.E.; Vlahos, N.F. Uterine myomas: An overview of development, clinical features, and management. Obstret. Gynecol. 2004, 104, 393–406. [Google Scholar] [CrossRef]
- Kim, T.; Purdy, M.P.; Kendall-Rauchfuss, L.; Habermann, E.B.; Bews, K.A.; Glasgow, A.E.; Khan, Z. Myomectomy associated blood transfusion risk and morbidity after surgery. Fertil. Steril. 2020, 114, 175–184. [Google Scholar] [CrossRef]
- Flynn, M.; Jamison, M.; Datta, S.; Myers, E. Health care resource use for uterine fibroid tumors in the United States. Am. J. Obstet. Gynecol. 2006, 195, 955–964. [Google Scholar] [CrossRef]
- Zepiridis, L.I.; Grimbizis, G.F.; Tarlatzis, B.C. Infertility and uterine fibroids. Best Pract. Res. Clin. Obstret. Gynaecol. 2016, 34, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.; Ellenson, L.; Ronnett, B. Blaustein’s Pathology of the Female Genital Tract. Int. J. Gynecol. Pathol. 2016. [Google Scholar] [CrossRef]
- Rosai, J.; Ackerman, L. Rosai and Ackerman’s Surgical Pathology, 10th ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1508–1513. [Google Scholar]
- Avritscher, R.; Iyer, R.B.; Ro, J.; Whitman, G. Lipoleiomyoma of the uterus. AJR Am. J. Roentgenol. 2001, 177, 856. [Google Scholar] [CrossRef] [PubMed]
- Myles, J.L.; Hart, W.R. Apoplectic leiomyomas of the uterus. A clinicopathologic study of five distinctive hemorrhagic leiomyomas associated with oral contraceptive usage. Am. J. Surg. Pathol. 1985, 9, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Ciarmela, P.; Islam, M.S.; Lamanna, P.; Tranquilli, A.L.; Castellucci, M. Healthy and pathological changes of myometrium: Pregnant myometrium, uterine fibroids and leiomyosarcoma. Rev. Argent. Anatomía Clínica 2012, 4, 7–13. [Google Scholar] [CrossRef]
- Toledo, G.; Oliva, E. Smooth muscle tumors of the uterus: A practical approach. Arch. Pathol. Lab. Med. 2008, 132, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Leppert, P.C.; Catherino, W.H.; Segars, J.H. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am. J. Obstret. Gynecol. 2006, 195, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Norian, J.; McCarthy-Keith, D.; Britten, J.; Catherino, W.H. Why leiomyomas are called fibroids: The central role of extracellular matrix in symptomatic women. Semin. Reprod. Med. 2010, 28, 169–179. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens-structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Fujita, M. Histological and biochemical studies of collagen in human uterine leiomyomas. Hokkaido J. Med. Sci. 1985, 60, 602–615. [Google Scholar] [PubMed]
- Arici, A.; Sozen, I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil. Steril. 2000, 73, 1006–1011. [Google Scholar] [CrossRef]
- Norian, J.M.; Malik, M.; Parker, C.Y.; Joseph, D.; Leppert, P.C.; Segars, J.H.; Catherino, W.H. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod. Sci. 2009, 16, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Friedman, A.J.; Peck, K.; Nowak, R.A. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J. Clin. Endocrinol. Metab. 1994, 79, 900–906. [Google Scholar] [PubMed]
- Giuliani, A.; Greco, S.; Pacile, S.; Zannotti, A.; Delli Carpini, G.; Tromba, G.; Giannubilo, S.R.; Ciavattini, A.; Ciarmela, P. Advanced 3D Imaging of Uterine Leiomyoma’s Morphology by Propagation-based Phase-Contrast Microtomography. Sci. Rep. 2019, 9, 10580. [Google Scholar] [CrossRef]
- Walker, C.L.; Stewart, E.A. Uterine fibroids: The elephant in the room. Science 2005, 308, 1589–1592. [Google Scholar] [CrossRef]
