Neuroprotective Natural Products for Alzheimer’s Disease
Abstract
:1. Introduction
2. Neuroprotective Mechanisms of Natural Products for AD
2.1. Overview of the Mechanisms Underlying AD
2.2. Neuroprotective Strategies for AD
2.3. Neuroprotective Effects from Natural Products
2.3.1. Anti-Oxidative Neuroprotective Activity from Natural Products for AD
2.3.2. Anti-Neuroinflammatory Neuroprotective Activity from Natural Products for AD
2.3.3. Anti-Aβ Aggregation Neuroprotective Activity from Natural Sources for AD
2.3.4. Neuroprotective Activity Targeting Tau Protein from Natural Products for AD
2.3.5. Neuroprotective Activity Targeting Cholinergic Neurotransmission from Natural Sources for AD
3. Neuroprotective Natural Products for AD
3.1. Neuroprotective Natural Products from Medicinal Plants for AD
3.1.1. Pistacia Genus
3.1.2. Panax Ginseng
3.1.3. Phyllanthus Genus
3.1.4. Ginkgo biloba L. (Ginkgoaceae)
3.1.5. Hibiscus sabdariffa L. (Malvaceae)
3.1.6. Hedera nepalensis K. (Araliaceae)
3.1.7. Salvia miltiorrhiza B. (Lamiaceae)
3.1.8. Nardostachys jatamansi D. (Caprifoliaceae)
3.1.9. Viscum album L. (Santalaceae)
3.1.10. Bacopa monnieri L. (Plantaginaceae)
3.1.11. Convolvulus pluricaulis C. (Convolvulaceae)
3.1.12. Centella asiatica L. (Apiaceae)
3.1.13. Uncaria rhynchophylla M. (Rubiaceae)
3.1.14. Glycyrrhiza inflata B. (Fabaceae)
3.1.15. Alpinia Oxyphylla-Schisandra Chinensis Herb Pair (ASHP)
3.1.16. Buchanania axillaris D. (Anacardiaceae), Hemidesmus indicus L. (Apocynaceae) and Rhus mysorensis G. (Anacardiaceae)
3.1.17. Coriandrum sativum L. (Apiaceae), N. Jatamansi, Polygonum multiflorum T. (Polygonaceae), Rehmannia glutinosa G. (Plantaginaceae), and Sorbus commixta H. (Rosaceae)
3.1.18. Bojungikgi-Tang (BJIGT; Bu Zhong Yi Qi Tang in China, Hochuekkito in Japan)
3.1.19. Fuzhisan (FZS)
3.2. Neuroprotective Natural Products from Food for AD
3.2.1. Momordica charantia L. (Cucurbitaceae)
3.2.2. Benincasa hispida L. (Cucurbitaceae)
3.2.3. Allium sativum L. (Alliaceae)
3.2.4. Curcuma longa L. (Zingiberaceae)
3.2.5. Zingiber officinale R. (Zingiberaceae)
3.2.6. Punica granatum (Pomegranate)
3.2.7. Oryza sativa (Rice Berry)
3.2.8. Vitis vinifera L. (Grape) and Red Wine
3.2.9. Nuts Including Almond, Hazelnut, and Walnut
3.3. Neuroprotective Natural Products from Marine Sources for AD
3.3.1. Marine Macroalgae (Seaweeds)
3.3.2. Spirulina Cyanobacteria
3.3.3. Thalassospira Profundimaris
4. Problems and Concerns with Natural Products for AD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| Aβ | amyloid β peptide |
| NFT | neurofibrillary tangle |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| Nrf2 | nuclear-factor erythroid 2-related factor |
| ARE | antioxidant response element |
| NF-κΒ | nuclear factor κΒ |
| MAPK | mitogen-activated protein kinase |
| IL-6 | interleukin 6 |
| LPS | lipopolysaccharide |
| EGCG | epigallocatechin-3-gallate |
| ERK | extracellular signal-regulated kinase |
| APP | amyloid precursor protein |
| SP | senile plaques |
| AICD | APP intracellular domain |
| BACE | beta-site amyloid precursor protein cleaving enzyme |
| CTF-β | carboxy-terminal fragment beta |
| PP2A | protein phosphatase 2A |
| CDK-5 | cyclin-dependent kinase 5 |
| GSK-3 | glycogen synthase kinase-3 |
| ACh | acetylcholine |
| AChE | acetylcholinesterase |
| ChAT | choline acetyltransferase |
| BDNF | brain-derived neurotrophic factor |
| PKC | protein kinase C |
| NGF | nerve growth factor |
| PPAR-α | peroxisome proliferator-activated receptor alpha |
| AChT | acetylcholine transferase |
| sAPPa | soluble amyloid precursor protein a |
| MEPA | methanolic extract of Phyllanthus acidus |
| EGb | Ginkgo biloba |
| CAT | catalase |
| SOD | superoxide dismutase |
| GSH | glutathione |
| GST | glutathione S-transferase |
| BuChE | butyrylcholinesterase |
| T2D | type II diabetes |
| AGE | aged garlic extract |
References
- Hippius, H.; Neundorfer, G. The discovery of Alzheimer′s disease. Dialogues Clin. Neurosci. 2003, 5, 101–108. [Google Scholar]
- 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020. [CrossRef]
- Kawas, C.H.; Corrada, M.M. Alzheimer’s and dementia in the oldest-old: A century of challenges. Curr. Alzheimer Res. 2006, 3, 411–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; van Duijn, C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, B.D.; Leurgans, S.E.; Hebert, L.E.; Scherr, P.A.; Yaffe, K.; Bennett, D.A. Contribution of Alzheimer disease to mortality in the United States. Neurology 2014, 82, 1045–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Silva, M.V.F.; Loures, C.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; Carvalho, M.D.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef] [Green Version]
- Shal, B.; Ding, W.; Ali, H.; Kim, Y.S.; Khan, S. Anti-neuroinflammatory Potential of Natural Products in Attenuation of Alzheimer’s Disease. Front. Pharm. 2018, 9, 548. [Google Scholar] [CrossRef]
- Schenk, D.; Basi, G.S.; Pangalos, M.N. Treatment strategies targeting amyloid beta-protein. Cold Spring Harb. Perspect Med. 2012, 2, a006387. [Google Scholar] [CrossRef] [Green Version]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Carroll, N.J. Dietary Modulation of Oxidative Stress in Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 1583. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Gaiteri, C.; Bodea, L.G.; Wang, Z.; McElwee, J.; Podtelezhnikov, A.A.; Zhang, C.; Xie, T.; Tran, L.; Dobrin, R.; et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 2013, 153, 707–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, D.O.; Wightman, E.L. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Goa, K.L. Galantamine: A review of its use in Alzheimer’s disease. Drugs 2000, 60, 1095–1122. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Potential medicinal plants for CNS disorders: An overview. Phytother. Res. 2006, 20, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Xiao, J. Natural Products for Treatment of Alzheimer’s Disease and Related Diseases: Understanding their Mechanism of Action. Curr. Neuropharmacol. 2013, 11, 337. [Google Scholar] [CrossRef]
- Akram, M.; Nawaz, A. Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regen Res. 2017, 12, 660–670. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.D.C. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Xie, Y.; Meng, X.; Kang, J. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Sig. Transduct Target Ther. 2019, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer′s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; Jacks, R.L.; Diamond, M.I. Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 2009, 284, 12845–12852. [Google Scholar] [CrossRef] [Green Version]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments in Alzheimer Disease: An Update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef] [Green Version]
- Longo, F.M.; Massa, S.M. Neuroprotective strategies in Alzheimer’s disease. NeuroRx 2004, 1, 117–127. [Google Scholar] [CrossRef]
- Niikura, T.; Tajima, H.; Kita, Y. Neuronal cell death in Alzheimer′s disease and a neuroprotective factor, humanin. Curr. Neuropharmacol. 2006, 4, 139–147. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, J.; Zhang, X.; Wang, S.; Viola, K.L.; Chow, F.E.; Zhang, Y.; Lippa, C.; Klein, W.L.; Gong, Y. Amyloid Beta Oligomers Target to Extracellular and Intracellular Neuronal Synaptic Proteins in Alzheimer’s Disease. Front. Neurol. 2019, 10, 1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Du, Y.; Zhang, Y.; Huang, Z.; Fu, M.; Li, J.; Pang, Y.; Lei, P.; Wang, Y.T.; Song, W.; et al. MKP-1 reduces Abeta generation and alleviates cognitive impairments in Alzheimer’s disease models. Signal. Transduct Target. 2019, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Quiroz-Baez, R.; Ferrera, P.; Rosendo-Gutierrez, R.; Moran, J.; Bermudez-Rattoni, F.; Arias, C. Caspase-12 activation is involved in amyloid-beta protein-induced synaptic toxicity. J. Alzheimers Dis. 2011, 26, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.W.; Chen, Y.; Huang, X.; Zhou, F.; Wang, W.; Xian, B.; Zhang, X.; Masliah, E.; Chen, Q.; et al. Appoptosin is a novel pro-apoptotic protein and mediates cell death in neurodegeneration. J. Neurosci. 2012, 32, 15565–15576. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Liu, F.; Gong, C.X.; Grundke-Iqbal, I. Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer′s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Black, I.B. Trophic regulation of synaptic plasticity. J. Neurobiol. 1999, 41, 108–118. [Google Scholar] [CrossRef]
- Tonnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [Green Version]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Olila, D.; Olwa, O.; Opuda-Asibo, J. Antibacterial and antifungal activities of extracts of Zanthoxylum chalybeum and Warburgia ugandensis, Ugandan medicinal plants. Afr. Health Sci. 2001, 1, 66–72. [Google Scholar] [PubMed]
- Venkatesan, R.; Ji, E.; Kim, S.Y. Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: A comprehensive review. Biomed. Res. Int. 2015, 2015, 814068. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid Med. Cell Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Kerr, F.; Sofola-Adesakin, O.; Ivanov, D.K.; Gatliff, J.; Gomez Perez-Nievas, B.; Bertrand, H.C.; Martinez, P.; Callard, R.; Snoeren, I.; Cocheme, H.M.; et al. Direct Keap1-Nrf2 disruption as a potential therapeutic target for Alzheimer’s disease. PLoS Genet. 2017, 13, e1006593. [Google Scholar] [CrossRef] [Green Version]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, D.M. Antioxidant Therapies for Neuroprotection-A Review. J. Clin. Med. 2019, 8, 1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, K.N.; Hovland, A.R.; Cole, W.C.; Prasad, K.C.; Nahreini, P.; Edwards-Prasad, J.; Andreatta, C.P. Multiple antioxidants in the prevention and treatment of Alzheimer disease: Analysis of biologic rationale. Clin. Neuropharmacol. 2000, 23, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Chandran, R.; Sajeesh, T.; Parimelazhagan, T. Total Phenolic Content, Anti-Radical property and HPLC profiles of Caralluma diffusa (Wight) N.E. Br. J. Biol. Act. Prod. Nat. 2014, 4, 188–195. [Google Scholar] [CrossRef]
