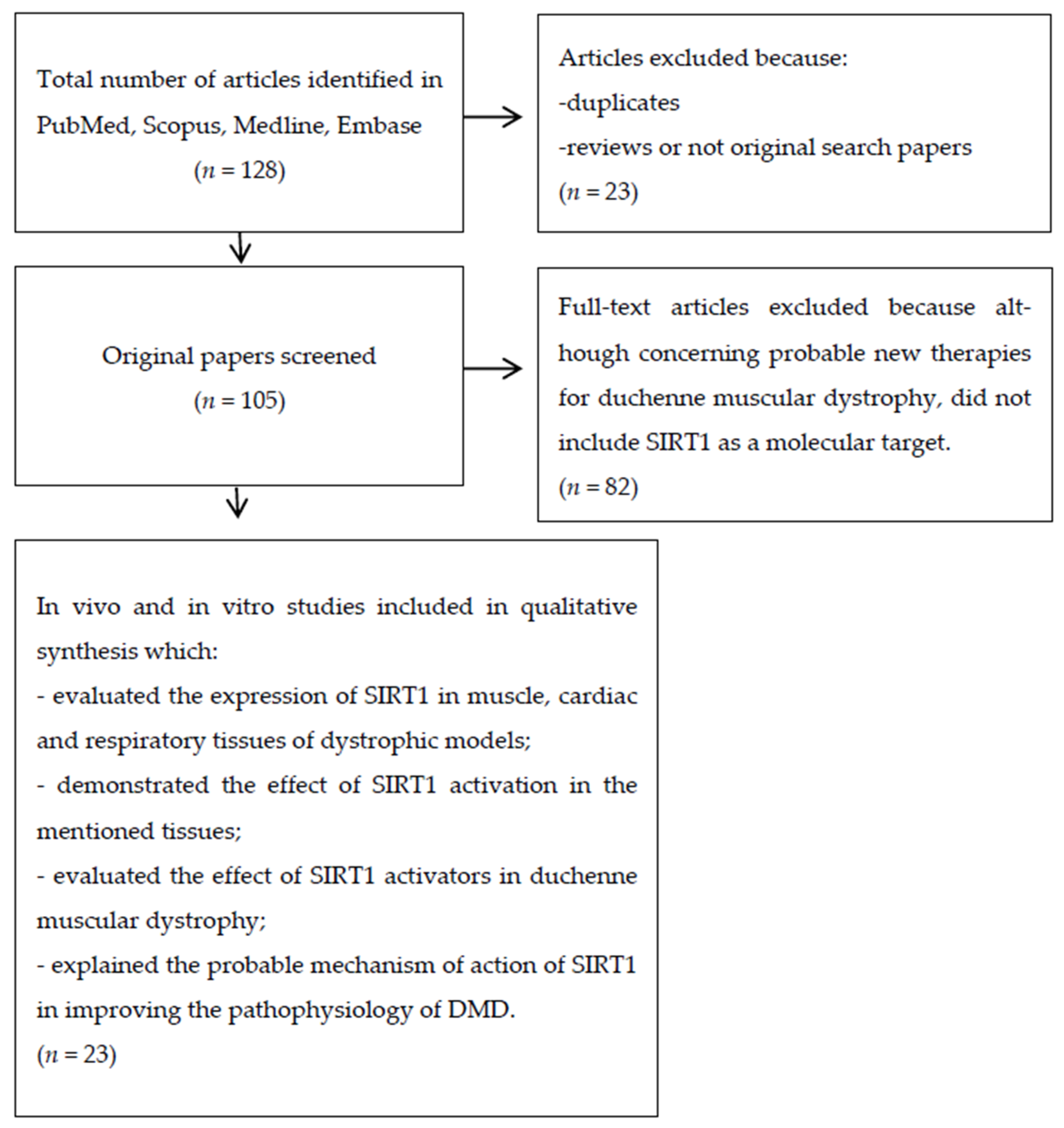
A Systematic Review on the Role of SIRT1 in Duchenne Muscular Dystrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
- -
- evaluated the expression of SIRT1 in muscle, cardiac and respiratory tissues of dystrophic models;
- -
- demonstrated the effect of SIRT1 activation in the mentioned tissues;
- -
- evaluated the effect of SIRT1 activators in Duchenne muscular dystrophy;
- -
- explained the potential mechanisms of action of SIRT1 in improving the pathophysiology of DMD.