- Hulboy, D.L.; Rudolph, L.A.; Matrisian, L.M. Matrix metalloproteinases as mediators of reproductive function. Mol. Hum. Reprod. 1997, 3, 27–45. [Google Scholar] [CrossRef]
- Ohara, N. Sex steroidal modulation of collagen metabolism in uterine leiomyomas. Clin. Exp. Obstet. Gynecol. 2009, 36, 10. [Google Scholar]
- Protic, O.; Toti, P.; Islam, M.S.; Occhini, R.; Giannubilo, S.R.; Catherino, W.H.; Cinti, S.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016, 364, 415–427. [Google Scholar] [CrossRef]
- Flake, G.P.; Andersen, J.; Dixon, D. Etiology and pathogenesis of uterine leiomyomas: A review. Environ. Health Perspect. 2003, 111, 1037–1054. [Google Scholar] [CrossRef]
- Kiechle-Schwarz, M.; Sreekantaiah, C.; Berger, C.S.; Pedron, S.; Medchill, M.T.; Surti, U.; Sandberg, A.A. Nonrandom cytogenetic changes in leiomyomas of the female genitourinary tract. A report of 35 cases. Cancer Genet. Cytogenet. 1991, 53, 125–136. [Google Scholar] [CrossRef]
- Rein, M.S.; Friedman, A.J.; Barbieri, R.L.; Pavelka, K.; Fletcher, J.A.; Morton, C.C. Cytogenetic abnormalities in uterine leiomyomata. Obstet. Gynecol. 1991, 77, 923–926. [Google Scholar]
- Meloni, A.M.; Surti, U.; Contento, A.M.; Davare, J.; Sandberg, A.A. Uterine leiomyomas: Cytogenetic and histologic profile. Obstet. Gynecol. 1992, 80, 209–217. [Google Scholar] [PubMed]
- Cha, P.C.; Takahashi, A.; Hosono, N.; Low, S.K.; Kamatani, N.; Kubo, M.; Nakamura, Y. A genome-wide association study identifies three loci associated with susceptibility to uterine fibroids. Nat. Genet. 2011, 43, 447–450. [Google Scholar] [CrossRef]
- Ligon, A.H.; Scott, I.C.; Takahara, K.; Greenspan, D.S.; Morton, C.C. PCOLCE deletion and expression analyses in uterine leiomyomata. Cancer Genet. Cytogenet. 2002, 137, 133–137. [Google Scholar] [CrossRef]
- Ptacek, T.; Song, C.; Walker, C.L.; Sell, S.M. Physical mapping of distinct 7q22 deletions in uterine leiomyoma and analysis of a recently annotated 7q22 candidate gene. Cancer Genet. Cytogenet. 2007, 174, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Velagaleti, G.V.; Tonk, V.S.; Hakim, N.M.; Wang, X.; Zhang, H.; Erickson-Johnson, M.R.; Medeiros, F.; Oliveira, A.M. Fusion of HMGA2 to COG5 in uterine leiomyoma. Cancer Genet. Cytogenet. 2011, 202, 11–16. [Google Scholar] [CrossRef]
- Mäkinen, N.; Mehine, M.; Tolvanen, J.; Kaasinen, E.; Li, Y.; Lehtonen, H.J.; Gentile, M.; Yan, J.; Enge, M.; Taipale, M. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011, 334, 252–255. [Google Scholar] [CrossRef]
- Nezhad, M.H.; Drieschner, N.; Helms, S.; Meyer, A.; Tadayyon, M.; Klemke, M.; Belge, G.; Bartnitzke, S.; Burchardt, K.; Frantzen, C. 6p21 rearrangements in uterine leiomyomas targeting HMGA1. Cancer Genet. Cytogenet. 2010, 203, 247–252. [Google Scholar] [CrossRef]
- Sandberg, A.A. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: Leiomyoma. Cancer Genet. Cytogenet. 2005, 158, 1–26. [Google Scholar] [CrossRef]
- El-Gharib, M.N.; Elsobky, E.S. Cytogenetic aberrations and the development of uterine leiomyomata. J. Obstet. Gynaecol. Res. 2010, 36, 101–107. [Google Scholar] [CrossRef]
- Mehine, M.; Kaasinen, E.; Makinen, N.; Katainen, R.; Kampjarvi, K.; Pitkanen, E.; Heinonen, H.R.; Butzow, R.; Kilpivaara, O.; Kuosmanen, A.; et al. Characterization of uterine leiomyomas by whole-genome sequencing. N. Engl. J. Med. 2013, 369, 43–53. [Google Scholar] [CrossRef]