- Cosme, P.; Rodriguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Andrade Teles, R.B.; Diniz, T.C.; Costa Pinto, T.C.; de Oliveira Junior, R.G.; Gama, E.S.M.; de Lavor, E.M.; Fernandes, A.W.C.; de Oliveira, A.P.; de Almeida Ribeiro, F.P.R.; da Silva, A.A.M.; et al. Flavonoids as Therapeutic Agents in Alzheimer’s and Parkinson’s Diseases: A Systematic Review of Preclinical Evidences. Oxid. Med. Cell Longev. 2018, 2018, 7043213. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, J.R.; Narayanasamy, P. Neuroprotection through flavonoid: Enhancement of the glyoxalase pathway. Redox Biol. 2018, 14, 465–473. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. [Google Scholar]
- Calabrese, V.; Butterfield, D.A.; Stella, A.M. Nutritional antioxidants and the heme oxygenase pathway of stress tolerance: Novel targets for neuroprotection in Alzheimer’s disease. Ital. J. Biochem. 2003, 52, 177–181. [Google Scholar]
- Esteban-Fernandez, A.; Rendeiro, C.; Spencer, J.P.; Del Coso, D.G.; de Llano, M.D.; Bartolome, B.; Moreno-Arribas, M.V. Neuroprotective Effects of Selected Microbial-Derived Phenolic Metabolites and Aroma Compounds from Wine in Human SH-SY5Y Neuroblastoma Cells and Their Putative Mechanisms of Action. Front. Nutr. 2017, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, M.R.; Ferreira, G.C.; Schuck, P.F. Protective effect of carnosic acid against paraquat-induced redox impairment and mitochondrial dysfunction in SH-SY5Y cells: Role for PI3K/Akt/Nrf2 pathway. Toxicol. Vitr. 2016, 32, 41–54. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, A.J.; Lowe, G.L. Carotenoids-Antioxidant Properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullan, K.; Williams, M.A.; Cardwell, C.R.; McGuinness, B.; Passmore, P.; Silvestri, G.; Woodside, J.V.; McKay, G.J. Serum concentrations of vitamin E and carotenoids are altered in Alzheimer’s disease: A case-control study. Alzheimers Dement. Transl. Res. Clin. Interv. 2017, 3, 432–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obulesu, M.; Dowlathabad, M.R.; Bramhachari, P.V. Carotenoids and Alzheimer’s Disease: An insight into therapeutic role of retinoids in animal models. Neurochem. Int. 2011, 59, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Chen, H.; Wang, Y.; Schneider, J.A.; Willett, W.C.; Morris, M.C. Dietary carotenoids related to risk of incident Alzheimer dementia (AD) and brain AD neuropathology: A community-based cohort of older adults. Am. J. Clin. Nutr. 2020, 113, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Shin, M.; Kim, S.; Lee, S.B. Recent Advances in Studies on the Therapeutic Potential of Dietary Carotenoids in Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2018, 2018, 4120458. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments for Alzheimer’s disease. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dal Pra, I.; Chiarini, A.; Gui, L.; Chakravarthy, B.; Pacchiana, R.; Gardenal, E.; Whitfield, J.F.; Armato, U. Do astrocytes collaborate with neurons in spreading the "infectious" abeta and Tau drivers of Alzheimer’s disease? Neuroscientist 2015, 21, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Von Bernhardi, R.; Eugenin-von Bernhardi, L.; Eugenin, J. Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.S.; Yu, J.T.; Jiang, T.; Zhu, X.C.; Tan, L. The NLRP3 inflammasome in Alzheimer’s disease. Mol. Neurobiol. 2013, 48, 875–882. [Google Scholar] [CrossRef]
- Streit, W.J.; Mrak, R.E.; Griffin, W.S. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflamm. 2004, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Garcez, M.L.; Mina, F.; Bellettini-Santos, T.; Carneiro, F.G.; Luz, A.P.; Schiavo, G.L.; Andrighetti, M.S.; Scheid, M.G.; Bolfe, R.P.; Budni, J. Minocycline reduces inflammatory parameters in the brain structures and serum and reverses memory impairment caused by the administration of amyloid beta (1–42) in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 77, 23–31. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. Erratum: NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Li, H.; Liu, Q.; Liu, F.; Tang, L.; Li, C.; Yuan, Y.; Zhan, Y.; Xu, W.; Li, W.; et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell Signal. 2011, 23, 883–892. [Google Scholar] [CrossRef]
- Longpre, F.; Garneau, P.; Christen, Y.; Ramassamy, C. Protection by EGb 761 against beta-amyloid-induced neurotoxicity: Involvement of NF-kappaB, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free Radic Biol. Med. 2006, 41, 1781–1794. [Google Scholar] [CrossRef]
- Boissiere, F.; Hunot, S.; Faucheux, B.; Duyckaerts, C.; Hauw, J.J.; Agid, Y.; Hirsch, E.C. Nuclear translocation of NF-kappaB in cholinergic neurons of patients with Alzheimer’s disease. Neuroreport 1997, 8, 2849–2852. [Google Scholar] [CrossRef]
- Ju Hwang, C.; Choi, D.Y.; Park, M.H.; Hong, J.T. NF-kappaB as a Key Mediator of Brain Inflammation in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2019, 18, 3–10. [Google Scholar] [CrossRef]
- Cooper, E.L.; Ma, M.J. Alzheimer Disease: Clues from traditional and complementary medicine. J. Tradit Complement. Med. 2017, 7, 380–385. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.-L.; Liu, R.; Li, X.-X.; Li, J.-F.; Zhang, L. Neuroprotective, Anti-Amyloidogenic and Neurotrophic Effects of Apigenin in an Alzheimer’s Disease Mouse Model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef]
- Wollen, K.A. Alzheimer’s disease: The pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Altern. Med. Rev. 2010, 15, 223–244. [Google Scholar]
- Olajide, O.A.; Bhatia, H.S.; de Oliveira, A.C.; Wright, C.W.; Fiebich, B.L. Inhibition of Neuroinflammation in LPS-Activated Microglia by Cryptolepine. Evid. Based Complement. Altern. Med. 2013, 2013, 459723. [Google Scholar] [CrossRef] [Green Version]
- He, F.Q.; Qiu, B.Y.; Li, T.K.; Xie, Q.; Cui, D.J.; Huang, X.L.; Gan, H.T. Tetrandrine suppresses amyloid-beta-induced inflammatory cytokines by inhibiting NF-kappaB pathway in murine BV2 microglial cells. Int. Immunopharmacol. 2011, 11, 1220–1225. [Google Scholar] [CrossRef]
- Park, S.E.; Sapkota, K.; Kim, S.; Kim, H.; Kim, S.J. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br. J. Pharm. 2011, 164, 1008–1025. [Google Scholar] [CrossRef] [Green Version]
- Velagapudi, R.; Ajileye, O.O.; Okorji, U.; Jain, P.; Aderogba, M.A.; Olajide, O.A. Agathisflavone isolated from Anacardium occidentale suppresses SIRT1-mediated neuroinflammation in BV2 microglia and neurotoxicity in APPSwe-transfected SH-SY5Y cells. Phytother. Res. 2018, 32, 1957–1966. [Google Scholar] [CrossRef] [Green Version]
- Rezai-Zadeh, K.; Ehrhart, J.; Bai, Y.; Sanberg, P.R.; Bickford, P.; Tan, J.; Shytle, R.D. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J. Neuroinflamm. 2008, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.H.; Choi, Y.H.; Moon, S.K.; Kim, W.J.; Kim, G.Y. Quercetin inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-kappaB pathway and activating the Nrf2-dependent HO-1 pathway. Int. Immunopharmacol. 2013, 17, 808–813. [Google Scholar] [CrossRef]
- Seong, K.J.; Lee, H.G.; Kook, M.S.; Ko, H.M.; Jung, J.Y.; Kim, W.J. Epigallocatechin-3-gallate rescues LPS-impaired adult hippocampal neurogenesis through suppressing the TLR4-NF-kappaB signaling pathway in mice. Korean J. Physiol. Pharm. 2016, 20, 41–51. [Google Scholar] [CrossRef]
- Olajide, O.A.; Kumar, A.; Velagapudi, R.; Okorji, U.P.; Fiebich, B.L. Punicalagin inhibits neuroinflammation in LPS-activated rat primary microglia. Mol. Nutr. Food Res. 2014, 58, 1843–1851. [Google Scholar] [CrossRef]
- Velagapudi, R.; Lepiarz, I.; El-Bakoush, A.; Katola, F.O.; Bhatia, H.; Fiebich, B.L.; Olajide, O.A. Induction of Autophagy and Activation of SIRT-1 Deacetylation Mechanisms Mediate Neuroprotection by the Pomegranate Metabolite Urolithin A in BV2 Microglia and Differentiated 3D Human Neural Progenitor Cells. Mol. Nutr. Food Res. 2019, 63, e1801237. [Google Scholar] [CrossRef] [Green Version]
- Infante-Garcia, C.; Ramos-Rodriguez, J.J.; Delgado-Olmos, I.; Gamero-Carrasco, C.; Fernandez-Ponce, M.T.; Casas, L.; Mantell, C.; Garcia-Alloza, M. Long-Term Mangiferin Extract Treatment Improves Central Pathology and Cognitive Deficits in APP/PS1 Mice. Mol. Neurobiol. 2017, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Dong, Q.X.; Zhu, J.; Sun, X.; Zhang, L.F.; Qiu, M.; Yu, X.L.; Liu, R.T. Resveratrol Rescues Tau-Induced Cognitive Deficits and Neuropathology in a Mouse Model of Tauopathy. Curr. Alzheimer Res. 2019, 16, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-kappaB pathways in BV2 cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory Effects of Curcumin in Microglial Cells. Front. Pharm. 2018, 9, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.A.; Tong, M.L.; Zhao, B.; Zhu, G.; Xi, D.H.; Yang, J.P. Parthenolide ameliorates intracerebral hemorrhage-induced brain injury in rats. Phytother. Res. 2020, 34, 153–160. [Google Scholar] [CrossRef]
- Qiang, W.; Cai, W.; Yang, Q.; Yang, L.; Dai, Y.; Zhao, Z.; Yin, J.; Li, Y.; Li, Q.; Wang, Y.; et al. Artemisinin B Improves Learning and Memory Impairment in AD Dementia Mice by Suppressing Neuroinflammation. Neuroscience 2018, 395, 1–12. [Google Scholar] [CrossRef]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.; Sun, J.; Zhang, K.; Liu, J. Ginkgo Biloba for Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Top. Med. Chem. 2016, 16, 520–528. [Google Scholar] [CrossRef]
- Leone, S.; Recinella, L.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Leporini, L.; Brunetti, L.; Menghini, L. Phytotherapic use of the Crocus sativus L. (Saffron) and its potential applications: A brief overview. Phytother. Res. 2018, 32, 2364–2375. [Google Scholar] [CrossRef]