2.3. Literature Search and Selection of Articles
2.4. Data Extraction
3. Results
3.1. Overview of Literature Search Results
3.2. Description of Articles Included in the Systematic Review
3.2.1. Main Outcomes Obtained by In Vivo Animal Studies
Effects of SIRT1 Activation in Skeletal Muscle
Effects of SIRT1 Activation in Cardiac Tissue
Effects of SIRT1 Activation in Diaphragm
3.2.2. Main Outcomes Obtained by In Vitro Studies
SIRT1 Effects in C2C12 Myoblast Cells
SIRT1 Effects in Cardiomyocytes
Main Outcomes Obtained by In Vitro Human Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoxha, M. Duchenne Muscular Dystrophy: Focus on Arachidonic Acid Metabolites. Biomed. Pharmacother. 2019, 110, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Lecompte, S.; Abou-Samra, M.; Boursereau, R.; Noel, L.; Brichard, S.M. Skeletal Muscle Secretome in Duchenne Muscular Dystrophy:A Pivotal Anti-Inflammatory Role of Adiponectin. Cell. Mol. Life Sci. 2017, 74, 2487–2501. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Guzman, O.R.; Rodriguez-Cruz, M.; Escobar Cedillo, R.E. Systemic Inflammation in Duchenne Muscular Dystrophy: Association with Muscle Function and Nutritional status. Biomed. Res. Int. 2015, 2015, 891972. [Google Scholar] [CrossRef] [PubMed]
- Deconinnck, N.; Dan, B. Pathophysiology of Duchenne Muscular Dystrophy: Current Hypotheses. Pediatr. Neurol. 2007, 36, 1–7. [Google Scholar] [CrossRef]
- Kuno, A.; Horio, Y. Review Article SIRT1: A Novel Target for the Treatment of Muscular Dystrophies. Oxidative Med. Cell. Longev. 2016, 2016, 6714686. [Google Scholar] [CrossRef]
- Ljubiic, V.; Burt, M.; Jasmin, B.J. The Therapeutic Potential of Skeletal Muscle Plasticity in Duchenne Muscular Dystrophy: Phenotypic Modifiers as Pharmacologic Targets. FASEB J. 2014, 28, 548–568. [Google Scholar] [CrossRef]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part1: Diagnosis, and Pharmacological and Psychosocial Management. Lancet Neurol. 2010, 9, 77–93. [Google Scholar] [CrossRef]
- Capogrosso, R.F.; Cozzoli, A.; Mantuanoa, P.; Camerino, G.M.; Massari, A.M.; Sblendorio, V.T.; deBellis, M.; Tamma, R.; Giustino, A.; Nico, B.; et al. Assessment of Resveratrol, Apocynin and Taurine on Mechanical-Metabolic Uncoupling and Oxidative Stressina Mouse Model of Duchenne Muscular Dystrophy: A Comparison with the Gold Standard—Methyl Prednisolone. Pharm. Res. 2016, 106, 101–113. [Google Scholar] [CrossRef]
- Malik, V.; Rodino-Klapac, L.R.; Mendell, J.R. Emerging Drugs for Duchenne Muscular Dystrophy. Exprt. Opin. Emerg. Drugs 2012, 17, 261–277. [Google Scholar] [CrossRef]
- DeLuca, A. Pre-Clinical Drug Testsin the Mdx Mouseasa Model of Dystrophinopathies: An Overview. Acta Myol. 2012, 31, 40–47. [Google Scholar]
- Tidball, J.G.; Wehling-Henricks, M. Damage and Inflammation in Muscular Dystrophy: Potential Implications and Relationships with Autoimmune Myositis. Curr. Opin. Rheumatol. 2005, 17, 707. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Kumar, A. Therapeutic Targeting of Signaling Pathways in Muscular Dystrophy. J. Mol. Med. 2010, 88, 155. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule Activators of Sirtuins Extend Saccharomyces Cerevisiae Lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Chang, H.C.; Guarente, L. SIRT1 and Other Sirtuinsin Metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wierman, M.B.; Smith, J.S. Yeast Sirtuins and the Regulation of Aging. FEMS Yeast Res. 2013, 25, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as Regulators of Metabolism and Healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef]
- Baur, J.A.; Ungvari, Z.; Minor, R.K.; Le Couteur, D.G.; deCabo, R. Are Sirtuins Viable Targets for Improving Healthspan and Lifespan? Nat. Rev. Drug Discov. 2012, 11, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by ActivatingSIRT1and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Granchi, C.; Minutolo, F. Activators of Sirtuin-1and their Involvmentin Cardioprotection. Med. Chem. 2018, in press. [Google Scholar]
- Rahman, S.; Islam, R. Mammalian Sirt1: Insights on Ist Biological Functions. Cell Comm. Signal. 2011, 9, 11. [Google Scholar] [CrossRef]
- North, B.; Verdin, E. Sirtuins: Sir2-Related NAD-Dependent Protein Deacetylases. Genome Biol. 2004, 5, 224. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Yang, D.I.; Lin, T.K.; Shaw, F.Z.; Liou, C.W.; Chuang, Y.C. Roles of Oxidative Stress, Apoptosis, PGC-1a and Mitochondrial Biogenesis in Cerebral Ischemia. Int. J. Mol. Sci. 2011, 12, 7199–7215. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.Y.; Wu, Z.; Boss, O. Transcriptional Co-Activator PGC-1a Drives the Formation of Slow-Twitch Muscle Fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK Regulates Energy Expenditure by Modulating NAD+Metabolism and SIRT1Activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Jiang, L.Q.; Deshmukh, A.S.; Mataki, C.; Coste, A.; Lagouge, M.; Zierath, J.R.; Auwerx, J. Interdependence of AMPK and SIRT1 for Metabolic Adaptation to Fasting and Exercise in Skeletal Muscle. Cell Metab. 2010, 11, 213–219. [Google Scholar] [CrossRef]
- Gerhart-Hines, Z.; Rodgers, J.T.; Bare, O.; Lerin, C.; Kim, S.H.; Mostoslavsky, R.; Alt, F.W.; Wu, Z.; Puigserver, P. Metabolic Control of Muscle Mitochondrial Function and Fatty Acid Oxidation through SIRT1/PGC-1alpha. EMBO J. 2007, 26, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic coactivator PGC-1α. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Sandri, M.; Lin, J.; Handschin, C.; Yang, W.; Arany, Z.P.; Lecker, S.H.; Goldberg, A.L.; Spiegelman, B.M. PGC-1alpha Protects Skeletal Muscle from Atrophy by Suppressing Foxo3ActionandAtrophyspecificGeneTranscription. Proc. Natl. Acad. Sci. USA 2006, 103, 16260–16265. [Google Scholar] [CrossRef]