- Mehine, M.; Makinen, N.; Heinonen, H.R.; Aaltonen, L.A.; Vahteristo, P. Genomics of uterine leiomyomas: Insights from high-throughput sequencing. Fertil. Steril. 2014, 102, 621–629. [Google Scholar] [CrossRef]
- Marsh, E.E.; Lin, Z.; Yin, P.; Milad, M.; Chakravarti, D.; Bulun, S.E. Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertil. Steril. 2008, 89, 1771–1776. [Google Scholar] [CrossRef]
- Navarro, A.; Yin, P.; Monsivais, D.; Lin, S.M.; Du, P.; Wei, J.J.; Bulun, S.E. Genome-Wide DNA Methylation Indicates Silencing of Tumor Suppressor Genes in Uterine Leiomyoma. PLoS ONE 2012, 7, e33284. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.H.; Torng, P.L.; Hsiao, S.M.; Jeng, Y.M.; Chen, M.W.; Chen, C.A. Histone Deacetylase 6 Regulates Estrogen Receptor α in Uterine Leiomyoma. Reprod. Sci. 2011, 18, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Mas, A.; Diamond, M.P.; Al-Hendy, A. The Mechanism and Function of Epigenetics in Uterine Leiomyoma Development. Reprod. Sci. 2016, 23, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Obijuru, L.; Laser, J.; Aris, V.; Lee, P.; Mittal, K.; Soteropoulos, P.; Wei, J.J. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer 2007, 46, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Matsuo, H.; Xu, Q.; Chen, W.; Wang, J.; Maruo, T. Concentration-dependent effects of a selective estrogen receptor modulator raloxifene on proliferation and apoptosis in human uterine leiomyoma cells cultured in vitro. Hum. Reprod. 2007, 22, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, B.; Milev, I.; Minkov, I.; Dimitrova, I.; Bradford, A.P.; Baev, V. Characterization of the uterine leiomyoma microRNAome by deep sequencing. Genomics 2012, 93, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Wlodarczyk, M.; Zgliczynski, S.; Lozinski, T.; Walczak, K.; Czekierdowski, A. The Role of miRNA and Related Pathways in Pathophysiology of Uterine Fibroids-From Bench to Bedside. Int. J. Mol. Sci. 2020, 21, 3016. [Google Scholar] [CrossRef]
- Nothnick, W.B. Non-coding RNAs in Uterine Development, Function and Disease. Adv. Exp. Med. Biol. 2016, 886, 171–189. [Google Scholar] [CrossRef]
- Baranov, V.S.; Osinovskaya, N.S.; Yarmolinskaya, M.I. Pathogenomics of Uterine Fibroids Development. Int. J. Mol. Sci. 2019, 20, 6151. [Google Scholar] [CrossRef]
- McWilliams, M.M.; Chennathukuzhi, V.M. Recent Advances in Uterine Fibroid Etiology. Semin. Reprod. Med. 2017, 35, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Mallik, S.; Maulik, U. MiRNA-TF-gene network analysis through ranking of biomolecules for multi-informative uterine leiomyoma dataset. J. Biomed. Inf. 2015, 57, 308–319. [Google Scholar] [CrossRef]
- Ciarmela, P.; Bloise, E.; Gray, P.C.; Carrarelli, P.; Islam, M.S.; De Pascalis, F.; Severi, F.M.; Vale, W.; Castellucci, M.; Petraglia, F. Activin-A and myostatin response and steroid regulation in human myometrium: Disruption of their signalling in uterine fibroid. J. Clin. Endocrinol. Metab. 2011, 96, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Moro, E.; Degli Esposti, E.; Borghese, G.; Manzara, F.; Zanello, M.; Raimondo, D.; Gava, G.; Arena, A.; Casadio, P.; Meriggiola, M.C.; et al. The Impact of Hormonal Replacement Treatment in Postmenopausal Women with Uterine Fibroids: A State-of-the-Art Review of the Literature. Medicina (Kaunas) 2019, 55, 549. [Google Scholar] [CrossRef] [PubMed]
- Sozen, I.; Arici, A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil. Steril. 2002, 78, 1–12. [Google Scholar] [CrossRef]
