Pathogenesis of Endometriosis: The Origin of Pain and Subfertility
Abstract
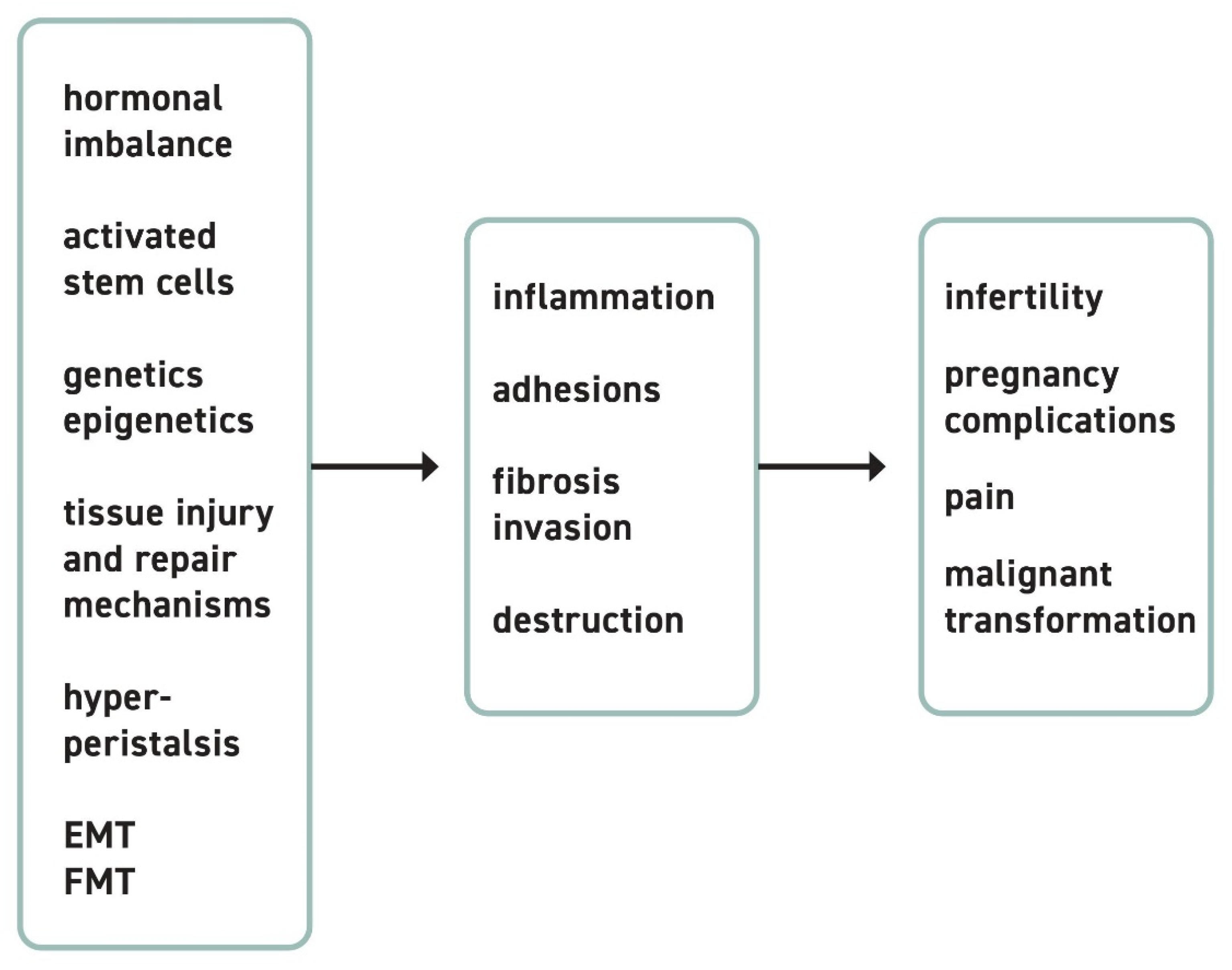
1. Pathogenesis of Endometriotic Lesions
2. Pathophysiologic Origin of Pain
2.1. EM-Associated Pain
2.2. Principles of Pain Development
2.3. Pathogenesis of Specific Forms of Pain
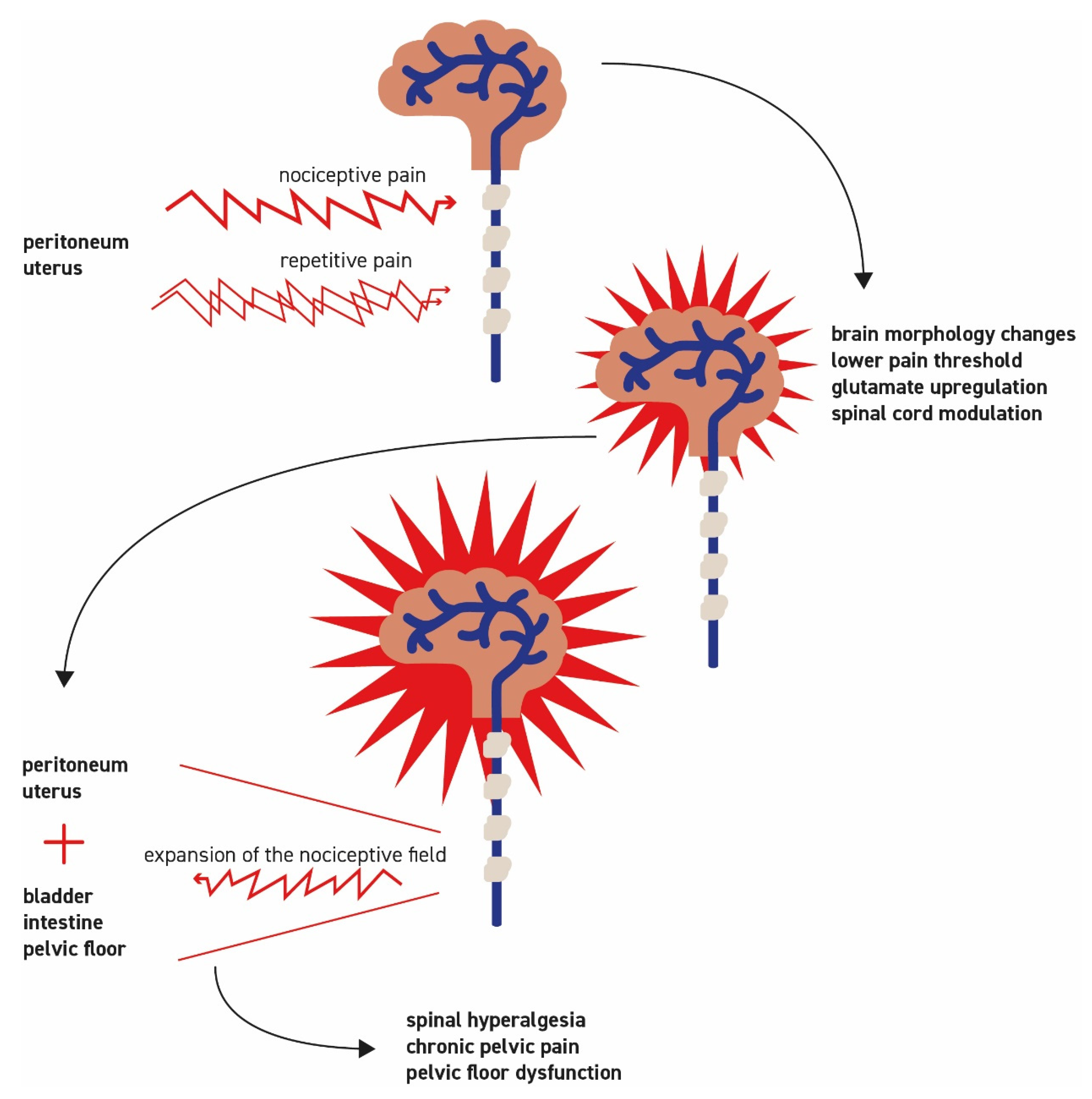
2.4. Neurogenic Inflammation
2.5. Development of Central Sensitization with Spinal Hyperalgesia
3. Pathogenesis of EM-Associated Sub/Infertility
3.1. Peritoneal Lesions and Peritoneal Fluid
3.2. Endometriomas and the Ovaries
3.3. AM and Fertility
3.4. Surgical Treatment and Assisted Reproductive Technology (ART)
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leyendecker, G.; Herbertz, M.; Kunz, G.; Mall, G. Endometriosis results from the dislocation of basal endometrium. Hum. Reprod. 2002, 17, 2725–2736. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110.43. [Google Scholar]
- Porpora, M.G.; Scaramuzzino, S.; Sangiuliano, C.; Piacenti, I.; Bonanni, V.; Piccioni, M.G.; Ostuni, R.; Masciullo, L.; Benedetti Panici, P.L. High prevalence of autoimmune diseases in women with endometriosis: A case-control study. Gynecol. Endocrinol. 2020, 36, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, H.; Weil, C.; Chodick, G.; Shalev, V.; Eisenberg, V.H. Evidence for an association between endometriosis, fibromyalgia, and autoimmune diseases. Am. J. Reprod. Immunol. 2019, 81, e13095. [Google Scholar] [CrossRef] [PubMed]
- Young, V.J.; Brown, J.K.; Saunders, P.T.; Horne, A.W. The role of the peritoneum in the pathogenesis of endometriosis. Hum. Reprod. Update 2013, 19, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Scheerer, C.; Bauer, P.; Chiantera, V.; Sehouli, J.; Kaufmann, A.; Mechsner, S. Characterization of endometriosis-associated immune cell infiltrates (EMaICI). Arch. Gynecol. Obstet. 2016, 294, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Chen, Y.H.; Chang, H.Y.; Au, H.K.; Tzeng, C.R.; Huang, Y.H. Chronic Niche Inflammation in Endometriosis-Associated Infertility: Current Understanding and Future Therapeutic Strategies. Int. J. Mol. Sci. 2018, 19, 2385. [Google Scholar] [CrossRef]
- Vercellini, P.; Crosignani, P.; Somigliana, E.; Viganò, P.; Buggio, L.; Bolis, G.; Fedele, L. The ‘incessant menstruation’ hypothesis: A mechanistic ovarian cancer model with implications for prevention. Hum. Reprod. 2011, 26, 2262–2273. [Google Scholar] [CrossRef]
- Shigesi, N.; Kvaskoff, M.; Kirtley, S.; Feng, Q.; Fang, H.; Knight, J.C.; Missmer, S.A.; Rahmioglu, N.; Zondervan, K.T.; Becker, C.M. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 486–503. [Google Scholar] [CrossRef]