- Wenz, T.; Rossi, S.G.; Rotundo, R.L.; Spiegelman, B.M.; Moraes, C.T. Increased Muscle PGC-1alpha Expression Protects from Sarcopenia and Metabolic Disease during Aging. Proc. Natl. Acad. Sci. USA 2009, 106, 20405–20410. [Google Scholar] [CrossRef]
- Handschin, C.; Kobayashi, Y.M.; Chin, S.; Seale, P.; Campbell, K.P.; Spiegelman, B.M. PGC-1alpha Regulates the Neuromuscular Junction Program and Ameliorates Duchenne Muscular Dystrophy. Genes Dev. 2007, 21, 770–783. [Google Scholar] [CrossRef]
- Boots, A.W.; Wilms, L.C.; Swennen, E.L.; Kleinjans, J.C.; Bast, A.; Haenen, G.R. In Vitro and Ex Vivo Anti-Inflammatory Activity of Quercetin in Healthy volunteers. Nutrition 2008, 24, 703–710. [Google Scholar] [CrossRef]
- Han, J.J.; Hao, J.; Kim, C.H.; Hong, J.S.; Ahn, H.Y.; Lee, Y.S. Quercetin Prevents cardiac Hypertrophy Induced by Pressure Overloadin Rats. J. Vet. Med. Sci. 2009, 71, 737–743. [Google Scholar] [CrossRef]
- Vásquez-Garzón, V.R.; Arellanes-Robledo, J.; García-Román, R.; Aparicio-Rautista, D.I.; Villa-Treviño, S. Inhibition of Reactive Oxygen Species and Pre-Neoplastic Lesions by Quercetin through an Antioxidant defense Mechanism. Free Radic. Res. 2009, 43, 128–137. [Google Scholar] [CrossRef]
- Ballmann, C.; Denney, T.S.; Beyers, R.J.; Quindry, T.; Romero, M.; Amin, R.; Selsby, J.T.; Quindry, J.C. Lifelong Quercetin Enrichment and Cardioprotection in Mdx/Utrn+/−Mice. J. Physiol. Heart Circ. Physiol. 2017, 312, H128–H140. [Google Scholar] [CrossRef] [PubMed]
- Abou-Samra, M.; Lecompte, S.; Schakman, O.; Noel, L.; Many, M.C.; Gailly, P.; Brichard, S.M. Involvement of Adiponectin in the Pathogenesis of Dystrophinopathy. Skelet. Muscle 2015, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, D.; Iwahara, N.; Sebori, R.; Hosoda, R.; Shimohama, S.; Kuno, A.; Horio, Y. SIRT1 Deficiency Interferes with Membrane Resealing after Cell Membrane Injury. PLoS ONE 2019, 14, e0218329. [Google Scholar] [CrossRef]
- Hulmi, J.J.; Hentilä, J.; De Ruisseau, K.C.; Oliveira, B.M.; Papaioannou, K.G.; Autio, R.; Kujala, U.M.; Ritvos, O.; Kainulainen, H.; Korkmaz, A.; et al. Effects of Muscular Dystrophy, Exercise and Blocking Activin Receptor IIB Ligand so the Unfolded Protein Response and Oxidative Stress. Free Radic. Biol. Med. 2016, 99, 308–322. [Google Scholar] [CrossRef]
- Chalkiadaki, A.; Igarashi, M.; Nasamu, A.S.; Knezevic, J.; Guarante, L. Muscle-Specific SIRT1 Gain-of-Function Increases Slow Twitch Fibers and Ameliorates Pathophysiology in a Mouse Model of Duchenne Muscular Dystrophy. PLoS Genet. 2014, 10, e1004490. [Google Scholar] [CrossRef] [PubMed]
- Ljubicic, V.; Burt, M.; Lunde, J.A.; Jasmin, B.J. Resveratrol Induces Expression of the Slow, Oxidative Phenotype in Mdx Mouse Muscle Together with Enhanced Activity of the SIRT1-PGC-1Axis. Am. J. Physiol. Cell Physiol. 2014, 307, C66–C82. [Google Scholar] [CrossRef]
- Gordon, B.S.; Delgado Diaz, D.C.; Kostek, M.C. Resveratrol Decreases Inflammation and Increases Utrophin Gene Expression in the Mdx Mouse Model of Duchenne Muscular Dystrophy. Clin. Nutr. 2013, 32, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Tanno, M.; Horio, Y. The Effects of Resveratrol and SIRT1Activation on Dystrophic Cardiomyopathy. Ann. N. Y. Acad. Sci. 2015, 1348, 46–54. [Google Scholar] [CrossRef]
- Hori, Y.S.; Kuno, A.; Hosoda, R.; Tanno, M.; Miura, T.; Shimamoto, K.; Horio, Y. Resveratrol Ameliorates Muscular Pathology in the Dystrophic Mdx Mouse, a Model for Duchenne Muscular Dystrophy. J. Pharm. Exp. 2011, 338, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Hori, Y.S.; Hosoda, R.; Tanno, M.; Miura, T.; Shimamoto, K.; Horio, Y. Resveratrol Improves Cardiomyopathy in Dystrophin-deficient Mice through SIRT1 Protein-mediated Modulation of p300Protein. J. Biol. Chem. 2013, 288, 5963–5972. [Google Scholar] [CrossRef] [PubMed]
- Sebori, R.; Kuno, A.; Hosoda, R.; Hayashi, T.; Horio, Y. Resveratrol Decreases Oxidative Stress by Restoring Mitophagy and Improves the Pathophysiology of Dystrophin-Deficient mdx Mice. Oxidative Med. Cell. Longev. 2018, 29, 9179270. [Google Scholar] [CrossRef]
- Kuno, A.; Hosoda, R.; Sebori, R.; Hayashi, T.; Sakuragi, T.; Tanabe, M.; Horio, Y. Resveratrol Ameliorates Mitophagy Disturbance and Improves Cardiac Pathophysiology of Dystrophin deficient mdx Mice. Sci. Rep. 2018, 8, 15555. [Google Scholar] [CrossRef]
- Selsby, J.T.; Ballmann, C.G.; Spaulding, H.R.; Ross, J.V.; Quindry, J.C. Oral Quercetin Administration Transiently Protects Respiratory Function in Dystrophin-Deficient Mice. J. Physiol. 2016, 594, 6037–6053. [Google Scholar] [CrossRef] [PubMed]
- Camerino, G.M.; Cannone, M.; Giustino, A.; Massari, A.M.; Capogrosso, R.F.; Cozzoli, A.; de Luca, A. Gene Expressionin Mdx Mouse Muscle in Relation to Age and Exercise: Aberrant Mechanical–Metabolic Coupling and Implications for Pre-Clinical Studies in Duchenne Muscular Dystrophy. Hum. Mol. Genet. 2014, 21, 5720–5732. [Google Scholar] [CrossRef] [PubMed]
- Ljubicic, V.; Khogali, S.; Renaud, J.M.; Jasmin, B.J. Chronic AMPK Stimulation Attenuates Adaptive Signaling in Dystrophic Skeletal Muscle. Am. J. Physiol. Cell Physiol. 2012, 302, C110–C121. [Google Scholar] [CrossRef]