- Ciarmela, P.; Wiater, E.; Vale, W. Activin-A in myometrium: Characterization of the actions on myometrial cells. Endocrinology 2008, 149, 2506–2516. [Google Scholar] [CrossRef] [PubMed]
- Ciarmela, P.; Wiater, E.; Smith, S.M.; Vale, W. Presence, actions, and regulation of myostatin in rat uterus and myometrial cells. Endocrinology 2009, 150, 906–914. [Google Scholar] [CrossRef]
- Hatthachote, P.; Gillespie, J.I. Complex interactions between sex steroids and cytokines in the human pregnant myometrium: Evidence for an autocrine signaling system at term. Endocrinology 1999, 140, 2533–2540. [Google Scholar] [CrossRef]
- Litovkin, K.V.; Domenyuk, V.P.; Bubnov, V.V.; Zaporozhan, V.N. Interleukin-6-174G/C polymorphism in breast cancer and uterine leiomyoma patients: A population-based case control study. Exp. Oncol. 2007, 29, 295–298. [Google Scholar]
- Kurachi, O.; Matsuo, H.; Samoto, T.; Maruo, T. Tumor necrosis factor-α expression in human uterine leiomyoma and its down-regulation by progesterone. J. Clin. Endocrinol. Metab. 2001, 86, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Syssoev, K.A.; Kulagina, N.V.; Chukhlovin, A.B.; Morozova, E.B.; Totolian, A.A. Expression of mRNA for chemokines and chemokine receptors in tissues of the myometrium and uterine leiomyoma. Bull. Exp. Biol. Med. 2008, 145, 84–89. [Google Scholar] [CrossRef]
- Sozen, I.; Olive, D.L.; Arici, A. Expression and hormonal regulation of monocyte chemotactic protein-1 in myometrium and leiomyomata. Fertil. Steril. 1998, 69, 1095–1102. [Google Scholar] [CrossRef]
- Nair, S.; Al-Hendy, A. Adipocytes enhance the proliferation of human leiomyoma cells via TNF-α proinflammatory cytokine. Reprod. Sci. 2011, 18, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Bodner-Adler, B.; Bodner, K.; Kimberger, O.; Czerwenka, K.; Leodolter, S.; Mayerhofer, K. Expression of matrix metalloproteinases in patients with uterine smooth muscle tumors: An immunohistochemical analysis of MMP-1 and MMP-2 protein expression in leiomyoma, uterine smooth muscle tumor of uncertain malignant potential, and leiomyosarcoma. J. Soc. Gynecol. Investig. 2004, 11, 182–186. [Google Scholar] [CrossRef]
- Wolanska, M.; Sobolewski, K.; Bańkowski, E.; Jaworski, S. Matrix metalloproteinases of human leiomyoma in various stages of tumor growth. Gynecol. Obstet. Investig. 2004, 58, 14–18. [Google Scholar] [CrossRef]
- Bogusiewicz, M.; Stryjecka-Zimmer, M.; Postawski, K.; Jakimiuk, A.J.; Rechberger, T. Activity of matrix metalloproteinase-2 and-9 and contents of their tissue inhibitors in uterine leiomyoma and corresponding myometrium. Gynecol. Endocrinol. 2007, 23, 541–546. [Google Scholar] [CrossRef]
- Walker, C.L.; Hunter, D.; Everitt, J.I. Uterine leiomyoma in the Eker rat: A unique model for important diseases of women. Genes Chromosomes Cancer 2003, 38, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D.; Walker, C.L. The Eker rat: Establishing a genetic paradigm linking renal cell carcinoma and uterine leiomyoma. Curr. Mol. Med. 2004, 4, 813–824. [Google Scholar] [CrossRef]
- Andersen, J.; Barbieri, R.L. Abnormal gene expression in uterine leiomyomas. J. Soc. Gynecol. Investig. 1995, 2, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Ohara, N.; Wang, J.; Matsuo, H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum. Reprod. Update 2004, 10, 207–220. [Google Scholar] [CrossRef]