- Mazumder, A.G.; Sharma, P.; Patial, V.; Singh, D. Crocin Attenuates Kindling Development and Associated Cognitive Impairments in Mice via Inhibiting Reactive Oxygen Species-Mediated NF-kappaB Activation. Basic Clin. Pharm. Toxicol. 2017, 120, 426–433. [Google Scholar] [CrossRef]
- Choi, S.K.; Park, Y.S.; Choi, D.K.; Chang, H.I. Effects of astaxanthin on the production of NO and the expression of COX-2 and iNOS in LPS-stimulated BV2 microglial cells. J. Microbiol. Biotechnol. 2008, 18, 1990–1996. [Google Scholar] [PubMed]
- Kim, J.E.; You, D.J.; Lee, C.; Ahn, C.; Seong, J.Y.; Hwang, J.I. Suppression of NF-kappaB signaling by KEAP1 regulation of IKKbeta activity through autophagic degradation and inhibition of phosphorylation. Cell Signal. 2010, 22, 1645–1654. [Google Scholar] [CrossRef]
- Kent, S.A.; Spires-Jones, T.L.; Durrant, C.S. The physiological roles of tau and Ab: Implications for Alzheimer’s disease pathology and therapeutics. Acta Neurophathol. 2020, 140, 417–447. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.; Chen, S.W.; Williamson, P.T.F.; Cascella, R.; Perni, M.; Jarvis, J.A.; Cecchi, C.; Vendruscolo, M.; Chiti, F.; Cremades, N.; et al. Structural basis of membrane disruption and cellular toxicity by alpha-synuclein oligomers. Science 2017, 358, 1440–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Hong, S.; Shepardson, N.E.; Walsh, D.M.; Shankar, G.M.; Selkoe, D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 2009, 62, 788–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Salminen, A.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Inflammation in Alzheimer’s disease: Amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog. Neurobiol. 2009, 87, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.; Hauptmann, S.; Scherping, I.; Meinhardt, J.; Rhein, V.; Drose, S.; Brandt, U.; Fandrich, M.; Muller, W.E.; Gotz, J. Oligomeric and fibrillar species of beta-amyloid (A beta 42) both impair mitochondrial function in P301L tau transgenic mice. J. Mol. Med. 2008, 86, 1255–1267. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.L.; Bajouco, L.M.; Mota, S.I.; Auberson, Y.P.; Oliveira, C.R.; Rego, A.C. Amyloid beta peptide 1-42 disturbs intracellular calcium homeostasis through activation of GluN2B-containing N-methyl-d-aspartate receptors in cortical cultures. Cell Calcium 2012, 51, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Briley, D.; Ghirardi, V.; Woltjer, R.; Renck, A.; Zolochevska, O.; Taglialatela, G.; Micci, M.A. Preserved neurogenesis in non-demented individuals with AD neuropathology. Sci. Rep. 2016, 6, 27812. [Google Scholar] [CrossRef] [Green Version]
- Vergallo, A.; Megret, L.; Lista, S.; Cavedo, E.; Zetterberg, H.; Blennow, K.; Vanmechelen, E.; De Vos, A.; Habert, M.O.; Potier, M.C.; et al. Plasma amyloid beta 40/42 ratio predicts cerebral amyloidosis in cognitively normal individuals at risk for Alzheimer’s disease. Alzheimers Dement. 2019, 15, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Satir, T.M.; Agholme, L.; Karlsson, A.; Karlsson, M.; Karila, P.; Illes, S.; Bergstrom, P.; Zetterberg, H. Partial reduction of amyloid beta production by beta-secretase inhibitors does not decrease synaptic transmission. Alzheimers Res. 2020, 12, 63. [Google Scholar] [CrossRef]
- Song, G.; Yang, H.; Shen, N.; Pham, P.; Brown, B.; Lin, X.; Hong, Y.; Sinu, P.; Cai, J.; Li, X.; et al. An Immunomodulatory Therapeutic Vaccine Targeting Oligomeric Amyloid-beta. J. Alzheimers Dis. 2020, 77, 1639–1653. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.F.; Kost, J.; Tariot, P.N.; Aisen, P.S.; Cummings, J.L.; Vellas, B.; Sur, C.; Mukai, Y.; Voss, T.; Furtek, C.; et al. Randomized Trial of Verubecestat for Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2018, 378, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Budd Haeberlein, S.; O’Gorman, J.; Chiao, P.; Bussiere, T.; von Rosenstiel, P.; Tian, Y.; Zhu, Y.; von Hehn, C.; Gheuens, S.; Skordos, L.; et al. Clinical Development of Aducanumab, an Anti-Abeta Human Monoclonal Antibody Being Investigated for the Treatment of Early Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2017, 4, 255–263. [Google Scholar] [CrossRef]
- Deshpande, P.; Gogia, N.; Singh, A. Exploring the efficacy of natural products in alleviating Alzheimer’s disease. Neural. Regen Res. 2019, 14, 1321–1329. [Google Scholar] [CrossRef]
- Lee, J.H.; Ahn, N.H.; Choi, S.B.; Kwon, Y.; Yang, S.H. Natural Products Targeting Amyloid Beta in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2341. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharm. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Cole, S.L.; Vassar, R. The Alzheimer’s disease beta-secretase enzyme, BACE1. Mol. Neurodegener. 2007, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Tyler, S.J.; Dawbarn, D.; Wilcock, G.K.; Allen, S.J. alpha- and beta-secretase: Profound changes in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002, 299, 373–376. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.; Li, J.; Niu, Q. Ginsenoside Rg1 ameliorates hippocampal long-term potentiation and memory in an Alzheimer’s disease model. Mol. Med. Rep. 2016, 13, 4904–4910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, F.; Chen, X.; Huang, T.; Lue, L.F.; Luddy, J.S.; Yan, S.S. Multi-faced neuroprotective effects of Ginsenoside Rg1 in an Alzheimer mouse model. Biochim. Biophys. Acta 2012, 1822, 286–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Li, C.; Wang, X.; Yang, Z.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. 2,2’,4’-trihydroxychalcone from Glycyrrhiza glabra as a new specific BACE1 inhibitor efficiently ameliorates memory impairment in mice. J. Neurochem. 2010, 114, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Jeon, S.Y.; Lee, H.J.; Kim, S.I.; Song, K.S. A beta-secretase (BACE1) inhibitor hispidin from the mycelial cultures of Phellinus linteus. Planta Med. 2004, 70, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Giunta, B.; Bickford, P.C.; Fountain, M.; Tan, J.; Shytle, R.D. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer’s disease. Int. J. Pharm. 2010, 389, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.Y.; Bae, K.; Seong, Y.H.; Song, K.S. Green tea catechins as a BACE1 (beta-secretase) inhibitor. Bioorg. Med. Chem. Lett. 2003, 13, 3905–3908. [Google Scholar] [CrossRef]
- Youn, K.; Lee, J.; Ho, C.-T.; Jun, M. Discovery of polymethoxyflavones from black ginger (Kaempferia parviflora) as potential β-secretase (BACE1) inhibitors. J. Funct. Foods 2016, 20, 567–574. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; He, W.; Chen, Y. Berberine Alleviates Amyloid-Beta Pathology in the Brain of APP/PS1 Transgenic Mice via Inhibiting beta/gamma-Secretases Activity and Enhancing alpha-Secretases. Curr. Alzheimer Res. 2018, 15, 1045–1052. [Google Scholar] [CrossRef]
- Xu, Y.J.; Mei, Y.; Qu, Z.L.; Zhang, S.J.; Zhao, W.; Fang, J.S.; Wu, J.; Yang, C.; Liu, S.J.; Fang, Y.Q.; et al. Ligustilide Ameliorates Memory Deficiency in APP/PS1 Transgenic Mice via Restoring Mitochondrial Dysfunction. Biomed. Res. Int. 2018, 2018, 4606752. [Google Scholar] [CrossRef] [Green Version]
- Tamamis, P.; Adler-Abramovich, L.; Reches, M.; Marshall, K.; Sikorski, P.; Serpell, L.; Gazit, E.; Archontis, G. Self-assembly of phenylalanine oligopeptides: Insights from experiments and simulations. Biophys. J. 2009, 96, 5020–5029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Edresi, S.; Alsalahat, I.; Freeman, S.; Aojula, H.; Penny, J. Resveratrol-mediated cleavage of amyloid beta1-42 peptide: Potential relevance to Alzheimer’s disease. Neurobiol. Aging 2020, 94, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Sun, W.; Liu, F. Brazilin inhibits the Zn(2+)-mediated aggregation of amyloid beta-protein and alleviates cytotoxicity. J. Inorg. Biochem. 2017, 177, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Jett, S.D.; Chi, E.Y. Curcumin Attenuates Amyloid-beta Aggregate Toxicity and Modulates Amyloid-beta Aggregation Pathway. ACS Chem. Neurosci. 2016, 7, 56–68. [Google Scholar] [CrossRef]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer’s beta-amyloid fibrils in vitro. Biochim. Biophys. Acta 2004, 1690, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Ladiwala, A.R.; Dordick, J.S.; Tessier, P.M. Aromatic small molecules remodel toxic soluble oligomers of amyloid beta through three independent pathways. J. Biol. Chem. 2011, 286, 3209–3218. [Google Scholar] [CrossRef] [Green Version]
- Anandhan, A.; Tamilselvam, K.; Radhiga, T.; Rao, S.; Essa, M.M.; Manivasagam, T. Theaflavin, a black tea polyphenol, protects nigral dopaminergic neurons against chronic MPTP/probenecid induced Parkinson’s disease. Brain Res. 2012, 1433, 104–113. [Google Scholar] [CrossRef]
- Tapia-Rojas, C.; Cabezas-Opazo, F.; Deaton, C.A.; Vergara, E.H.; Johnson, G.V.W.; Quintanilla, R.A. It’s all about tau. Prog. Neurobiol. 2019, 175, 54–76. [Google Scholar] [CrossRef]
- Kopke, E.; Tung, Y.C.; Shaikh, S.; Alonso, A.C.; Iqbal, K.; Grundke-Iqbal, I. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J. Biol. Chem. 1993, 268, 24374–24384. [Google Scholar] [CrossRef]
- Lauretti, E.; Pratico, D. Alzheimer’s disease: Phenotypic approaches using disease models and the targeting of tau protein. Expert Opin. Targets 2020, 24, 319–330. [Google Scholar] [CrossRef]
- Min, S.W.; Cho, S.H.; Zhou, Y.; Schroeder, S.; Haroutunian, V.; Seeley, W.W.; Huang, E.J.; Shen, Y.; Masliah, E.; Mukherjee, C.; et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 2010, 67, 953–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, S.N.; Funk, K.E.; Wan, Y.; Liao, Z.; Davies, P.; Kuret, J.; Yang, A.J. Dual modification of Alzheimer’s disease PHF-tau protein by lysine methylation and ubiquitylation: A mass spectrometry approach. Acta Neuropathol. 2012, 123, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Huseby, C.J.; Hoffman, C.N.; Cooper, G.L.; Cocuron, J.C.; Alonso, A.P.; Thomas, S.N.; Yang, A.J.; Kuret, J. Quantification of Tau Protein Lysine Methylation in Aging and Alzheimer’s Disease. J. Alzheimers Dis. 2019, 71, 979–991. [Google Scholar] [CrossRef]