- Hollinger, K.; Shanely, R.A.; Quindry, J.C.; Selsby, J.T. Long-Term Quercetin Dietary Enrichment Decreases Muscle Injury in Mdx Mice. Clin. Nutr. 2015, 34, 515–522. [Google Scholar] [CrossRef]
- Spaulding, H.R.; Ballmann, C.G.; Quindry, J.C.; Selsby, J.T. Long-Term Quercetin Dietary Enrichment Partially Protects Dystrophic Skeletal Muscle. PLoS ONE 2016, 11, e0168293. [Google Scholar] [CrossRef] [PubMed]
- Ballmann, C.; Denney, T.; Beyers, R.J.; Quindry, T.; Romero, M.; Selsby, J.T.; Quindry, J.C. Long Term Dietary Quercetin Enrichment as a Cardioprotective Counter measure in Mdx Mice. Exp. Physiol. 2017, 102, 635–649. [Google Scholar] [CrossRef]
- Gordon, B.S.; Delgado-Diaz, D.C.; Carson, J.; Fayad, R.; Wilson, L.B.; Kostek, M.C. Resveratrol Improves Muscle Function but Not Oxidative Capacity in Young Mdx Mice. Can. J. Physiol. Pharm. 2014, 92, 243–251. [Google Scholar] [CrossRef]
- Ballmann, C.; Hollinger, K.; Selsby, J.T.; Amin, R.; Quindry, J.C. Histological and Biochemical Outcomes of Cardiac Pathologyinmdx Mice with Dietary Quercetin Enrichment. Exp. Physiol. 2015, 100, 12–22. [Google Scholar] [CrossRef]
- Selsby, J.T.; Morine, K.J.; Pendrak, K.; Barton, E.R.; Sweeney, H.L. Rescue of Dystrophic Skeletal MusclebyPGC-1aInvolves a Fast to Slow Fiber Type Shift in the mdx Mouse. PLoS ONE 2012, 7, e30063. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Mariño, G.; Bennetzen, M.V. Spermidine and Resveratrol Induce Autophagy by Distinct Pathways Convergingonthe Acetylproteome. J. Cell Biol. 2011, 192, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and Its Effects on the Vascular System. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef]

| No | Study | Type of Animal | Experimental Design | Event | Parameters Assessed | Outcomes |
|---|---|---|---|---|---|---|
| 1 | Hulmi, J.J. et al. “Effects of muscular dystrophy, exercise and blocking activin receptor IIB ligands on the unfolded protein response and oxidative stress.” Free Radical Biology and Medicine 99 (2016) 308–322 | mdx mice/wild type mice as control | (1) Comparison between mdx and wild type mice regarding: endoplasmatic reticulum (ER) stress and unfolded protein response (UPR); the ratio of phosphorylated-SIRT1 (p-SIRT1) and SIRT1; acetylated lysine levels. (2) Seven weeks of voluntary exercise and/or soluble activin receptor-Fc (sAcvR2B-Fc) administration. | Dystrophin deficiency in skeletal muscle. | ER stress/UPR; Phosphorylated SIRT1 levels; Acetylated protein lysine residues levels. | (1) Compared to wild type mice, in mdx mice UPR response is activated in the ER, also p-SIRT1 levels are increased 1.7 times and total SIRT1 levels decreased leading to increased p-SIRT1/SIRT1. Further more acetylated protein lysine residues are increased 1.6 times in mdx mice compared to the wild type ones. All these conditions contribute to the onset of DMD pathophysiology. (2) Voluntary exercise alone or combined with sAcvR2B-Fc administration leads to increased p-SIRT1 which in turn lead to redox regulation [38]. |
| 2 | Capogrosso, R.F. et al. “Assessment of resveratrol, apocynin and taurine on mechanical-metabolic uncoupling and oxidative stress in a mouse model of duchenne muscular dystrophy: A comparison with the gold Standard, α -methyl prednisolone.” Pharmacological Research 106 (2016) 101–113 | mdx mice/wild type mice as control | Resveratrol (100 mg/kg i.p 5 days/week); apocinin (38 mg/kg/day per os); taurine (1 g/kg/day per os); α-methyl prednisolone (1 mg/kg i.p., 5 days/week) were administered for 4 weeks in parallel with twice a week exercise (30 min running on a horizontal treadmill). | Evaluation of resveratrol, apocinin and taurine in comparison with methyl prednisolone (PDN) in ameliorating DMD. | Body weight; fore-limb force; plasma levels of creatine kinase and lactate dehydrogenase; ROS levels; NADPH oxidase (NOX) activity; SIRT1 expression. | (1) Body weight: In wt untreated mice, the increment of body weight was lower (+7 g) compared to untreated mdx mice (+8.5/+11.8 g). mdx mice treated with apocynine or taurine showed no great differences compared to the untreated ones. Conversely resveratrol (+7 g), and especially PDN (+3 g) lowered dhe body weight increment. (2) Forelimb force: similar values for untreated wt and mdx mice; increased values for treated mdx mice with increased recovery score (RS): apocynin 35%; taurine 55%; PDN 58%; resveratrol 60%. (3) Creatine kinase: resveratrol reduced CK levels with a rescue score of 64%; taurine, apocynine and PDN did not affect CK levels. (4) Lactate dehydrogenase: resveratrol and taruine reduced LDH with a rescue score of 82% and 47%, respectively; apocynine and PDN did not affect LDH levels. (5) SIRT1 expression: all the treatments led to an unexpected reduction in SIRT1 expression, probably due to an ROS reduction after the treatment [8]. |
| 3 | Kuno, A. et al. “The effects of resveratrol and SIRT1 activation on dystrophic cardiomyopathy”. Ann. N.Y. Ann. N. Y. Acad. Sci. 1348 (2015) 46–54 | mdx mice/wild type mice as control. | Resveratrol (4 g/kg chow ad libitum) for 32 weeks, beginning at the age of 9 weeks. Estimated intake of resveratrol: 500 mg/kg/day. | Resveratrol’s effect on cardiomyopathy due to muscular dystrophy. | Motion of the left ventricular wall; cardiac hypertrophy; myocardial mRNA levels of B-type natriuretic peptide; average life span; cardiomyocyte cross-sectional area; transcription co-activator p300. | Resveratrol treatment suppressed cardiac hypertrophy due to a suppressed increase in heart weight body weight ratio, reduced myocardial mRNA levels of atrial natriuretic peptide and cardiomyocites cross-sectional area, compared to untreated mice. It also preserved cardiac function and reduced tissue fibrosis in diseased heart [42]. |