- Sadan, O.; Van Iddekinge, B.; Van Gelderen, C.J.; Savage, N.; Becker, P.J.; Van Der Walt, L.A.; Robinson, M. Oestrogen and progesterone receptor concentrations in leiomyoma and normal myometrium. Ann. Clin. Biochem. 1987, 24, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Sefton, E.C. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol. Cell. Endocrinol. 2011, 358, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Brandon, D.D.; Bethea, C.L.; Strawn, E.Y.; Novy, M.J.; Burry, K.A.; Harrington, M.S.; Erickson, T.E.; Warner, C.; Keenan, E.J.; Clinton, G.M. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am. J. Obstet. Gynecol. 1993, 169, 78–85. [Google Scholar] [CrossRef]
- Marelli, G.; Codegoni, A.M.; Bizzi, A. Estrogen and progesterone receptors in leiomyomas and normal uterine tissues during reproductive life. Acta Eur. Fertil. 1989, 20, 19–22. [Google Scholar]
- Viville, B.; Charnock-Jones, D.S.; Sharkey, A.M.; Wetzka, B.; Smith, S.K. Distribution of the A and B forms of the progesterone receptor messenger ribonucleic acid and protein in uterine leiomyomata and adjacent myometrium. Hum. Reprod. 1997, 12, 815–822. [Google Scholar] [CrossRef][Green Version]
- Ying, Z.; Weiyuan, Z. Dual actions of progesterone on uterine leiomyoma correlate with the ratio of progesterone receptor A:B. Gynecol. Endocrinol. 2009, 25, 520–523. [Google Scholar] [CrossRef]
- Fujimoto, J.; Hirose, R.; Ichigo, S.; Sakaguchi, H.; Li, Y.; Tamaya, T. Expression of progesterone receptor form A and B mRNAs in uterine leiomyoma. Tumor Biol. 1998, 19, 126–131. [Google Scholar] [CrossRef]
- Protic, O.; Islam, M.S.; Greco, S.; Giannubilo, S.R.; Lamanna, P.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; Hinz, B.; Ciarmela, P. Activin A in Inflammation, Tissue Repair, and Fibrosis: Possible Role as Inflammatory and Fibrotic Mediator of Uterine Fibroid Development and Growth. Semin. Reprod. Med. 2017, 35, 499–509. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D.A. Mechanisms of fibrogenesis. Exp. Biol. Med. 2008, 233, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Prunotto, M.; Desmouliere, A.; Varga, J.; De Wever, O.; Mareel, M.; Gabbiani, G. Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling. Am. J. Pathol. 2012, 180, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Derm. 2007, 127, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Kacperczyk, J.; Bartnik, P.; Romejko-Wolniewicz, E.; Dobrowolska-Redo, A. Postmyomectomic Uterine Rupture Despite Cesarean Section. Anticancer Res. 2016, 36, 1011–1013. [Google Scholar]
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Lech, M.; Anders, H.J. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta 2013, 1832, 989–997. [Google Scholar] [CrossRef]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.J.; Ruckerl, D.; Cook, P.C.; Jones, L.H.; Finkelman, F.D.; van Rooijen, N.; MacDonald, A.S.; Allen, J.E. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 2011, 332, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.J.; Ruckerl, D.; Thomas, G.D.; Hewitson, J.P.; Duncan, S.; Brombacher, F.; Maizels, R.M.; Hume, D.A.; Allen, J.E. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J. Exp. Med. 2013, 210, 2477–2491. [Google Scholar] [CrossRef]
- Berse, B.; Brown, L.F.; Van de Water, L.; Dvorak, H.F.; Senger, D.R. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol. Biol. Cell 1992, 3, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Chujo, S.; Shirasaki, F.; Kondo-Miyazaki, M.; Ikawa, Y.; Takehara, K. Role of connective tissue growth factor and its interaction with basic fibroblast growth factor and macrophage chemoattractant protein-1 in skin fibrosis. J. Cell Physiol. 2009, 220, 189–195. [Google Scholar] [CrossRef]