- Sharma, A.; Weber, D.; Raupbach, J.; Dakal, T.C.; Fliessbach, K.; Ramirez, A.; Grune, T.; Wullner, U. Advanced glycation end products and protein carbonyl levels in plasma reveal sex-specific differences in Parkinson’s and Alzheimer’s disease. Redox Biol. 2020, 34, 101546. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.X. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 2005, 22, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Hanger, D.P.; Anderton, B.H.; Noble, W. Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 2009, 15, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ruan, Y.Y.; Xu, H.; Shi, X.M.; Wang, Z.X.; Hu, Y.L. Safflower yellow reduces lipid peroxidation, neuropathology, tau phosphorylation and ameliorates amyloid beta-induced impairment of learning and memory in rats. Biomed. Pharm. 2015, 76, 153–164. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Yan, X.; Qin, K.; Shi, M.; Lin, T.; Zhu, Y.; Kang, T.; Zhao, G. Protective effects of ginsenoside Rd against okadaic acid-induced neurotoxicity in vivo and in vitro. J. Ethnopharmacol. 2011, 138, 135–141. [Google Scholar] [CrossRef]
- Karakani, A.M.; Riazi, G.; Mahmood Ghaffari, S.; Ahmadian, S.; Mokhtari, F.; Jalili Firuzi, M.; Zahra Bathaie, S. Inhibitory effect of corcin on aggregation of 1N/4R human tau protein in vitro. Iran. J. Basic Med. Sci. 2015, 18, 485–492. [Google Scholar]
- Feng, J.H.; Cai, B.C.; Guo, W.F.; Wang, M.Y.; Ma, Y.; Lu, Q.X. Neuroprotective effects of Tongmai Yizhi Decoction () against Alzheimer’s disease through attenuating cyclin-dependent kinase-5 expression. Chin. J. Integr Med. 2017, 23, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, Y.; Jiang, Y.; Ding, J.; Li, L. Geniposide ameliorates learning memory deficits, reduces tau phosphorylation and decreases apoptosis via GSK3beta pathway in streptozotocin-induced alzheimer rat model. Brain Pathol. 2014, 24, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; He, Z.; Peng, A.; Zhang, X.; Cheng, B.; Sun, Y.; Zheng, L.; Huang, K. Effects of several quinones on insulin aggregation. Sci. Rep. 2014, 4, 5648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.H.; Chen, I.C.; Lin, H.Y.; Chen, H.C.; Lin, C.H.; Lin, T.H.; Weng, Y.T.; Chao, C.Y.; Wu, Y.R.; Lin, J.Y.; et al. The aqueous extract of Glycyrrhiza inflata can upregulate unfolded protein response-mediated chaperones to reduce tau misfolding in cell models of Alzheimer’s disease. Drug Des. Devel. 2016, 10, 885–896. [Google Scholar] [CrossRef] [Green Version]
- Azimi, A.; Ghaffari, S.M.; Riazi, G.H.; Arab, S.S.; Tavakol, M.M.; Pooyan, S. alpha-Cyperone of Cyperus rotundus is an effective candidate for reduction of inflammation by destabilization of microtubule fibers in brain. J. Ethnopharmacol. 2016, 194, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Bijari, N.; Balalaie, S.; Akbari, V.; Golmohammadi, F.; Moradi, S.; Adibi, H.; Khodarahmi, R. Effective suppression of the modified PHF6 peptide/1N4R Tau amyloid aggregation by intact curcumin, not its degradation products: Another evidence for the pigment as preventive/therapeutic "functional food". Int. J. Biol. Macromol 2018, 120, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.C.; Kwan, P.; Cheung, S.K.K.; Ho, A.; Baum, L. Effects of Resveratrol and Morin on Insoluble Tau in Tau Transgenic Mice. Transl. Neurosci. 2018, 9, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, G.K.; Shwartz, D.; Losev, Y.; Arad, E.; Shemesh, C.; Pichinuk, E.; Engel, H.; Raveh, A.; Jelinek, R.; Cooper, I.; et al. Purpurin modulates Tau-derived VQIVYK fibrillization and ameliorates Alzheimer’s disease-like symptoms in animal model. Cell Mol. Life Sci. 2020, 77, 2795–2813. [Google Scholar] [CrossRef]
- Ghasemzadeh, S.; Riazi, G.H. Inhibition of Tau amyloid fibril formation by folic acid: In-vitro and theoretical studies. Int. J. Biol. Macromol. 2020, 154, 1505–1516. [Google Scholar] [CrossRef]
- Shin, S.J.; Park, Y.H.; Jeon, S.G.; Kim, S.; Nam, Y.; Oh, S.M.; Lee, Y.Y.; Moon, M. Red Ginseng Inhibits Tau Aggregation and Promotes Tau Dissociation In Vitro. Oxid Med. Cell Longev. 2020, 2020, 7829842. [Google Scholar] [CrossRef]
- Gold, P.E. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol. Learn. Mem. 2003, 80, 194–210. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, M.; Kukreja, H.; Chugh, R.; Silakari, O.; Singh, D. Acetylcholinesterase inhibitors as Alzheimer therapy: From nerve toxins to neuroprotection. Eur. J. Med. Chem. 2013, 70, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Eyjolfsdottir, H.; Eriksdotter, M.; Linderoth, B.; Lind, G.; Juliusson, B.; Kusk, P.; Almkvist, O.; Andreasen, N.; Blennow, K.; Ferreira, D.; et al. Targeted delivery of nerve growth factor to the cholinergic basal forebrain of Alzheimer’s disease patients: Application of a second-generation encapsulated cell biodelivery device. Alzheimers Res. 2016, 8, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, D.; Kaur Dogra, A.; Tahrani, A.; Herrmann, F.; Wink, M. Extracts from Traditional Chinese Medicinal Plants Inhibit Acetylcholinesterase, a Known Alzheimer’s Disease Target. Molecules 2016, 21, 1161. [Google Scholar] [CrossRef] [Green Version]
- Ohba, T.; Yoshino, Y.; Ishisaka, M.; Abe, N.; Tsuruma, K.; Shimazawa, M.; Oyama, M.; Tabira, T.; Hara, H. Japanese Huperzia serrata extract and the constituent, huperzine A, ameliorate the scopolamine-induced cognitive impairment in mice. Biosci. Biotechnol. Biochem. 2015, 79, 1838–1844. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Wu, J.; Chen, Y. Chemical Constituents of Plants from the Genus Phlegmariurus. Chem. Biodivers. 2016, 13, 269–274. [Google Scholar] [CrossRef]
- Geromichalos, G.D.; Lamari, F.N.; Papandreou, M.A.; Trafalis, D.T.; Margarity, M.; Papageorgiou, A.; Sinakos, Z. Saffron as a source of novel acetylcholinesterase inhibitors: Molecular docking and in vitro enzymatic studies. J. Agric. Food Chem. 2012, 60, 6131–6138. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.B.; Zhao, T.; Muna, S.S.; Jin, H.M.; Park, J.I.; Jo, K.S.; Lee, B.H.; Chae, S.W.; Kim, S.Y.; Park, S.H.; et al. Therapeutic potential of Gastrodia elata Blume for the treatment of Alzheimer’s disease. Neural Regen Res. 2013, 8, 1061–1070. [Google Scholar] [CrossRef]
- Teng, Y.; Zhang, M.Q.; Wang, W.; Liu, L.T.; Zhou, L.M.; Miao, S.K.; Wan, L.H. Compound danshen tablet ameliorated abeta25-35-induced spatial memory impairment in mice via rescuing imbalance between cytokines and neurotrophins. BMC Complement. Altern. Med. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.Q.; Zhang, L.; Yang, C.; Rong, C.P.; He, W.Q.; Zhang, C.X.; Li, S.; Su, R.Y.; Chang, X.; Qin, J.H.; et al. Alleviating effects of Bushen-Yizhi formula on ibotenic acid-induced cholinergic impairments in rat. Rejuvenation Res. 2015, 18, 111–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, M.; Kim, H.G.; Choi, J.G.; Oh, H.; Lee, P.K.; Ha, S.K.; Kim, S.Y.; Park, Y.; Huh, Y.; Oh, M.S. Corrigendum to ”6-Shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia” [BBRC 449 (2014) 8-13]. Biochem. Biophys. Res. Commun. 2020, 521, 545. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Xu, P.; Song, S.; Liu, P.; Chi, T.; Ji, X.; Jin, G.; Qiu, S.; Hou, Y.; et al. Xanthoceras sorbifolia extracts ameliorate dendritic spine deficiency and cognitive decline via upregulation of BDNF expression in a rat model of Alzheimer’s disease. Neurosci. Lett. 2016, 629, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Kim, J.Y.; Lee, Y.; Chun, H.J.; Choi, Y.W.; Shin, H.K.; Choi, B.T.; Kim, C.M.; Lee, J. PMC-12, a traditional herbal medicine, enhances learning memory and hippocampal neurogenesis in mice. Neurosci. Lett. 2016, 617, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sun, Z.; Liu, Y.; Jia, Y.; Zhang, B.; Zhang, J. Resveratrol improves cognition and reduces oxidative stress in rats with vascular dementia. Neural Regen Res. 2013, 8, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.F.; Li, N.; Wang, Q.; Cheng, X.J.; Li, X.M.; Liu, T.T. Resveratrol decreases the insoluble Abeta1-42 level in hippocampus and protects the integrity of the blood-brain barrier in AD rats. Neuroscience 2015, 310, 641–649. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Rizak, J.D.; Wu, S.; Wang, Z.; He, R.; Su, M.; Qin, D.; Wang, J.; Hu, X. Resveratrol Attenuates Formaldehyde Induced Hyperphosphorylation of Tau Protein and Cytotoxicity in N2a Cells. Front. Neurosci. 2016, 10, 598. [Google Scholar] [CrossRef] [Green Version]
- Schmatz, R.; Mazzanti, C.M.; Spanevello, R.; Stefanello, N.; Gutierres, J.; Correa, M.; da Rosa, M.M.; Rubin, M.A.; Chitolina Schetinger, M.R.; Morsch, V.M. Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur. J. Pharm. 2009, 610, 42–48. [Google Scholar] [CrossRef]
- Rahvar, M.; Nikseresht, M.; Shafiee, S.M.; Naghibalhossaini, F.; Rasti, M.; Panjehshahin, M.R.; Owji, A.A. Effect of oral resveratrol on the BDNF gene expression in the hippocampus of the rat brain. Neurochem. Res. 2011, 36, 761–765. [Google Scholar] [CrossRef]
- Howes, M.J.; Perry, N.S.; Houghton, P.J. Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother. Res. 2003, 17, 1–18. [Google Scholar] [CrossRef]
- Ansari, N.; Khodagholi, F. Natural products as promising drug candidates for the treatment of Alzheimer’s disease: Molecular mechanism aspect. Curr. Neuropharmacol. 2013, 11, 414–429. [Google Scholar] [CrossRef] [Green Version]
- D’Onofrio, G.; Sancarlo, D.; Ruan, Q.; Yu, Z.; Panza, F.; Daniele, A.; Greco, A.; Seripa, D. Phytochemicals in the Treatment of Alzheimer’s Disease: A Systematic Review. Curr. Drug Targets 2017, 18, 1487–1498. [Google Scholar] [CrossRef]