- Keckstein, J.; Ulrich, U.; Kandolf, O.; Wiesinger, H.; Wustlich, M. Laparoscopic therapy of intestinal endometriosis and the ranking of drug treatment. Zentralbl. Gynakol. 2003, 125, 259–266. [Google Scholar] [PubMed]
- Leyendecker, G.; Kunz, G.; Noe, M.; Herbertz, M.; Mall, G. Endometriosis: A dysfunction and disease of the archimetra. Hum. Reprod. Update 1998, 4, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Bilgicyildirim, A.; Inacker, M.; Stalf, T.; Huppert, P.; Mall, G.; Böttcher, B.; Wildt, L. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch. Gynecol. Obstet. 2015, 291, 917–932. [Google Scholar] [CrossRef]
- Dueholm, M.; Lundorf, E.; Hansen, E.S.; Sørensen, J.S.; Ledertoug, S.; Olesen, F. Magnetic resonance imaging and transvaginal ultrasonography for the diagnosis of adenomyosis. Fertil. Steril. 2001, 76, 588–594. [Google Scholar] [CrossRef]
- Exacoustos, C.; Luciano, D.; Corbett, B.; De Felice, G.; Di Feliciantonio, M.; Luciano, A.; Zupi, E. The uterine junctional zone: A 3-dimensional ultrasound study of patients with endometriosis. Am. J. Obstet. Gynecol. 2013, 209, 248.e1–248.e7. [Google Scholar] [CrossRef] [PubMed]
- Cousins, F.L.; Gargett, C.E. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.G.; Sillem, M.; Plendl, J.; Chiantera, V.; Sehouli, J.; Mechsner, S. Myofibroblasts Are Evidence of Chronic Tissue Microtrauma at the Endometrial-Myometrial Junctional Zone in Uteri with Adenomyosis. Reprod. Sci. 2017, 24, 1410–1418. [Google Scholar] [CrossRef]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The Immunopathophysiology of Endometriosis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef]
- Barcena de Arellano, M.L.; Gericke, J.; Reichelt, U.; Okuducu, A.F.; Ebert, A.D.; Chiantera, V.; Schneider, A.; Mechsner, S. Immunohistochemical characterization of endometriosis-associated smooth muscle cells in human peritoneal endometriotic lesions. Hum. Reprod. 2011, 26, 2721–2730. [Google Scholar] [CrossRef]
- Mechsner, S.; Bartley, J.; Infanger, M.; Loddenkemper, C.; Herbel, J.; Ebert, A.D. Clinical management and immunohistochemical analysis of umbilical endometriosis. Arch. Gynecol. Obstet. 2009, 280, 235–242. [Google Scholar] [CrossRef]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial stem/progenitor cells: The first 10 years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef]
- Rei, C.; Williams, T.; Feloney, M. Endometriosis in a Man as a Rare Source of Abdominal Pain: A Case Report and Review of the Literature. Case Rep. Obstet. Gynecol. 2018, 2018, 2083121. [Google Scholar] [CrossRef]