- Rappolee, D.A.; Mark, D.; Banda, M.J.; Werb, Z. Wound macrophages express TGF-alpha and other growth factors in vivo: Analysis by mRNA phenotyping. Science 1988, 241, 708–712. [Google Scholar] [CrossRef]
- Shimokado, K.; Raines, E.W.; Madtes, D.K.; Barrett, T.B.; Benditt, E.P.; Ross, R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell 1985, 43, 277–286. [Google Scholar] [CrossRef]
- Willenborg, S.; Lucas, T.; van Loo, G.; Knipper, J.A.; Krieg, T.; Haase, I.; Brachvogel, B.; Hammerschmidt, M.; Nagy, A.; Ferrara, N.; et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012, 120, 613–625. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Resolution of liver fibrosis: Basic mechanisms and clinical relevance. Semin. Liver Dis. 2015, 35, 119–131. [Google Scholar] [CrossRef]
- Khalil, N.; Bereznay, O.; Sporn, M.; Greenberg, A.H. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J. Exp. Med. 1989, 170, 727–737. [Google Scholar] [CrossRef]
- Said, E.A.; Dupuy, F.P.; Trautmann, L.; Zhang, Y.; Shi, Y.; El-Far, M.; Hill, B.J.; Noto, A.; Ancuta, P.; Peretz, Y.; et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 2010, 16, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Shouval, D.S.; Biswas, A.; Goettel, J.A.; McCann, K.; Conaway, E.; Redhu, N.S.; Mascanfroni, I.D.; Al Adham, Z.; Lavoie, S.; Ibourk, M.; et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 2014, 40, 706–719. [Google Scholar] [CrossRef]
- Zigmond, E.; Bernshtein, B.; Friedlander, G.; Walker, C.R.; Yona, S.; Kim, K.W.; Brenner, O.; Krauthgamer, R.; Varol, C.; Muller, W.; et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 2014, 40, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Saini, J.; McPhee, J.S.; Al-Dabbagh, S.; Stewart, C.E.; Al-Shanti, N. Regenerative function of immune system: Modulation of muscle stem cells. Ageing Res. Rev. 2016, 27, 67–76. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Stables, M.J.; Shah, S.; Camon, E.B.; Lovering, R.C.; Newson, J.; Bystrom, J.; Farrow, S.; Gilroy, D.W. Transcriptomic analyses of murine resolution-phase macrophages. Blood 2011, 118, e192–e208. [Google Scholar] [CrossRef]
- Varga, T.; Mounier, R.; Horvath, A.; Cuvellier, S.; Dumont, F.; Poliska, S.; Ardjoune, H.; Juban, G.; Nagy, L.; Chazaud, B. Highly Dynamic Transcriptional Signature of Distinct Macrophage Subsets during Sterile Inflammation, Resolution, and Tissue Repair. J. Immunol. 2016, 196, 4771–4782. [Google Scholar] [CrossRef]
- Varga, T.; Mounier, R.; Patsalos, A.; Gogolak, P.; Peloquin, M.; Horvath, A.; Pap, A.; Daniel, B.; Nagy, G.; Pintye, E.; et al. Macrophage PPARgamma, a Lipid Activated Transcription Factor Controls the Growth Factor GDF3 and Skeletal Muscle Regeneration. Immunity 2016, 45, 1038–1051. [Google Scholar] [CrossRef]
- Heredia, J.E.; Mukundan, L.; Chen, F.M.; Mueller, A.A.; Deo, R.C.; Locksley, R.M.; Rando, T.A.; Chawla, A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 2013, 153, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; O’Dea, E.L.; Doedens, A.; Kim, J.W.; Weidemann, A.; Stockmann, C.; Asagiri, M.; Simon, M.C.; Hoffmann, A.; Johnson, R.S. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010, 24, 491–501. [Google Scholar] [CrossRef]