| 4 | Chalkiadaki, A. et al. “Muscle-Specific SIRT1 Gain-of-Function Increases Slow Twitch Fibers and Ameliorates Pathophysiology in aMouse Model of Duchenne Muscular Dystrophy”. PLoS Genetics (2014) | Muscle-specific transgenic mice (TG)/SIRT1 muscle-specific knockout mice (MckKO). | SIRT1 muscle transgenic mice were crossed to mdx mice. | Effect of SIRT1 overexpression in muscles. | Muscle wasting; alteration of fiber composition; PGC-1α activity; utrophin levels. | Mice with overexpressed SIRT1 (TG mice) oppose atrophy gene program reversing muscle hypertrophy of dystrophic muscle, improved fiber shift from fast-to-slow twitch, increased utrophin levels and increased PGC-1α levels compared to MckKO [39]. |
| 5 | Fujiwara, D. et al. “SIRT1 deficiency interferes with membrane resealing after cell membrane injury”. PLoS ONE 14(6) (2019). | Skeletal muscle-specific SIRT1 knockout (SIRT1-MKO)/wild type mice as control. | Skeletal muscle-specific SIRT1 knockout mice (SIRT1 MKO) were generated. | Effect of lack of SIRT1 in skeletal muscles. | Exercise capacity; myofiber damage evaluation; membrane repair; serum levels of creatine kinase (CK) and lactate dehydrogenase activities (LDH). | SIRT1-MKO mice shows a similar pathophysiology to mild dystrophic mice. In SIRT1-MKO mice, high serum CK and LDH levels were observed, compared to the WT ones. Increased numbers of centrally nucleated small myofibers and decreased numbers of middle-sized myofibers were also observed in SIRT1-MKO mice compared to WT mice [37]. |
| 6 | Gordon, B.S. et al. “Resveratrol decreases inflammation and increases utrophin gene expression in the mdx mouse model of duchenne muscular dystrophy”. Clinical Nutrition 32 (2013) 104–111. | mdx mice. | Resveratrol (0, 10, 100, or 500 mg/kg) everyday for 10 days beginning at the age of 5 weeks. | Effect of resveratrol in reducing inflammation caused by DMD. | SIRT1 gene expression; active dose of resveratrol; immune cell infiltration; macrophage infiltration; IL-6, PGC-1α, and utrophin levels. |
Resveratrol treatment improved SIRT1 gene expression, especially at the dose of 100 mg/kg where the increase is about 60 ± 10%. So, the most effective dose (100 mg/kg) was used for further analysis:
|
| 7 | Hori, Y.S. et al. “Resveratrol Ameliorates Muscular Pathology in the Dystrophic mdx Mouse, a Model for Duchenne Muscular Dystrophy” The Journal of pharmacology and experimental therapeutics (2011) 338:784–794 | mdx mice/wild type mice as control | Resveratrol (4 g/kg meal) mixed with powder meal and orally administered ad libitum for 32 weeks, beginning at the age of 9 weeks. | Resveratrol’s effect in mdx mice. | Preservation of muscle mass; inhibition of oxidative stress and fibrosis. |
After resveratrol treatment, the following parameters were assessed, compared to mdx untreated mice:
|
| 8 | Ljubicic, V. et al. “Resveratrol induces expression of the slow, oxidative phenotype in mdx mouse muscle together with enhanced activity of the SIRT1-PGC-1 axis”. Am J Physiol Cell Physiol (2014) 307: C66–C82. | mdx mice/wild type mice as control | -First protocol: resveratrol (100 mg/kg/day with diet) for 6 weeks. Moderate dose and short duration (MDSD). -Second protocol: resveratrol (500 mg/kg/day with diet) for 12 weeks. High dose and long duration (HDLD). | Effect of resveratrol in muscle remodeling by stimulating the slow, oxidative myogenic program in dystrophic skeletal muscle. | SIRT-1 activity; PGC-1α activity. | Resveratrol treatment promotes SIRT1 and PGC-1α signaling, enhancing the expression of the slow, oxidative phenotype in mdx mice. The dose of 100 mg/kg/day maximizes the effect [40]. |
| 9 | Kuno, A. et al. “Resveratrol Improves Cardiomyopathy in Dystrophin-deficient Mice through SIRT1 Protein-mediated Modulation of p300 Protein”. The Journal of Biological chemistry, vol. 288 (2013) 5963–5972 | mdx mice/control mice | Resveratrol (4 g/kg) mixed with powder meal and orally administered ad libitum for 32 weeks, beginning at the age of 9 weeks. | Effect of SIRT1 modulation, through long-term resveratrol treatment, in cardiomyopathy. | p300levels; SIRT1 activity; heart weight. |
Resveratrol treatment ameliorated cardiomyopathy and cardiac function in mdx mice. Below are some of the parameters assessed that proved it:
|
| 10 | Sebori, R. et al. “Resveratrol Decreases Oxidative Stress by Restoring Mitophagyand Improves the Pathophysiology of Dystrophin-Deficientmdx Mice”. Oxidative Medicine and Cellular Longevity Volume (2018) | mdx mice/control mice | Resveratrol (0.04, 0.4, and 4 g/kg food) for 56 weeks. | Resveratrol effect in dystrophin-deficient mdx mice through oxidative stress reduction. | Creatine kinase levels; physical activities; autophagy. | In treated mdx mice reduced myofiber wasting and enhanced muscular maturation were observed at the dose of 4 g/kg food of resveratrol compared to untreated mdx mice. Additionally, reduced creatine kinase levels and increased physical activity at the dose of 0.4 g/kg food of resveratrol were shown, compared to untreated mdx mice [45]. |