- Ibrahim, M.G.; Sillem, M.; Plendl, J.; Taube, E.T.; Schüring, A.; Götte, M.; Chiantera, V.; Sehouli, J.; Mechsner, S. Arrangement of myofibroblastic and smooth muscle-like cells in superficial peritoneal endometriosis and a possible role of transforming growth factor beta 1 (TGFβ1) in myofibroblastic metaplasia. Arch. Gynecol. Obstet. 2019, 299, 489–499. [Google Scholar] [CrossRef]
- Abrao, M.S.; Podgaec, S.; Dias, J.A., Jr.; Averbach, M.; Garry, R.; Ferraz Silva, L.F.; Carvalho, F.M. Deeply infiltrating endometriosis affecting the rectum and lymph nodes. Fertil. Steril. 2006, 86, 543–547. [Google Scholar] [CrossRef]
- Mechsner, S.; Weichbrodt, M.; Riedlinger, W.F.; Bartley, J.; Kaufmann, A.M.; Schneider, A.; Köhler, C. Estrogen and progestogen receptor positive endometriotic lesions and disseminated cells in pelvic sentinel lymph nodes of patients with deep infiltrating rectovaginal endometriosis: A pilot study. Hum. Reprod. 2008, 23, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Delbandi, A.A.; Mahmoudi, M.; Shervin, A.; Heidari, S.; Kolahdouz-Mohammadi, R.; Zarnani, A.H. Evaluation of apoptosis and angiogenesis in ectopic and eutopic stromal cells of patients with endometriosis compared to non-endometriotic controls. BMC Womens Health 2020, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Treloar, S.A.; O’Connor, D.T.; O’Connor, V.M.; Martin, N.G. Genetic influences on endometriosis in an Australian twin sample. Fertil. Steril. 1999, 71, 701–710. [Google Scholar] [CrossRef]
- Saha, R.; Pettersson, H.J.; Svedberg, P.; Olovsson, M.; Bergqvist, A.; Marions, L.; Tornvall, P.; Kuja-Halkola, R. Heritability of endometriosis. Fertil. Steril. 2015, 104, 947–952. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Treloar, S.A.; Lin, J.; Weeks, D.E.; Nyholt, D.R.; Mangion, J.; MacKay, I.J.; Cardon, L.R.; Martin, N.G.; Kennedy, S.H. Significant evidence of one or more susceptibility loci for endometriosis with near-Mendelian inheritance on chromosome 7p13–15. Hum. Reprod. 2007, 22, 717–728. [Google Scholar] [CrossRef]
- Sapkota, Y.; Fassbender, A.; Bowdler, L.; Fung, J.N.; Peterse, D.O.D.; Montgomery, G.W.; Nyholt, D.R.; D’Hooghe, T.M. Independent Replication and Meta-Analysis for Endometriosis Risk Loci. Twin Res. Hum. Genet. 2015, 18, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.S.; Mäkinen, N.; Harris, H.R.; Rahmioglu, N.; Uimari, O.; Cook, J.P.; Shigesi, N.; Ferreira, T.; Velez-Edwards, D.R.; Edwards, T.L.; et al. Genome-wide association and epidemiological analyses reveal common genetic origins between uterine leiomyomata and endometriosis. Nat. Commun. 2019, 10, 4857. [Google Scholar] [CrossRef] [PubMed]