- Gondin, J.; Theret, M.; Duhamel, G.; Pegan, K.; Mathieu, J.R.; Peyssonnaux, C.; Cuvellier, S.; Latroche, C.; Chazaud, B.; Bendahan, D.; et al. Myeloid HIFs are dispensable for resolution of inflammation during skeletal muscle regeneration. J. Immunol. 2015, 194, 3389–3399. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Holness, C.L.; Simmons, D.L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993, 81, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Khan, K.N.; Kitajima, M.; Hiraki, K.; Moriyama, S.; Masuzaki, H.; Samejima, T.; Fujishita, A.; Ishimaru, T. Differential infiltration of macrophages and prostaglandin production by different uterine leiomyomas. Hum. Reprod. 2006, 21, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Fujishita, A.; Sekine, I.; Ishimaru, T.; Masuzaki, H. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum. Reprod. 2010, 25, 642–653. [Google Scholar] [CrossRef]
- Sozen, I.; Senturk, L.M.; Arici, A. Effect of gonadotropin-releasing hormone agonists on monocyte chemotactic protein-1 production and macrophage infiltration in leiomyomatous uterus. Fertil. Steril. 2001, 76, 792–796. [Google Scholar] [CrossRef]
- Aleem, F.A.; Predanic, M. The hemodynamic effect of GnRH agonist therapy on uterine leiomyoma vascularity: A prospective study using transvaginal color Doppler sonography. Gynecol. Endocrinol. 1995, 9, 253–258. [Google Scholar] [CrossRef]
- Matta, W.H.; Stabile, I.; Shaw, R.W.; Campbell, S. Doppler assessment of uterine blood flow changes in patients with fibroids receiving the gonadotropin-releasing hormone agonist Buserelin. Fertil. Steril. 1988, 49, 1083–1085. [Google Scholar] [CrossRef]
- Kitaya, K.; Yasuo, T. Leukocyte density and composition in human cycling endometrium with uterine fibroids. Hum. Immunol. 2010, 71, 158–163. [Google Scholar] [CrossRef]
- Rasko, J.E.; Ben, H. Granulocyte-macrophage colony stimulating factor. In The Cytokine Handbook; Thomson, A., Ed.; Academic Press: London, UK, 1994; pp. 343–369. [Google Scholar]
- Andreutti, D.; Gabbiani, G.; Neuville, P. Early granulocyte-macrophage colony-stimulating factor expression by alveolar inflammatory cells during bleomycin-induced rat lung fibrosis. Lab. Investig. 1998, 78, 1493–1502. [Google Scholar] [PubMed]
- Rubbia-Brandt, L.; Sappino, A.P.; Gabbiani, G. Locally applied GM-CSF induces the accumulation of alpha-smooth muscle actin containing myofibroblasts. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1991, 60, 73–82. [Google Scholar] [CrossRef]
- Xing, Z.; Gauldie, J.; Tremblay, G.M.; Hewlett, B.R.; Addison, C. Intradermal transgenic expression of granulocyte-macrophage colony-stimulating factor induces neutrophilia, epidermal hyperplasia, Langerhans’ cell/macrophage accumulation, and dermal fibrosis. Lab. Investig. A J. Tech. Methods Pathol. 1997, 77, 615. [Google Scholar]
- Xing, Z.; Tremblay, G.M.; Sime, P.J.; Gauldie, J. Overexpression of granulocyte-macrophage colony-stimulating factor induces pulmonary granulation tissue formation and fibrosis by induction of transforming growth factor-beta 1 and myofibroblast accumulation. Am. J. Pathol. 1997, 150, 59–66. [Google Scholar] [PubMed]
- Xing, Z.; Ohkawara, Y.; Jordana, M.; Graham, F.; Gauldie, J. Transfer of granulocyte-macrophage colony-stimulating factor gene to rat lung induces eosinophilia, monocytosis, and fibrotic reactions. J. Clin. Investig. 1996, 97, 1102–1110. [Google Scholar] [CrossRef]
- Border, W.A.; Noble, N.A. Transforming growth factor beta in tissue fibrosis. N. Engl. J. Med. 1994, 331, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.B. Transforming growth factor-beta: Activity and efficacy in animal models of wound healing. Wound Repair Regen 1995, 3, 408–418. [Google Scholar] [CrossRef]