| 11 | Kuno, A. et al. “Resveratrol Ameliorates Mitophagy Disturbance and Improves Cardiac Pathophysiology of Dystrophin deficient mdx Mice”. Scientific reports (2018) 8:15555 | mdx mice/ control mice | Resveratrol (0.04, 0.4, or 4 g/kg food) ad libitum, mixed with powdered meal for 56 weeks, beginning at the age of 9 weeks. | Benefits of resveratrol in cardiac diseases in mdx mice, by promoting mitophagy. | Mitochondrial DNA (mtDNA); autophagy of damaged mitochondria (mitophagy) level; ROS levels. | Mdx mice shows lower levels of cardiac mtDNA compared to control mice. Resveratrol treatment improved cardiomyopathy, increasing mitophagy and decreasing mtDNA deletion and ROS levels compared to untreated mdx mice. The most effective dose is 0.4 g/kg food of resveratrol [46]. |
| 12 | Ballmann, C. et al. “Lifelong quercetin enrichment and cardioprotection in Mdx/Utrn/mice”. Am J Physiol Heart CircPhysiol (2017) 312: H128–H140. | dystrophin-deficient mice heterozygous for a utrophin mutation (Mdx/Utrn+/−)/ control mice | 0.2% quercetin enriched diet, provided ad libitum for 8 months, beginning at the age of 2 months. | Cardioprotective effect in dystrophic hearts of quercetin, a SIRT1/PGC-1α activator. | Functional cardiac assessment; cardiac expression of utrophin, fibronectin, inflammatory markers. |
Quercetin treatment results in a reduction in the pathological remodeling of the dystrophic heart; below are listed some of the parameters assessed:
|
| 13 | Selsby, J.T. et al. “Oral quercetin administration transiently protects respiratory function in dystrophin-deficient mice”. J Physiol 594.20 (2016) pp 6037–6053 | mdx mice/control mice | 0.2% quercetin enriched diet, provided ad libitum for 12 months, beginning at the age of 2 months. | The benefits of dietary quercetin supplementation in respiratory function of dystrophin-deficient mice. | Respiratory function; histological parameters; biochemical parameters. | Quercetin treatment improved respiratory function; in fact respiratory frequency was increased compared to untreated mdx mice and was similar to control mice for the first 6–8 months of age, but beyond the 8th month there is not any improvement. The maximum value was obtained at 8 months of age where the frequency of treated mdx mice was ±115, versus ±95 for untreated mdx mice. Quercetin treatment also improved other parameters of respiratory function such as tidal volume, with a 20-fold increase, minute ventilation, with a 50-fold increase, peak inspiratory flow, with a 25-fold increase, and peak expiratory flow, with a 40-fold increase, compared to untreated mdx mice [47]. |
| 14 | Abou-Samra, M. et al. “Involvement of adiponectin in the pathogenesis of dystrophinopathy”. Skeletal Muscle (2015) 5:25. | mdx-ApN mice/mdx mice/wild type mice as control | Mice overexpressing adiponectin (ApN mice) are crossed with mdx mice in order to obtain mdx mice overexpressing ApN (mdx-ApN mice). | Effect of adiponectin in counteracting muscle degeneration. | Global force or resistance; muscle damage markers in plasma; plasma creatine kinase (CK) and lactate dehydrogenase (LDH); utrophin levels. |
Different parameters were assessed in the three group of animals:
|
| 15 | Camerino, G., M. et al. “Gene expression in mdx mouse muscle in relation to age and exercise: aberrant mechanical–metabolic coupling and implications for pre-clinical studies in Duchenne muscular dystrophy”. Human Molecular Genetics, (2014) 1–13. | mdx mice/wild type mice as control | Twice a week, mdx and control mice undergo to 30 min running on a horizontal treadmill at 12 m/min, with a break of 48–72 h between each session. The exercise lasts for 4 or 12 weeks. | Age and exercise -related gene expression in mdx mice muscles. | Effect of exercise on in vivo performance; analysis of gene expression in the GC muscles of mdx. |
The effect of exercise on in vivo performance were demonstrated with parameters such as:
|
| 16 | Ljubicic, V. et al. “Chronic AMPK stimulation attenuates adaptive signaling in dystrophic skeletal muscle”. Am J Physiol Cell Physiol (2012) 302: C110–C121 | mdx mice | AMP-activated protein kinase (AMPK) activator 5-aminoimidazole-4-carboxamide-1—D-ribofuranoside (AICAR; 500 mg/kg/day) for 30 days, and then half of the animals were subjected to treadmill running to induce acute AMPK signaling. | Effects of induced phenotype shift in dystrophic skeletal muscle in the subsequent intracellular signaling. | Evaluation of mRNA levels of phenotypic modifiers, including PPARγ coactivator-1α (PGC-1α) and SIRT1. | In mdx mice, treadmill running induces PGC-1α, and SIRT1 mRNAs, that in turn promotes the slow, oxidative myogenic program. Additionally, acute stress-induced expression of PGC-1α and SIRT1 is attenuated by AMPK stimulation. These data suggest the importance of the axis PPGC-1α/SIRT1 as a novel therapeutic target for DMD [49]. |
| 17 | Hollinger, K. et al. “Long-term quercetin dietary enrichment decreases muscle injury in mdx mice”. Clinical Nutrition (2015) 34(3):515–522 | mdx mice | 0.2% quercetin enriched diet, provided ad libitum, for 6 months, beginning at the age of 3 months. | Effect of long-term quercetin enriched diet in decreasing muscle injury in diaphragm of dystrophic mice. | In a diaphragm strip: number of muscle fibers, percentage of fibers with centralized nuclei, fibrotic area, and expression of oxidative genes. | Quercetin-treated mdx mice show a better muscle profile with an increase of 24% in muscle fibers, a reduction of 34% in fibers with centralized nuclei; a 31% reduction in immune cell infiltration and a 47% decrease in fibrotic area, all compared to untreated mdx mice. Utrophin levels are unchanged [50]. |