- Zelenko, Z.; Aghajanova, L.; Irwin, J.C.; Giudice, L.C. Nuclear receptor, coregulator signaling, and chromatin remodeling pathways suggest involvement of the epigenome in the steroid hormone response of endometrium and abnormalities in endometriosis. Reprod. Sci. 2012, 19, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Giampaolino, P.; Della Corte, L.; Foreste, V.; Barra, F.; Ferrero, S.; Bifulco, G. Dioxin and endometriosis: A new possible relation based on epigenetic theory. Gynecol. Endocrinol. 2020, 36, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Khan, Z.; Zanfagnin, V.; Correa, L.F.; Delaney, A.A.; Daftary, G.S. Epigenetic Modulation of Collagen 1A1: Therapeutic Implications in Fibrosis and Endometriosis. Biol. Reprod. 2016, 94, 87. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D.; Stangret, A.; Ruiz-Ruiz, C.; Olivares, E.G.; Soriţău, O.; Suşman, S.; Szewczyk, G. Estrogen- and Progesterone (P4)-Mediated Epigenetic Modifications of Endometrial Stromal Cells (EnSCs) and/or Mesenchymal Stem/Stromal Cells (MSCs) in the Etiopathogenesis of Endometriosis. Stem. Cell Rev. Rep. 2021. [Google Scholar] [CrossRef] [PubMed]
- Nazri, H.M.; Imran, M.; Fischer, R.; Heilig, R.; Manek, S.; Dragovic, R.A.; Kessler, B.M.; Zondervan, K.T.; Tapmeier, T.T.; Becker, C.M. Characterization of exosomes in peritoneal fluid of endometriosis patients. Fertil. Steril. 2020, 113, 364–373.e2. [Google Scholar] [CrossRef]
- De Graaff, A.A.; D’Hooghe, T.M.; Dunselman, G.A.; Dirksen, C.D.; Hummelshoj, L.; Simoens, S. The significant effect of endometriosis on physical, mental and social wellbeing: Results from an international cross-sectional survey. Hum. Reprod. 2013, 28, 2677–2685. [Google Scholar] [CrossRef]
- Greene, R.; Stratton, P.; Cleary, S.D.; Ballweg, M.L.; Sinaii, N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil. Steril. 2009, 91, 32–39. [Google Scholar] [CrossRef]
- Ballweg, M.L. Impact of endometriosis on women’s health: Comparative historical data show that the earlier the onset, the more severe the disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 201–218. [Google Scholar] [CrossRef]
- Mechsner, S. Endometriosis: An often unrecognized pain disorder. Schmerz 2016, 30, 477–490. [Google Scholar] [CrossRef]
- Nanda, A.K.T.; Banerjee, P.; Dutta, M.; Wangdi, T.; Sharma, P.; Chaudhury, K.; Jana, S.K. Cytokines, Angiogenesis, and Extracellular Matrix Degradation are Augmented by Oxidative Stress in Endometriosis. Ann. Lab. Med. 2020, 40, 390–397. [Google Scholar] [CrossRef]