- Vyalov, S.; Desmouliere, A.; Gabbiani, G. GM-CSF-induced granulation tissue formation: Relationships between macrophage and myofibroblast accumulation. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1993, 63, 231–239. [Google Scholar] [CrossRef]
- Ciebiera, M.; Wlodarczyk, M.; Wrzosek, M.; Meczekalski, B.; Nowicka, G.; Lukaszuk, K.; Ciebiera, M.; Slabuszewska-Jozwiak, A.; Jakiel, G. Role of Transforming Growth Factor beta in Uterine Fibroid Biology. Int. J. Mol. Sci. 2017, 18, 2435. [Google Scholar] [CrossRef]
- Fallowfield, J.A.; Mizuno, M.; Kendall, T.J.; Constandinou, C.M.; Benyon, R.C.; Duffield, J.S.; Iredale, J.P. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J. Immunol. 2007, 178, 5288–5295. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Filardi, E.; Puig-Kroger, A.; Blanco, F.J.; Nieto, C.; Bragado, R.; Palomero, M.I.; Bernabeu, C.; Vega, M.A.; Corbi, A.L. Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood 2011, 117, 5092–5101. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Catherino, W.H.; Protic, O.; Janjusevic, M.; Gray, P.C.; Giannubilo, S.R.; Ciavattini, A.; Lamanna, P.; Tranquilli, A.L.; Petraglia, F.; et al. Role of activin-A and myostatin and their signaling pathway in human myometrial and leiomyoma cell function. J. Clin. Endocrinol. Metab. 2014, 99, E775–E785. [Google Scholar] [CrossRef]
- Hubner, G.; Werner, S. Serum growth factors and proinflammatory cytokines are potent inducers of activin expression in cultured fibroblasts and keratinocytes. Exp. Cell Res. 1996, 228, 106–113. [Google Scholar] [CrossRef]
- Shao, L.; Frigon, N.L., Jr.; Sehy, D.W.; Yu, A.L.; Lofgren, J.; Schwall, R.; Yu, J. Regulation of production of activin A in human marrow stromal cells and monocytes. Exp. Hematol. 1992, 20, 1235–1242. [Google Scholar] [PubMed]
- Shao, L.E.; Frigon, N.L., Jr.; Yu, A.; Palyash, J.; Yu, J. Contrasting effects of inflammatory cytokines and glucocorticoids on the production of activin A in human marrow stromal cells and their implications. Cytokine 1998, 10, 227–235. [Google Scholar] [CrossRef]
- Takahashi, S.; Uchimaru, K.; Harigaya, K.; Asano, S.; Yamashita, T. Tumor necrosis factor and interleukin-1 induce activin A gene expression in a human bone marrow stromal cell line. Biochem. Biophys. Res. Commun 1992, 188, 310–317. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zannotti, A.; Greco, S.; Pellegrino, P.; Giantomassi, F.; Delli Carpini, G.; Goteri, G.; Ciavattini, A.; Ciarmela, P. Macrophages and Immune Responses in Uterine Fibroids. Cells 2021, 10, 982. https://doi.org/10.3390/cells10050982
Zannotti A, Greco S, Pellegrino P, Giantomassi F, Delli Carpini G, Goteri G, Ciavattini A, Ciarmela P. Macrophages and Immune Responses in Uterine Fibroids. Cells. 2021; 10(5):982. https://doi.org/10.3390/cells10050982
Chicago/Turabian StyleZannotti, Alessandro, Stefania Greco, Pamela Pellegrino, Federica Giantomassi, Giovanni Delli Carpini, Gaia Goteri, Andrea Ciavattini, and Pasquapina Ciarmela. 2021. "Macrophages and Immune Responses in Uterine Fibroids" Cells 10, no. 5: 982. https://doi.org/10.3390/cells10050982
APA StyleZannotti, A., Greco, S., Pellegrino, P., Giantomassi, F., Delli Carpini, G., Goteri, G., Ciavattini, A., & Ciarmela, P. (2021). Macrophages and Immune Responses in Uterine Fibroids. Cells, 10(5), 982. https://doi.org/10.3390/cells10050982