- Moeini, R.; Memariani, Z.; Asadi, F.; Bozorgi, M.; Gorji, N. Pistacia Genus as a Potential Source of Neuroprotective Natural Products. Planta Med. 2019, 85, 1326–1350. [Google Scholar] [CrossRef] [PubMed]
- Golchin, L.; Shabani, M.; Harandi, S.; Razavinasab, M. Pistachio supplementation attenuates motor and cognition impairments induced by cisplatin or vincristine in rats. Adv. Biomed. Res. 2015, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Hajpour Soq, K.; Yousefi, A.; Estakhr, P.; Dalvi, M.; Mousavi Khaneghah, A. Antioxidant activity of Pistacia atlantica var mutica kernel oil and it’s unsaponifiable matters. J. Food Sci. Technol. 2019, 56, 5336–5345. [Google Scholar] [CrossRef] [PubMed]
- Abdi Gorabi, S.; Mohammadzadeh, H.; Rostampour, M. The Effects of Ripe Pistachio Hulls Hydroalcoholic Extract and Aerobic Training on Learning and Memory in Streptozotocin-induced Diabetic Male Rats. Basic Clin. Neurosci. 2020, 11, 525–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholamhoseinian, A.; Moradi, M.N.; Sharifi-Far, F. Screening the methanol extracts of some Iranian plants for acetylcholinesterase inhibitory activity. Res. Pharm. Sci. 2009, 4, 105–112. [Google Scholar] [PubMed]
- Ammari, M.; Othman, H.; Hajri, A.; Sakly, M.; Abdelmelek, H. Pistacia lentiscus oil attenuates memory dysfunction and decreases levels of biomarkers of oxidative stress induced by lipopolysaccharide in rats. Brain Res. Bull. 2018, 140, 140–147. [Google Scholar] [CrossRef]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE Inhibitors from Plants and their Contribution to Alzheimer’s Disease Therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [Green Version]
- Adhami, H.R.; Farsam, H.; Krenn, L. Screening of medicinal plants from Iranian traditional medicine for acetylcholinesterase inhibition. Phytother. Res. 2011, 25, 1148–1152. [Google Scholar] [CrossRef]
- Pacifico, S.; Piccolella, S.; Marciano, S.; Galasso, S.; Nocera, P.; Piscopo, V.; Fiorentino, A.; Monaco, P. LC-MS/MS profiling of a mastic leaf phenol enriched extract and its effects on H2O2 and Abeta(25–35) oxidative injury in SK-B-NE(C)-2 cells. J. Agric. Food Chem. 2014, 62, 11957–11966. [Google Scholar] [CrossRef]
- Quartu, M.; Serra, M.P.; Boi, M.; Pillolla, G.; Melis, T.; Poddighe, L.; Del Fiacco, M.; Falconieri, D.; Carta, G.; Murru, E.; et al. Effect of acute administration of Pistacia lentiscus L. essential oil on rat cerebral cortex following transient bilateral common carotid artery occlusion. Lipids Health Dis. 2012, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Zahoor, M.; Zafar, R.; Rahman, N.U. Isolation and identification of phenolic antioxidants from Pistacia integerrima gall and their anticholine esterase activities. Heliyon 2018, 4, e01007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.S.; Yun, J.H.; Baek, I.S.; Leem, Y.H.; Kang, H.W.; Cho, H.K.; Lyu, Y.S.; Son, H.J.; Han, P.L. Oriental medicine Jangwonhwan reduces Abeta(1-42) level and beta-amyloid deposition in the brain of Tg-APPswe/PS1dE9 mouse model of Alzheimer disease. J. Ethnopharmacol. 2010, 128, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.H.; Lee, D.S.; Lee, D.J.; Kim, S.H.; Chung, S.; Yang, H.O. Effects of fermented ginseng on memory impairment and beta-amyloid reduction in Alzheimer’s disease experimental models. J. Ginseng Res. 2013, 37, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.R.; Yun, B.S.; In, O.H.; Sung, C.K. Comparative study of korean white, red, and black ginseng extract on cholinesterase inhibitory activity and cholinergic function. J. Ginseng Res. 2011, 35, 421–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.G.; Kim, N.; Huh, E.; Lee, H.; Oh, M.H.; Park, J.D.; Pyo, M.K.; Oh, M.S. White Ginseng Protects Mouse Hippocampal Cells Against Amyloid-Beta Oligomer Toxicity. Phytother. Res. 2017, 31, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Lee, S.T.; Oh, M.J.; Park, H.J.; Shim, J.Y.; Chu, K.; Kim, M. Improvement of cognitive deficit in Alzheimer’s disease patients by long term treatment with korean red ginseng. J. Ginseng Res. 2011, 35, 457–461. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.H.; Park, M.H.; Lee, J.H. Effect of Korean Red Ginseng on Cognitive Function and Quantitative EEG in Patients with Alzheimer’s Disease: A Preliminary Study. J. Altern Complement. Med. 2016, 22, 280–285. [Google Scholar] [CrossRef]
- Kim, H.J.; Jung, S.W.; Kim, S.Y.; Cho, I.H.; Kim, H.C.; Rhim, H.; Kim, M.; Nah, S.Y. Panax ginseng as an adjuvant treatment for Alzheimer’s disease. J. Ginseng Res. 2018, 42, 401–411. [Google Scholar] [CrossRef]
- Uddin, M.S.; Mamun, A.A.; Hossain, M.S.; Ashaduzzaman, M.; Noor, M.A.A.; Hossain, M.S.; Uddin, M.J.; Sarker, J.; Asaduzzaman, M. Neuroprotective effect of Phyllanthus acidus L. on learning and memory impairment in scopolamine-induced animal model of dementia and oxidative stress: Natural wonder for regulating the development and progression of Alzheimer’s disease. Adv. Alzheimers Dis. 2016, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Alagan, A.; Jantan, I.; Kumolosasi, E.; Ogawa, S.; Abdullah, M.A.; Azmi, N. Protective Effects of Phyllanthus amarus Against Lipopolysaccharide-Induced Neuroinflammation and Cognitive Impairment in Rats. Front. Pharm. 2019, 10, 632. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.S.; Mamun, A.A.; Hossain, M.S.; Akter, F.; Iqbal, M.A.; Asaduzzaman, M. Exploring the Effect of Phyllanthus emblica L. on Cognitive Performance, Brain Antioxidant Markers and Acetylcholinesterase Activity in Rats: Promising Natural Gift for the Mitigation of Alzheimer’s Disease. Ann. Neurosci. 2016, 23, 218–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, K.; Wang, Y.; Li, D.; Wang, Q.; Xie, S.; Wang, J.; Zuo, Z. Enhanced anti-amnestic effect of donepezil by Ginkgo biloba extract (EGb 761) via further improvement in pro-cholinergic and antioxidative activities. J. Ethnopharmacol. 2021, 269, 113711. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ye, M.; Guo, H. An Updated Review of Randomized Clinical Trials Testing the Improvement of Cognitive Function of Ginkgo biloba Extract in Healthy People and Alzheimer’s Patients. Front. Pharm. 2019, 10, 1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ude, C.; Schubert-Zsilavecz, M.; Wurglics, M. Ginkgo biloba extracts: A review of the pharmacokinetics of the active ingredients. Clin. Pharm. 2013, 52, 727–749. [Google Scholar] [CrossRef]
- El-Shiekh, R.A.; Ashour, R.M.; Abd El-Haleim, E.A.; Ahmed, K.A.; Abdel-Sattar, E. Hibiscus sabdariffa L.: A potent natural neuroprotective agent for the prevention of streptozotocin-induced Alzheimer’s disease in mice. Biomed. Pharm. 2020, 128, 110303. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, W.J.; Ismail, H.; Mehmood, F.; Mirza, B. Neuroprotective, antidiabetic and antioxidant effect of Hedera nepalensis and lupeol against STZ + AlCl3 induced rats model. Daru 2018, 26, 179–190. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Qian, S.S.; Zhang, Y.J.; Wang, R.Q. Salvia miltiorrhiza: A source for anti-Alzheimer’s disease drugs. Pharm. Biol. 2016, 54, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Yao, L.; Zhou, H.; Qu, S.; Zeng, X.; Zhou, D.; Zhou, Y.; Li, X.; Liu, Z. Neuroprotection against Abeta25-35-induced apoptosis by Salvia miltiorrhiza extract in SH-SY5Y cells. Neurochem. Int. 2014, 75, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.Y.; Jeon, B.H.; Kim, Y.C.; Lee, S.H.; Sohn, D.H.; Seo, G.S. PF2401-SF, standardized fraction of Salvia miltiorrhiza shows anti-inflammatory activity in macrophages and acute arthritis in vivo. Int. Immunopharmacol. 2013, 16, 160–164. [Google Scholar] [CrossRef]
- Hu, L.; Yu, J.; Li, F.; Chen, B.; Li, L.; Liu, G. Effects of Salvia miltorrhiza in neural differentiation of rat mesenchymal stem cells with optimized protocol. J. Ethnopharmacol. 2011, 136, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Pang, M.; Rong, L.; Zhou, W.; Wang, J.; Liu, C.; Wang, X. Effects of Salvia miltiorrhiza on neural differentiation of induced pluripotent stem cells. J. Ethnopharmacol. 2014, 153, 233–241. [Google Scholar] [CrossRef]
- Liu, Q.F.; Jeon, Y.; Sung, Y.W.; Lee, J.H.; Jeong, H.; Kim, Y.M.; Yun, H.S.; Chin, Y.W.; Jeon, S.; Cho, K.S.; et al. Nardostachys jatamansi Ethanol Extract Ameliorates Abeta42 Cytotoxicity. Biol. Pharm. Bull. 2018, 41, 470–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.F.; Lee, J.H.; Kim, Y.M.; Lee, S.; Hong, Y.K.; Hwang, S.; Oh, Y.; Lee, K.; Yun, H.S.; Lee, I.S.; et al. In Vivo Screening of Traditional Medicinal Plants for Neuroprotective Activity against Abeta42 Cytotoxicity by Using Drosophila Models of Alzheimer’s Disease. Biol. Pharm. Bull. 2015, 38, 1891–1901. [Google Scholar] [CrossRef] [Green Version]
- Szurpnicka, A.; Kowalczuk, A.; Szterk, A. Biological activity of mistletoe: In vitro and in vivo studies and mechanisms of action. Arch. Pharmacal. Res. 2020, 43, 593–629. [Google Scholar] [CrossRef]
- Russo, A.; Borrelli, F. Bacopa monniera, a reputed nootropic plant: An overview. Phytomedicine 2005, 12, 305–317. [Google Scholar] [CrossRef]
- Dhanasekaran, M.; Tharakan, B.; Holcomb, L.A.; Hitt, A.R.; Young, K.A.; Manyam, B.V. Neuroprotective mechanisms of ayurvedic antidementia botanical Bacopa monniera. Phytother. Res. 2007, 21, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Uabundit, N.; Wattanathorn, J.; Mucimapura, S.; Ingkaninan, K. Cognitive enhancement and neuroprotective effects of Bacopa monnieri in Alzheimer’s disease model. J. Ethnopharmacol. 2010, 127, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.K.; Bhattacharya, A.; Kumar, A.; Ghosal, S. Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother. Res. 2000, 14, 174–179. [Google Scholar] [CrossRef]