- Piacenti, I.; Viscardi, M.F.; Masciullo, L.; Sangiuliano, C.; Scaramuzzino, S.; Piccioni, M.G.; Muzii, L.; Benedetti Panici, P.; Porpora, M.G. Dienogest versus continuous oral levonorgestrel/EE in patients with endometriosis: What’s the best choice? Gynecol. Endocrinol. 2021, 1–5. [Google Scholar] [CrossRef]
- Anaf, V.; Simon, P.; El Nakadi, I.; Fayt, I.; Buxant, F.; Simonart, T.; Peny, M.O.; Noel, J.C. Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules. Hum. Reprod. 2000, 15, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Barcena de Arellano, M.L.; Mechsner, S. The peritoneum—An important factor for pathogenesis and pain generation in endometriosis. J. Mol. Med. 2014, 92, 595–602. [Google Scholar] [CrossRef]
- Arnold, J.; Barcena de Arellano, M.L.; Rüster, C.; Vercellino, G.F.; Chiantera, V.; Schneider, A.; Mechsner, S. Imbalance between sympathetic and sensory innervation in peritoneal endometriosis. Brain Behav. Immun. 2012, 26, 132–141. [Google Scholar] [PubMed]
- Hoffman, D. Central and peripheral pain generators in women with chronic pelvic pain: Patient centered assessment and treatment. Curr. Rheumatol. Rev. 2015, 11, 146–166. [Google Scholar] [CrossRef] [PubMed]
- Aredo, J.V.; Heyrana, K.J.; Karp, B.I.; Shah, J.P.; Stratton, P. Relating Chronic Pelvic Pain and Endometriosis to Signs of Sensitization and Myofascial Pain and Dysfunction. Semin. Reprod. Med. 2017, 35, 88–97. [Google Scholar] [CrossRef]
- As-Sanie, S.; Harris, R.E.; Napadow, V.; Kim, J.; Neshewat, G.; Kairys, A.; Williams, D.; Clauw, D.J.; Schmidt-Wilcke, T. Changes in regional gray matter volume in women with chronic pelvic pain: A voxel-based morphometry study. Pain 2012, 153, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Ng, N.; Wahl, K.; Orr, N.L.; Noga, H.; Williams, C.; Allaire, C.; Bedaiwy, M.A.; Yong, P.J. Endometriosis and Negative Perception of the Medical Profession. J. Obstet. Gynaecol. Can. 2020, 42, 248–255. [Google Scholar] [CrossRef]
- Strathy, J.H.; Molgaard, C.A.; Coulam, C.B.; Melton, L.J., 3rd. Endometriosis and infertility: A laparoscopic study of endometriosis among fertile and infertile women. Fertil. Steril. 1982, 38, 667–672. [Google Scholar] [CrossRef]
- Ferrero, S.; Esposito, F.; Abbamonte, L.H.; Anserini, P.; Remorgida, V.; Ragni, N. Quality of sex life in women with endometriosis and deep dyspareunia. Fertil. Steril. 2005, 83, 573–579. [Google Scholar] [CrossRef]
- De Ziegler, D.; Borghese, B.; Chapron, C. Endometriosis and infertility: Pathophysiology and management. Lancet 2010, 376, 730–738. [Google Scholar] [CrossRef]