| 18 | Spaulding, H., R. et al. “Long-Term Quercetin Dietary Enrichment Partially Protects Dystrophic Skeletal Muscle” PLoS ONE (2016) 11(12) | mdx mice/wild type mice as control | 0.2% quercetin-enriched diet, provided ad libitum, for 12 months, beginning at the age of 2 months. | Effect of long-term quercetin-enriched diet in preservation of limb muscle function through PGC-1α/SIRT1 pathway activation. | Physical activity; soleus and extensor digitorumlongus (EDL) muscle function; specific tension; fatigue resistance; muscle injury; fibrosis. | In quercetin-treated mdx mice, physical activity is increased. In total, 50% of loss of specific tension and fatigue resistance in the soleus is prevented, while in EDL muscle there are no big improvements. Relative muscle mass in the soleus is doubled in untreated mdx mice compared to control, and in the treated one it is 11% greater than in the untreated ones. In EDL muscle, the relative muscle mass of untreated mdx mice is 60% increased compared to control mice, but no improvements are observed after quercetin treatment [51]. |
| 19 | Ballmann, C. et al. “Long term dietary quercetin enrichment as a cardioprotective countermeasure in mdx mice”. Exp Physiol. (2017) 102(6):635–649. | mdx mice/control mice | 0.2% quercetin enriched diet, provided ad libitum, for 12 months, beginning at the age of 2 months. | Cardioprotective effect of long-term quercetin enriched diet in mdx mice. | Physical activity; cardiac function; percentage of damaged tissue; cardiac levels of fibronectin. |
Different parameters were assesed:
|
| 20 | Gordon, B.S. et al. “Resveratrol improves muscle function but not oxidative capacity in young mdx mice”. Can. J. Physiol. Pharmacol. Vol. 92, (2014) | mdx mice | Resveratrol (100 mg/kg suspended in 200 µL of water every other day) for 8 weeks beginning at the age of 4–5 weeks. | Resveratrol effects on muscle function, muscle pathology and oxidative capacity in young mdx mice. | Body grip strength; in situ muscle function; fibers total number; centralized nuclei cells; number of immune cells; utrophin quantification. |
Different parameters were assessed to identify the role of resveratrol treatment in muscle function:
|
| 21 | Ballmann, C. et al. “Histological and Biochemical Outcomes of Cardiac Pathology in mdx Mice with Dietary Quercetin Enrichment“. ExpPhysiol 100.1 (2015) 12–22 | Mice | Experiment 1: 0.2% quercetin enriched diet, ad libitum, for 6 months, beginning at the age of 3 weeks. Experiment 2: 0.2% quercetin enriched diet, ad libitum, for 6 months, beginning at the age of 3 months. | Effect of quercetin-enriched diet in preventing and rescuing cardiac pathology in mdx mice. | Mitochondrial biogenesis; cardiac remodeling; fibrotic area; antioxidant expression; utrophin levels. | Protocol 1: Prevention. Quercetin increases mitochondrial biogenesis in hearts and improves heart levels of SOD2, an endogenous antioxidant enzyme. Utrophin levels are also increased. Protocol 2: Rescue. Quercetin feeding leads to a reduction in heart remodeling. Additionally, TGF-β1 levels, a regulator of fibrosis, are decreased. All these findings suggest that oral quercetin may be a counter measure against cardiac and skeletal muscle pathology [54]. |
| 22 | Selsby, J.T. et al. “Rescue of Dystrophic Skeletal Muscle by PGC-1a Involves a Fast to Slow Fiber Type Shift in the mdx Mouse” PLoS ONE 7(1) (2012). | mdx mice | Overexpression induction of PGC-1α: (a) via injection of adeno-associated virus (AAV) (b) orally by administration of resveratrol (100 mg/kg/day) for eight weeks. | Effect of PGC-1α overexpression in rescuing dystrophic skeletal muscle. | Utrophin expression; type I myosin heavy chain expression; mitochondrial protein expression; SIRT-1 levels; muscle resistance; fatigue resistance. | Both treatments lead to an increased expression of PGC-1α, that in turn leads to increased utrophin levels, and type I myosin heavy chain as well. Mitochondrial protein expression and SIRT1 levels are also increased, while p38 and NRF-1 activity is reduced. All these events result in improved muscle and fatigue resistance [55]. |
| No | Study | Type of Cells | Experimental Design | Event | Parameters Assessed | Outcomes |
|---|---|---|---|---|---|---|
| 1 | Fujiwara, D. et al. “SIRT1 deficiency interferes with membrane resealing after cell membrane injury”. PLoS ONE 14(6) (2019). | C2C12 myoblast cells | Cells treatment with vehicle or SIRT1 inhibitors: nicotinammide (NAM) 10 mM or Ex527 10 μM, incubated for 12 h. RNAi-mediated knockdown was performed by transfection of Sirt1-siRNAor Control-siRNA, both 30 nM. | Effects of SIRT1 inhibition in membrane resealing of C2C12 myoblast cells. | Effects of SIRT1 on membrane repair. | Influx of fluorescent dye FM1-43 in cells after laser irradiation was used to monitor membrane resealing. In treated C2C12 cells, FM1-43 uptake is improved compared to untreated cells, suggesting that NAM and Ex527 inhibited membrane resealing through SIRT1 inhibition.Treatment of cells with Sirt1-siRNA was used to further confirm the role of SIRT1 in membrane resealing. Additionally, in this case, in treated cells, membrane resealing is inhibited due to inhibited aggregation of membrane vesicles at the injured site [37]. |