- Limpeanchob, N.; Jaipan, S.; Rattanakaruna, S.; Phrompittayarat, W.; Ingkaninan, K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J. Ethnopharmacol. 2008, 120, 112–117. [Google Scholar] [CrossRef]
- Sadhu, A.; Upadhyay, P.; Agrawal, A.; Ilango, K.; Karmakar, D.; Singh, G.P.; Dubey, G.P. Management of cognitive determinants in senile dementia of Alzheimer’s type: Therapeutic potential of a novel polyherbal drug product. Clin. Drug Investig. 2014, 34, 857–869. [Google Scholar] [CrossRef]
- Malik, J.; Karan, M.; Vasisht, K. Nootropic, anxiolytic and CNS-depressant studies on different plant sources of shankhpushpi. Pharm. Biol. 2011, 49, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Nahata, A.; Patil, U.K.; Dixit, V.K. Effect of Convulvulus pluricaulis Choisy. on learning behaviour and memory enhancement activity in rodents. Nat. Prod. Res. 2008, 22, 1472–1482. [Google Scholar] [CrossRef]
- Bihaqi, S.W.; Singh, A.P.; Tiwari, M. Supplementation of Convolvulus pluricaulis attenuates scopolamine-induced increased tau and amyloid precursor protein (AbetaPP) expression in rat brain. Indian J. Pharm. 2012, 44, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Sethiya, N.K.; Nahata, A.; Mishra, S.H.; Dixit, V.K. An update on Shankhpushpi, a cognition-boosting Ayurvedic medicine. Zhong Xi Yi Jie He Xue Bao 2009, 7, 1001–1022. [Google Scholar] [CrossRef] [PubMed]
- Shinomol, G.K.; Muralidhara; Bharath, M.M. Exploring the Role of “Brahmi” (Bacopa monnieri and Centella asiatica) in Brain Function and Therapy. Recent Pat. Endocr. Metab. Immune Drug Discov. 2011, 5, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Veerendra Kumar, M.H.; Gupta, Y.K. Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer’s disease in rats. Clin. Exp. Pharm. Physiol. 2003, 30, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Tsai, W.H.; Chen, C.J.; Pan, T.M. Centella asiatica extract protects against amyloid beta1-40-induced neurotoxicity in neuronal cells by activating the antioxidative defence system. J. Tradit Complement. Med. 2016, 6, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Dhanasekaran, M.; Holcomb, L.A.; Hitt, A.R.; Tharakan, B.; Porter, J.W.; Young, K.A.; Manyam, B.V. Centella asiatica extract selectively decreases amyloid beta levels in hippocampus of Alzheimer’s disease animal model. Phytother. Res. 2009, 23, 14–19. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, T.H. Natural product for the treatment of Alzheimer’s disease. J. Basic Clin. Physiol. Pharm. 2017, 28, 413–423. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Tang, N.Y.; Chiang, S.Y.; Hsieh, C.T.; Lin, J.G. Anticonvulsive and free radical scavenging actions of two herbs, Uncaria rhynchophylla (MIQ) Jack and Gastrodia elata Bl., in kainic acid-treated rats. Life Sci. 1999, 65, 2071–2082. [Google Scholar] [CrossRef]
- Tang, N.Y.; Liu, C.H.; Su, S.Y.; Jan, Y.M.; Hsieh, C.T.; Cheng, C.Y.; Shyu, W.C.; Hsieh, C.L. Uncaria rhynchophylla (miq) Jack plays a role in neuronal protection in kainic acid-treated rats. Am. J. Chin. Med. 2010, 38, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Iwasaki, K.; Furukawa, K.; Seki, T.; He, M.; Maruyama, M.; Tomita, N.; Kudo, Y.; Higuchi, M.; Saido, T.C.; et al. Uncaria rhynchophylla, a Chinese medicinal herb, has potent antiaggregation effects on Alzheimer’s beta-amyloid proteins. J. Neurosci. Res. 2006, 84, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.S.; Yu, J.X.; Chen, X.P.; Xu, R.X. Pharmacological actions of Uncaria alkaloids, rhynchophylline and isorhynchophylline. Acta Pharm. Sin. 2003, 24, 97–101. [Google Scholar]
- Xian, Y.F.; Lin, Z.X.; Mao, Q.Q.; Hu, Z.; Zhao, M.; Che, C.T.; Ip, S.P. Bioassay-Guided Isolation of Neuroprotective Compounds from Uncaria rhynchophylla against Beta-Amyloid-Induced Neurotoxicity. Evid. Based Complement. Altern. Med. 2012, 2012, 802625. [Google Scholar] [CrossRef]
- Kang, T.H.; Murakami, Y.; Matsumoto, K.; Takayama, H.; Kitajima, M.; Aimi, N.; Watanabe, H. Rhynchophylline and isorhynchophylline inhibit NMDA receptors expressed in Xenopus oocytes. Eur. J. Pharm. 2002, 455, 27–34. [Google Scholar] [CrossRef]
- Chen, C.M.; Weng, Y.T.; Chen, W.L.; Lin, T.H.; Chao, C.Y.; Lin, C.H.; Chen, I.C.; Lee, L.C.; Lin, H.Y.; Wu, Y.R.; et al. Aqueous extract of Glycyrrhiza inflata inhibits aggregation by upregulating PPARGC1A and NFE2L2-ARE pathways in cell models of spinocerebellar ataxia 3. Free Radic. Biol. Med. 2014, 71, 339–350. [Google Scholar] [CrossRef]
- Chiu, Y.J.; Lee, C.M.; Lin, T.H.; Lin, H.Y.; Lee, S.Y.; Mesri, M.; Chang, K.H.; Lin, J.Y.; Lee-Chen, G.J.; Chen, C.M. Chinese Herbal Medicine Glycyrrhiza inflataReduces Abeta Aggregation and Exerts Neuroprotection through Anti-Oxidation and Anti-Inflammation. Am. J. Chin. Med. 2018, 1–25. [Google Scholar] [CrossRef]
- Qi, Y.; Cheng, X.; Jing, H.; Yan, T.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Combination of schisandrin and nootkatone exerts neuroprotective effect in Alzheimer’s disease mice model. Metab. Brain Dis. 2019, 34, 1689–1703. [Google Scholar] [CrossRef] [PubMed]
- Penumala, M.; Zinka, R.B.; Shaik, J.B.; Mallepalli, S.K.R.; Vadde, R.; Amooru, D.G. Phytochemical profiling and in vitro screening for anticholinesterase, antioxidant, antiglucosidase and neuroprotective effect of three traditional medicinal plants for Alzheimer’s Disease and Diabetes Mellitus dual therapy. BMC Complement. Altern Med. 2018, 18, 77. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.S.; Kim, Y.J.; Sohn, E.; Yoon, J.; Kim, B.Y.; Jeong, S.J. Bojungikgi-Tang, a Traditional Herbal Formula, Exerts Neuroprotective Effects and Ameliorates Memory Impairments in Alzheimer’s Disease-Like Experimental Models. Nutrients 2018, 10, 1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.L.; Wang, D.S.; Zhao, B.Q.; Li, Q.; Qu, H.Y.; Zhang, T.; Zhou, J.P.; Sun, M.J. Effects of Chinese herbal medicine fuzhisan on aged rats. Exp. Gerontol. 2008, 43, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, D.; Duan, S.; Wang, J.; Bai, J.; Li, W. Analysis of fuzhisan and quantitation of baicalin and ginsenoside Rb(1) by HPLC-DAD-ELSD. Arch. Pharm. Res. 2009, 32, 989–996. [Google Scholar] [CrossRef]
- Bi, M.; Tong, S.; Zhang, Z.; Ma, Q.; Zhang, S.; Luo, Z.; Zhang, Y.; Li, X.; Wang, D. Changes in cerebral glucose metabolism in patients with mild-to-moderate Alzheimer’s disease: A pilot study with the Chinese herbal medicine fuzhisan. Neurosci. Lett. 2011, 501, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, D.; Wang, X.; Feng, H.; Tang, Y.; Sun, R.; Zheng, Y.; Dong, L.; Zhao, J.; Zhang, X.; et al. Effect and mechanism of fuzhisan and donepezil on the sirtuin 1 pathway and amyloid precursor protein metabolism in PC12 cells. Mol. Med. Rep. 2016, 13, 3539–3546. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Chen, S.L.; Chang, Y.T.; Chyuan, J.H.; Hsieh-Li, H.M. Administration of Momordica charantia Enhances the Neuroprotection and Reduces the Side Effects of LiCl in the Treatment of Alzheimer’s Disease. Nutrients 2018, 10, 1888. [Google Scholar] [CrossRef] [Green Version]
- Sepehri, H.; Hojati, A.; Safari, R. Effect of Bitter Melon on Spatial Memory of Rats Receiving a High-Fat Diet. J. Exp. Pharm. 2019, 11, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Veerendra Kumar, M.H.; Gupta, Y.K. Intracerebroventricular administration of colchicine produces cognitive impairment associated with oxidative stress in rats. Pharm. Biochem. Behav. 2002, 73, 565–571. [Google Scholar] [CrossRef]
- Mathew, B.; Biju, R. Neuroprotective effects of garlic a review. Libyan J. Med. 2008, 3, 23–33. [Google Scholar] [CrossRef]
- Sripanidkulchai, B. Benefits of aged garlic extract on Alzheimer’s disease: Possible mechanisms of action. Exp. Med. 2020, 19, 1560–1564. [Google Scholar] [CrossRef] [Green Version]
- Nillert, N.; Pannangrong, W.; Welbat, J.U.; Chaijaroonkhanarak, W.; Sripanidkulchai, K.; Sripanidkulchai, B. Neuroprotective Effects of Aged Garlic Extract on Cognitive Dysfunction and Neuroinflammation Induced by beta-Amyloid in Rats. Nutrients 2017, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, K.A.; Hwang, Y.J.; Hwang, I.G.; Song, J.; Jun Kim, Y. Low temperature-aged garlic extract suppresses psychological stress by modulation of stress hormones and oxidative stress response in brain. J. Chin. Med. Assoc. 2019, 82, 191–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khazdair, M.R.; Anaeigoudari, A.; Hashemzehi, M.; Mohebbati, R. Neuroprotective potency of some spice herbs, a literature review. J. Tradit. Complement. Med. 2019, 9, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Yamada, M. REVIEW: Curcumin and Alzheimer’s disease. CNS Neurosci. 2010, 16, 285–297. [Google Scholar] [CrossRef]
- Da Costa, I.M.; Freire, M.A.M.; de Paiva Cavalcanti, J.R.L.; de Araujo, D.P.; Norrara, B.; Moreira Rosa, I.M.M.; de Azevedo, E.P.; do Rego, A.C.M.; Filho, I.A.; Guzen, F.P. Supplementation with Curcuma longa Reverses Neurotoxic and Behavioral Damage in Models of Alzheimer’s Disease: A Systematic Review. Curr. Neuropharmacol. 2019, 17, 406–421. [Google Scholar] [CrossRef]
- Yuliani, S.; Mustofa; Partadiredja, G. The neuroprotective effects of an ethanolic turmeric (Curcuma longa L.) extract against trimethyltin-induced oxidative stress in rats. Nutr. Neurosci. 2019, 22, 797–804. [Google Scholar] [CrossRef]
- Kadri, Y.; Nciri, R.; Brahmi, N.; Saidi, S.; Harrath, A.H.; Alwasel, S.; Aldahmash, W.; El Feki, A.; Allagui, M.S. Protective effects of Curcuma longa against neurobehavioral and neurochemical damage caused by cerium chloride in mice. Env. Sci. Pollut. Res. Int. 2018, 25, 19555–19565. [Google Scholar] [CrossRef]