| 2 | Hori, Y.,S. et al. “Resveratrol Ameliorates Muscular Pathology in the Dystrophic mdx Mouse, a Model for Duchenne Muscular Dystrophy”. J Pharmacol. Exp. Ther. (2011) 338(3)784–94 | C2C12 myoblast cells | Pre-treatement with resveratrol (30 µM) and then treatment with TGF-β1 (10 ng/mL) | Effects of resveratrol in reducing ROS levels in myoblasts, through SIRT1 activation. | Intracellular ROS levels. | TGF-β1 treatment leads to increased ROS levels and fibronectin synthesis, suggesting oxidative damage. Resveratrol pre-treatment reverses all these effects through SIRT1 activation [43]. |
| 3 | Kuno, A. et al. “Resveratrol Improves Cardiomyopathy in Dystrophin-deficient Mice through SIRT1 Protein-mediated Modulation of p300 Protein”. THE JOURNAL OF BIOLOGICAL CHEMISTRY (2013). 5963–5972. | Cardiomyocytes of mdx mice. | Resveratrol (4 g/kg meal) mixed with powdered meal, orally administered ad libitum, for 32 weeks, beginning at the age of 9 weeks. | Downregulation of p300 as a cardioprotective mechanism of resveratrol, through SIRT1 activation, in DMD. | Activity of p300 protein; mechanism of p300 downregulation. | In untreated mdx mice the minimal Feret’s diameter of cardiomyocytes in the left ventricle was larger compared to those in control mice. Resveratrol treatment reverses the effect. Additionally, resveratrol and SIRT1 overexpression downregulate p300 activity in cardiomyocytes resulting in hypertrophy inhibition [44]. |
| 4 | Sebori, R. et al. “Resveratrol Decreases Oxidative Stress by Restoring Mitophagy and Improves the Pathophysiology of Dystrophin-Deficient mdx Mice”. Oxidative Medicine and Cellular Longevity (2018). | C2C12 myoblast cells | Cells incubation with vehicle or resveratrol 30 µM for 6 h. | Effects of resveratrol in autophagy/mitophagy processes in C2C12 myoblast cells. | Mitochondrial superoxide levels in C2C12 cells; mitophagy; autophagy. | Resveratrol treatment of C2C12 cells improves autophagosome production and autophagy [56,57]. To assess the role of resveratrol in ROS reduction, antimycin (AA), a mitochondria depolarizer, was used. AA increases mitochondrial ROS levels in C2C12 cells, while resveratrol treatment suppressed this effect. Additionally, resveratrol improves mitophagy and reduces damaged mitochondria from C2C12 cells [45]. |
| 5 | Kuno, A. et al. “Resveratrol Ameliorates Mitophagy Disturbance and Improves Cardiac Pathophysiology of Dystrophindeficient mdx Mice”. SCientifiCREPOrTS (2018) 8:15555 | H9C2 cardiomyocytes | Vehicle or resveratrol (30μM) for 24 h. | Effect of resveratrol in ameliorating cardiac pathology in mdx mice, through mitophagy mechanism. | Mitochondrial DNA (mtDNA) deletion, autophagy of damaged mitochondria (mitophagy). | Resveratrol treatment promotes FoxO3a activity in the nucleus of cardiomyocytes compared to untreated cells.Autophagy and mitophagy are promoted by FoxOs modulation leading overall to decreased ROS accumulation in the heart [46]. |
| 6 | Lecompte, S. et al. “Skeletal muscle secretomein Duchenne muscular dystrophy: a pivotal anti-inflammatory role of adiponectin”. Cell. Mol. Life Sci. (2017) 74:2487–2501 | Human myotubes of dystrophic patients/human myotubes as control | Primary cultures of human myotubes treated or not with adiponectin (ApN) after an inflammatory challenge. | Role of adiponectin in Duchenne muscular dystrophy. | ApN production; levels of some pro-inflammatory molecules like (TNFα, IL-17A, and CCL28), and anti-inflammatory ones like IL6. | In dystrophic myotubes, ApN mRNA and ApN levels were decreased by ~60% and ~15%, respectively, compared to control cells. The treatment of human myotubes with ApN leads to the downregulation of pro-inflammatory markers such as TNFα (~−30%) and to the upregulation of IL-6 (~+75%) that exerts anti-inflammatory properties. The ApN pathway involves the AMPK-SIRT1-PGC-1α axis, leading to utrophin A upregulation. Overall, these data suggest that ApN activity in DMD is regulated by the AMPK-SIRT1-PGC-1α pathway [2]. |
| 7 | Abou-Samra, M.A.; et al. “Involvement of adiponectin in the pathogenesis of dystrophinopathy”. Skeletal Muscle (2015) 5:25 | Human myotubes | Cells were treated with human recombinant TNFα (10 ng/mL) + interferon gamma (IFNγ) (10 ng/mL) and/or ApN (5μg/mL), at the indicated concentrations, for 24 h. | Effect of adiponectin in counteracting muscle degeneration. | Effects of adiponectin on inflamed human myotubes | Adiponectin treatment after the inflammatory stimuli of TNFα and IFNγ lead to a reduction in TNFα mRNAs. This positive effect of ApN is abrogated by siRNA silencing of genes encoding for AdipoR1, SIRT1, or PGC-1α. These data suggest that the SIRT1/PGC-1α axis plays a crucial role in the anti-inflammatory activity of ApN [35]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domi, E.; Hoxha, M.; Prendi, E.; Zappacosta, B. A Systematic Review on the Role of SIRT1 in Duchenne Muscular Dystrophy. Cells 2021, 10, 1380. https://doi.org/10.3390/cells10061380
Domi E, Hoxha M, Prendi E, Zappacosta B. A Systematic Review on the Role of SIRT1 in Duchenne Muscular Dystrophy. Cells. 2021; 10(6):1380. https://doi.org/10.3390/cells10061380
Chicago/Turabian StyleDomi, Elisa, Malvina Hoxha, Emanuela Prendi, and Bruno Zappacosta. 2021. "A Systematic Review on the Role of SIRT1 in Duchenne Muscular Dystrophy" Cells 10, no. 6: 1380. https://doi.org/10.3390/cells10061380
APA StyleDomi, E., Hoxha, M., Prendi, E., & Zappacosta, B. (2021). A Systematic Review on the Role of SIRT1 in Duchenne Muscular Dystrophy. Cells, 10(6), 1380. https://doi.org/10.3390/cells10061380