- Ganguli, M.; Chandra, V.; Kamboh, M.I.; Johnston, J.M.; Dodge, H.H.; Thelma, B.K.; Juyal, R.C.; Pandav, R.; Belle, S.H.; DeKosky, S.T. Apolipoprotein E polymorphism and Alzheimer disease: The Indo-US Cross-National Dementia Study. Arch. Neurol. 2000, 57, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Ng, T.P.; Chiam, P.C.; Lee, T.; Chua, H.C.; Lim, L.; Kua, E.H. Curry consumption and cognitive function in the elderly. Am. J. Epidemiol. 2006, 164, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Hishikawa, N.; Takahashi, Y.; Amakusa, Y.; Tanno, Y.; Tuji, Y.; Niwa, H.; Murakami, N.; Krishna, U.K. Effects of turmeric on Alzheimer’s disease with behavioral and psychological symptoms of dementia. Ayu 2012, 33, 499–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oboh, G.; Ademiluyi, A.O.; Akinyemi, A.J. Inhibition of acetylcholinesterase activities and some pro-oxidant induced lipid peroxidation in rat brain by two varieties of ginger (Zingiber officinale). Exp. Toxicol. Pathol. 2012, 64, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.E.; Shah, A.; Fagan, A.M.; Schwetye, K.E.; Parsadanian, M.; Schulman, R.N.; Finn, M.B.; Holtzman, D.M. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2006, 24, 506–515. [Google Scholar] [CrossRef]
- Rojanathammanee, L.; Puig, K.L.; Combs, C.K. Pomegranate polyphenols and extract inhibit nuclear factor of activated T-cell activity and microglial activation in vitro and in a transgenic mouse model of Alzheimer disease. J. Nutr. 2013, 143, 597–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subash, S.; Essa, M.M.; Al-Asmi, A.; Al-Adawi, S.; Vaishnav, R.; Braidy, N.; Manivasagam, T.; Guillemin, G.J. Pomegranate from Oman Alleviates the Brain Oxidative Damage in Transgenic Mouse Model of Alzheimer’s disease. J. Tradit Complement. Med. 2014, 4, 232–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, T.; Ma, H.; Liu, W.; Niesen, D.B.; Shah, N.; Crews, R.; Rose, K.N.; Vattem, D.A.; Seeram, N.P. Pomegranate’s Neuroprotective Effects against Alzheimer’s Disease Are Mediated by Urolithins, Its Ellagitannin-Gut Microbial Derived Metabolites. ACS Chem. Neurosci. 2016, 7, 26–33. [Google Scholar] [CrossRef]
- Pannangrong, W.; Wattanathorn, J.; Muchimapura, S.; Tiamkao, S.; Tong-Un, T. Purple rice berry is neuroprotective and enhances cognition in a rat model of Alzheimer’s disease. J. Med. Food 2011, 14, 688–694. [Google Scholar] [CrossRef]
- Peters, R.; Peters, J.; Warner, J.; Beckett, N.; Bulpitt, C. Alcohol, dementia and cognitive decline in the elderly: A systematic review. Age Ageing 2008, 37, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.; et al. Resveratrol and Grape Extract-loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef]
- Siahmard, Z.; Alaei, H.; Reisi, P.; Pilehvarian, A.A. The effect of red grape juice on Alzheimer’s disease in rats. Adv. Biomed. Res. 2012, 1, 63. [Google Scholar] [CrossRef]
- Sun, Q.; Jia, N.; Li, X.; Yang, J.; Chen, G. Grape seed proanthocyanidins ameliorate neuronal oxidative damage by inhibiting GSK-3beta-dependent mitochondrial permeability transition pore opening in an experimental model of sporadic Alzheimer’s disease. Aging (Albany Ny) 2019, 11, 4107–4124. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Nie, Y.; Zhang, X.; Tan, B.; Cao, H.; Chen, W.; Gao, W.; Chen, J.; Liang, Z.; Lai, H.; et al. Effects of grape seed proanthocyanidin on Alzheimer’s disease in vitro and in vivo. Exp. Med. 2016, 12, 1681–1692. [Google Scholar] [CrossRef] [Green Version]
- Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Aly, H.F.; El-Sherbiny, M.; Matloub, A.A.; Fouad, G.I. Therapeutic impact of grape leaves polyphenols on certain biochemical and neurological markers in AlCl3-induced Alzheimer’s disease. Biomed. Pharm. 2017, 93, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Gorji, N.; Moeini, R.; Memariani, Z. Almond, hazelnut and walnut, three nuts for neuroprotection in Alzheimer’s disease: A neuropharmacological review of their bioactive constituents. Pharm. Res. 2018, 129, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Valls-Pedret, C.; Cofan, M.; Sabate, J.; Serra-Mir, M.; Perez-Heras, A.M.; Arechiga, A.; Casaroli-Marano, R.P.; Alforja, S.; Sala-Vila, A.; et al. The Walnuts and Healthy Aging Study (WAHA): Protocol for a Nutritional Intervention Trial with Walnuts on Brain Aging. Front. Aging Neurosci. 2016, 8, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahaeddin, Z.; Yans, A.; Khodagholi, F.; Hajimehdipoor, H.; Sahranavard, S. Hazelnut and neuroprotection: Improved memory and hindered anxiety in response to intra-hippocampal Abeta injection. Nutr. Neurosci. 2017, 20, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, K.S.; Kasture, S.B.; Mengi, S.A. Efficacy study of Prunus amygdalus (almond) nuts in scopolamine-induced amnesia in rats. Indian J. Pharm. 2010, 42, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Haider, S.; Batool, Z.; Haleem, D.J. Nootropic and hypophagic effects following long term intake of almonds (Prunus amygdalus) in rats. Nutr. Hosp. 2012, 27, 2109–2115. [Google Scholar] [CrossRef]
- Batool, Z.; Sadir, S.; Liaquat, L.; Tabassum, S.; Madiha, S.; Rafiq, S.; Tariq, S.; Batool, T.S.; Saleem, S.; Naqvi, F.; et al. Repeated administration of almonds increases brain acetylcholine levels and enhances memory function in healthy rats while attenuates memory deficits in animal model of amnesia. Brain Res. Bull. 2016, 120, 63–74. [Google Scholar] [CrossRef]
- Batool, Z.; Agha, F.; Ahmad, S.; Liaquat, L.; Tabassum, S.; Khaliq, S.; Anis, L.; Sajid, I.; Emad, S.; Perveen, T.; et al. Attenuation of cadmium-induced decline in spatial, habituation and recognition memory by long-term administration of almond and walnut supplementation: Role of cholinergic function. Pak. J. Pharm. Sci. 2017, 30, 273–279. [Google Scholar] [PubMed]
- Pribis, P.; Bailey, R.N.; Russell, A.A.; Kilsby, M.A.; Hernandez, M.; Craig, W.J.; Grajales, T.; Shavlik, D.J.; Sabate, J. Effects of walnut consumption on cognitive performance in young adults. Br. J. Nutr. 2012, 107, 1393–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.; Cai, P.S.; Xiong, C.M.; Ruan, J.L. Neuroprotective effect of peptides extracted from walnut (Juglans Sigilata Dode) proteins on Abeta25-35-induced memory impairment in mice. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, T.; Zhang, J.; Xu, J.; Sun-Waterhouse, D.; Zhao, M.; Su, G. Effect of walnut protein hydrolysate on scopolamine-induced learning and memory deficits in mice. J. Food Sci. Technol. 2017, 54, 3102–3110. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Beneficial Effects of Walnuts on Cognition and Brain Health. Nutrients 2020, 12, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthaiyah, B.; Essa, M.M.; Chauhan, V.; Chauhan, A. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem. Res. 2011, 36, 2096–2103. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.; Silva, R.; Pinto, M.M.M.; Sousa, E. Marine Natural Products, Multitarget Therapy and Repurposed Agents in Alzheimer’s Disease. Pharmaceuticals 2020, 13, 242. [Google Scholar] [CrossRef]
- Alghazwi, M.; Kan, Y.Q.; Zhang, W.; Gai, W.P.; Garson, M.J.; Smid, S. Neuroprotective activities of natural products from marine macroalgae during 1999–2015. J. Appl. Phycol. 2016, 28, 3599–3616. [Google Scholar] [CrossRef]
- Ahn, B.R.; Moon, H.E.; Kim, H.R.; Jung, H.A.; Choi, J.S. Neuroprotective effect of edible brown alga Eisenia bicyclis on amyloid beta peptide-induced toxicity in PC12 cells. Arch. Pharm Res. 2012, 35, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Um, M.Y.; Lim, D.W.; Son, H.J.; Cho, S.; Lee, C. Phlorotannin-rich fraction from Ishige foliacea brown seaweed prevents the scopolamine-induced memory impairment via regulation of ERK-CREB-BDNF pathway. J. Funct. Foods 2018, 40, 110–116. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering Potential Applications of Cyanobacteria and Algal Metabolites in Biology, Agriculture and Medicine: Current Status and Future Prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef] [Green Version]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid. Based Complement. Altern. Med. 2020, 2020, 6565396. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Bescos, P.; Pinero-Estrada, E.; Villar del Fresno, A.M. Neuroprotection by Spirulina platensis protean extract and phycocyanin against iron-induced toxicity in SH-SY5Y neuroblastoma cells. Toxicol. Vitr. 2008, 22, 1496–1502. [Google Scholar] [CrossRef]
- Chen, J.C.; Liu, K.S.; Yang, T.J.; Hwang, J.H.; Chan, Y.C.; Lee, I.T. Spirulina and C-phycocyanin reduce cytotoxicity and inflammation-related genes expression of microglial cells. Nutr. Neurosci. 2012, 15, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Hou, M.F.; Huang, H.W.; Chang, F.R.; Yeh, C.C.; Tang, J.Y.; Chang, H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013, 13, 55. [Google Scholar] [CrossRef] [Green Version]
- Koh, E.J.; Kim, K.J.; Song, J.H.; Choi, J.; Lee, H.Y.; Kang, D.H.; Heo, H.J.; Lee, B.Y. Spirulina maxima Extract Ameliorates Learning and Memory Impairments via Inhibiting GSK-3beta Phosphorylation Induced by Intracerebroventricular Injection of Amyloid-beta 1-42 in Mice. Int. J. Mol. Sci. 2017, 18, 2401. [Google Scholar] [CrossRef] [Green Version]
- Koh, E.J.; Kim, K.J.; Choi, J.; Kang, D.H.; Lee, B.Y. Spirulina maxima extract prevents cell death through BDNF activation against amyloid beta 1-42 (Abeta1-42) induced neurotoxicity in PC12 cells. Neurosci. Lett. 2018, 673, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, Z.; Qian, P.Y.; Herrup, K. Marine bacterial extracts as a new rich source of drugs against Alzheimer’s disease. J. Neurochem. 2020, 152, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharm. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Arseneault, M.; Sanderson, T.; Murthy, V.; Ramassamy, C. Challenges for research on polyphenols from foods in Alzheimer’s disease: Bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem. 2008, 56, 4855–4873. [Google Scholar] [CrossRef]
- Zhao, D.; Simon, J.E.; Wu, Q. A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy. Crit. Rev. Food Sci. Nutr. 2020, 60, 597–625. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Martinoli, M.G. Considerations for the Use of Polyphenols as Therapies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharm. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovais, M.; Zia, N.; Ahmad, I.; Khalil, A.T.; Raza, A.; Ayaz, M.; Sadiq, A.; Ullah, F.; Shinwari, Z.K. Phyto-Therapeutic and Nanomedicinal Approaches to Cure Alzheimer’s Disease: Present Status and Future Opportunities. Front. Aging Neurosci. 2018, 10, 284. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; Chen, J.; Gao, J. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: Focus on recent advances. Asian J. Pharm. Sci. 2019, 14, 480–496. [Google Scholar] [CrossRef]
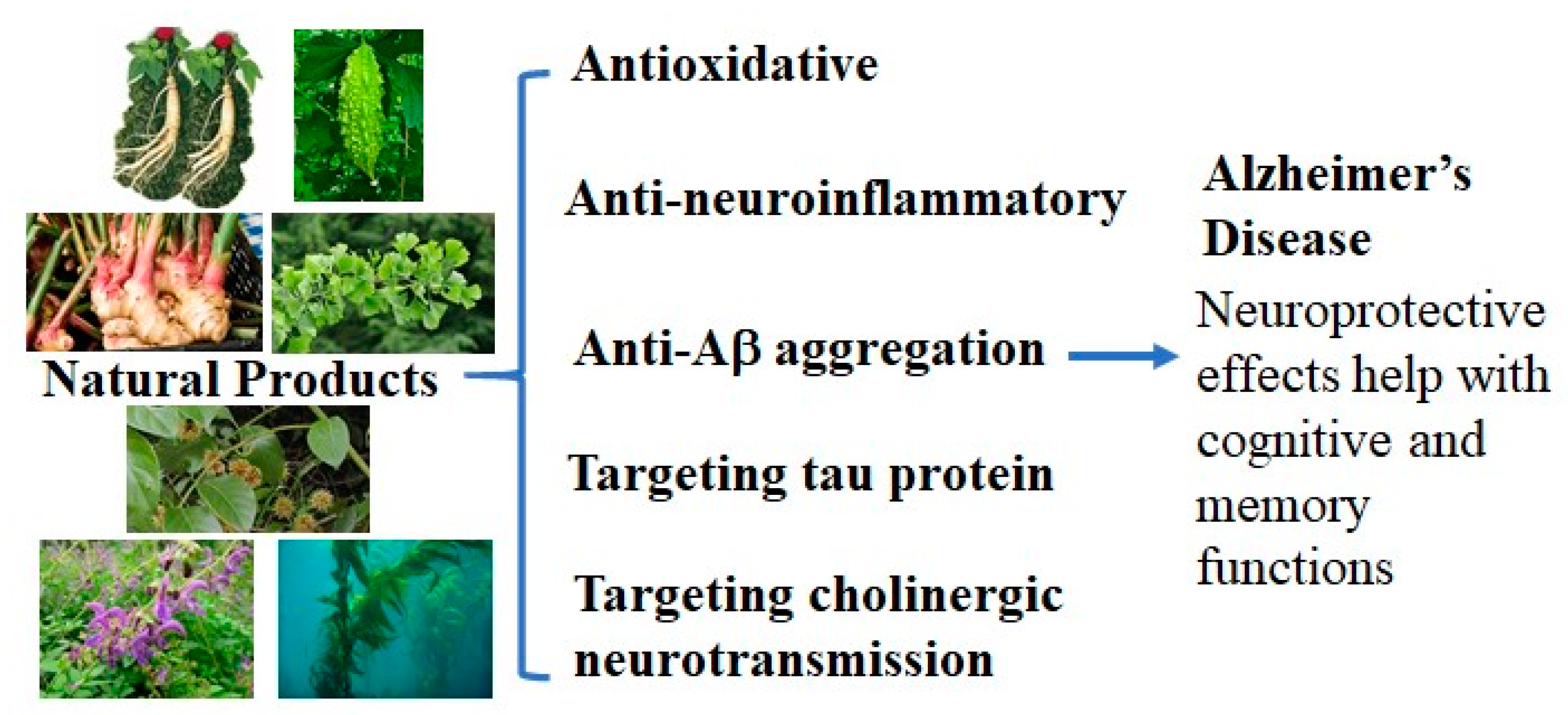
| Natural Products | Extract | Neuroprotective Effects Found | Research Status | References |
|---|---|---|---|---|
| Pistacia vera | Kernel | Inhibited cisplatin or vincristine-induced cognitive and motor impairments | In vivo, rats | [183] |
| Pistacia lentiscus | Essential oil | Attenuated lipopolysaccharide-induced memory impairment and decreased AChE activity and oxidative stress markers in brain tissue | Rats | [187] |
| Pistacia integerrima | Gall extracts | Radical scavenging and cholinesterase inhibitory activity | In vitro | [192] |
| Pistacia atlantica | Ethyl acetate and aqueous extracts | AChE inhibitory activity | In vitro | [182] |
| Panax ginseng | Root extracts | Reduced Aβ formation, inhibited AChE, restored the decreased synaptophysin and ChAT activity, reduced Aβ formation and aggregation | In vitro, mice and clinical trials | [193,194,195,196,197,198,199] |
| Phyllanthus acidus | Methanolic extract | Improvement of cognitive functions and reduced oxidative stress via elevating the level of brain antioxidant enzymes, as well as reducing lipid peroxidation and AChE activity | In vitro | [200] |
| Phyllanthus amarus, Cynodon dactylon | Methanolic Extract | Increased the levels of superoxide dismutase, catalase, and NADH dehydrogenase | Rats | [201] |
| Phyllanthus emblica | Ethanolic extract | Improved learning, memory, and antioxidant potential, as well as decreased AChE activity | Mice | [202] |
| Ginkgo biloba L. | Leaf extract (EGb) | Scavenged free radicals, prevented mitochondrial dysfunction, activated JNK and ERK pathways, and inhibited neuronal apoptosis | Scopolamine-induced AD rat model, clinical trials | [203,204,205] |
| Hibiscus sabdariffa L. | Anthocyanin-enriched extracts | Prevented memory impairment through the amelioration of STZ-induced neuroinflammation and amyloidogenesis | In vitro, mice | [207] |
| Hedera nepalensis K. | Crude extract | Increased the levels of catalase (CAT) and superoxide dismutase (SOD), while reducing glutathione (GSH) levels | Rats | [208] |
| Salvia miltiorrhiza B. | Root extract | Inhibited oxidative stress and the mitochondria-dependent apoptotic pathway. Inhibited iNOS expression and NO production. Induced neuron cell differentiation from rat mesenchymal stem cells. Promote the differentiation potential of iPSCs and enhanced the survival and neural differentiation of transplanted iPSCs-derived neurons. | In vitro, rat | [210,211,212,213] |
| Nardostachys jatamansi D. | Ethanolic extract | Inhibited Aβ-induced cell death | In vitro, Drosophila model | [214,215] |
| Viscum album L. | Extract | Significantly increased BDNF levels in the serum and diminished AlCl3-induced neurotoxicity | In vitro, mice | [216] |
| Bacopa monnieri L. | Extract | Decreased cholinergic degeneration and showed cognition-enhancing effects, protect neuronal cells from β-amyloid-induced damages by lowering ROS levels, inhibited AChE | Rats, clinical trials | [217,218,219,220,221,222] |
| Convolvulus pluricaulis C. | Ethanolic extract | Decreased tau and AβPP expression in the brain | Rats | [223,224,225,226] |
| Centella asiatica L. | Ethanolic extract | Reduced β-amyloid pathology and oxidative stress in the brain; protected neurons against the neurotoxicity induced by Aβ1–40, decreased ROS production, and activated the antioxidative defense system by increasing the activities of various related enzymes and enhancing levels of glutathione and glutathione disulfide | Mice | [227,228,229,230,231] |
| Uncaria rhynchophylla M. | Root extract | Showed free radical scavenging activity and inhibited lipid peroxidation; reduced microglial activation, nNOS, iNOS, and apoptosis; inhibited Aβ fibril formation and also dissemble preformed Aβ fibrils | Rats | [232,233,234,235,236,237] |
| Glycyrrhiza inflata B. | Aqueous extract | Reduction in ROS and tau misfolding, potent anti-Aβ aggregation and radical-scavenging activities. Suppressed the production of NO, TNFα, IL-1β, PGE2, and/or Iba1 | In vitro | [154,238,239] |
| Alpinia oxyphylla-Schisandra chinensis herb pair (ASHP) | Ethanolic extract | Inhibition of the TLR4/NF-kB/NLRP3 pathway; restored the activities of GST, COX-2, SOD, total antioxidant capacity, and iNOS, increased the levels of NO, GSH, and malondialdehyde | Mice | [240] |
| Buchanania axillaris D., Hemidesmus indicus L. and Rhus mysorensis G. | Methanolic extracts | Inhibit AChE, BuChE, and α- and β-glucosidase, neuroprotective effects against the cell death-induced oxidative stress | In vitro | [241] |
| Coriandrum sativum L., N. jatamansi, Polygonum multiflorum T., Rehmannia glutinosa G., and Sorbus commixta H. | Ethanolic extract | Neuroprotective effects against Aβ42 neurotoxicity | Drosophila AD models | [215] |
| Bojungikgi-tang (BJIGT) | Herbal formula | Inhibited Ab aggregation, enhanced BACE activity in vivo, and increased antioxidant activity; prevent the aggregation and expression of Aβ peptides, NeuN and BDNF in the hippocampus | In vitro, mice, clinical trials | [242] |
| Fuzhisan (FZS) | Herbal complex | Anti-apoptosis and anti-Aβ accumulation activity, enhancing ACh levels, and neurotrophic effects | Rats, Clinical tirals | [243,244,245,246] |
| Momordica charantia L. | Dried and ground fruit | Reduced gliosis, oligomeric Aβ level, tau hyperphosphorylation, and neuronal loss, Increasing the expression levels of synaptic-related protein and pS9-GSK3b | In vitro, rats | [247,248] |
| Benincasa hispida L. | Aqueous extract | Prevented SP formation; antioxidant scavenging actions; prevention of dentate granule cell destruction in the hippocampus and by preventing the extracellular SP deposition | Rats | [249] |
| Allium sativum L. | Aged garlic extract | Reduced the activation of microglia and IL-1 level, minimized the inflammatory response; suppressed psychological stress through modulation of stress hormones and oxidative stress response in the brain | Rats | [250,251,252,253] |
| Curcuma longa L. | Ethanolic extract | Attenuate CeCl3-induced oxidative stress, enhanced the activities of antioxidant enzymes, and decreased AChE activity | In vitro, mice, rats, clinical trials | [254,255,256,257,258,259,260,261,262] |
| Zingiber officinale R. | Root extract | Inhibited AChE, inhibited lipid peroxidation; reduced the overstimulation of NMDA receptors and prevented the production of free radicals | Rats | [263,264] |
| Punica granatum | Juice and extracts | Counteraced oxidative stress, reduced brain inflammation, decreased the accumulation of soluble Aβ42, and reduced amyloid deposition in the hippocampus | Mice | [265,266,267,268] |
| Oryza sativa | Dietary supplement | Decreased hippocampal AChE activity and lipid peroxidation products | Rats | [269] |
| Vitis vinifera L. | Juice, polyphenolic extract | Inhibited Aβ aggregation; antioxidative, anti-neuroinflammatory, and anti-amnesic activities | Rats | [270,271,272,273,274,275] |
| Hazelnut (Corylus avellana) | Kernel | Enhanced memory, reduced anxiety, and ameliorated neuroinflammation and apoptosis | Rats | [276,278,279] |
| Almond (Prunus dulcis) | Paste | Reduced AChE activity, lowered cholesterol and triglyceride levels; improved learning and memory with enhanced brain tryptophan monoamine levels and serotonergic turnover | Rats | [279,280,281,282] |
| Walnut (Juglans regia) | Defatted protein | Attenuated expression of proinflammatory cytokines, decreased level of AChE, significantly restored levels of antioxidant enzymes, and reduced expression of NF-κB | Rats | [283,284,285,286,287] |
| Eisenia bicyclis | Methanolic extract | Reduced intracellular ROS production in PC12 cells induced with Aβ25–35 | In vitro | [290] |
| Ishige foliacea | Phlorotannin-rich fraction | Reducing brain AChE activity, suppressed oxidative stress, and activated the ERK-BDNF-CREB signaling pathway | Mice | [291] |
| Spirulina platensis | Protein and aqueous extracts | Scavenged free radicals and prevented radical-mediated cell death; reduced cytotoxicity and inhibited the expression of inflammation-related genes like COX-2, TNF-α, IL-6, and iNOS | In vitro | [293,294,295] |
| Spirulina maxima | Ethanolic Extract | Decreased expression levels of hippocampal Aβ1–42, APP, and BACE1 decreased AChE activity, suppressed hippocampal oxidative stress, increased BDNF level, and activated BDNF/PI3K/Akt signaling pathways | Mice | [296,297,298] |
| Thalassospira profundimaris | Crude extract | Preserved synaptic structure; blocked cell cycle-related neuron death | In vitro, mice | [299] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. https://doi.org/10.3390/cells10061309
Chen X, Drew J, Berney W, Lei W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells. 2021; 10(6):1309. https://doi.org/10.3390/cells10061309
Chicago/Turabian StyleChen, Xin, Joshua Drew, Wren Berney, and Wei Lei. 2021. "Neuroprotective Natural Products for Alzheimer’s Disease" Cells 10, no. 6: 1309. https://doi.org/10.3390/cells10061309
APA StyleChen, X., Drew, J., Berney, W., & Lei, W. (2021). Neuroprotective Natural Products for Alzheimer’s Disease. Cells, 10(6), 1309. https://doi.org/10.3390/cells10061309