- Karande, V.C.; Pratt, D.E.; Rao, R.; Balin, M.; Gleicher, N. Elevated tubal perfusion pressures during selective salpingography are highly suggestive of tubal endometriosis. Fertil. Steril. 1995, 64, 1070–1073. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Carvalho, K.I.; Kallas, E.G.; Mechsner, S.; Baracat, E.C.; Abrão, M.S. Chemokines in the pathogenesis of endometriosis and infertility. J. Reprod. Immunol. 2013, 98, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Akande, V.A.; Hunt, L.P.; Cahill, D.J.; Jenkins, J.M. Differences in time to natural conception between women with unexplained infertility and infertile women with minor endometriosis. Hum. Reprod. 2004, 19, 96–103. [Google Scholar] [CrossRef]
- Marcoux, S.; Maheux, R.; Bérubé, S.; Endometriosis, C.C.G.O. Laparoscopic surgery in infertile women with minimal or mild endometriosis. N. Eng. J. Med. 1997, 337, 217–222. [Google Scholar] [CrossRef]
- Vercellini, P.; Somigliana, E.; Viganò, P.; Abbiati, A.; Barbara, G.; Crosignani, P.G. Surgery for endometriosis-associated infertility: A pragmatic approach. Hum. Reprod. 2009, 24, 254–269. [Google Scholar] [CrossRef]
- Halis, G.; Arici, A. Endometriosis and inflammation in infertility. Ann. N. Y. Acad. Sci. 2004, 1034, 300–315. [Google Scholar] [CrossRef]
- Xu, B.; Guo, N.; Zhang, X.M.; Shi, W.; Tong, X.H.; Iqbal, F.; Liu, Y.S. Oocyte quality is decreased in women with minimal or mild endometriosis. Sci. Rep. 2015, 5, 10779. [Google Scholar] [CrossRef]
- Pellicer, A.; Oliveira, N.; Ruiz, A.; Remohí, J.; Simón, C. Exploring the mechanism(s) of endometriosis-related infertility: An analysis of embryo development and implantation in assisted reproduction. Hum. Reprod. 1995, 10 (Suppl. S2), 91–97. [Google Scholar] [CrossRef]
- Garrido, N.; Navarro, J.; García-Velasco, J.; Remoh, J.; Pellice, A.; Simón, C. The endometrium versus embryonic quality in endometriosis-related infertility. Hum. Reprod. Update 2002, 8, 95–103. [Google Scholar] [CrossRef]
- Shebl, O.; Ebner, T.; Sommergruber, M.; Sir, A.; Tews, G. Anti muellerian hormone serum levels in women with endometriosis: A case-control study. Gynecol. Endocrinol. 2009, 25, 713–716. [Google Scholar] [CrossRef]
- Horikawa, T.; Nakagawa, K.; Ohgi, S.; Kojima, R.; Nakashima, A.; Ito, M.; Takahashi, Y.; Saito, H. The frequency of ovulation from the affected ovary decreases following laparoscopic cystectomy in infertile women with unilateral endometrioma during a natural cycle. J. Assist. Reprod. Genet. 2008, 25, 239–244. [Google Scholar] [CrossRef]
- Somigliana, E.; Ragni, G.; Benedetti, F.; Borroni, R.; Vegetti, W.; Crosignani, P.G. Does laparoscopic excision of endometriotic ovarian cysts significantly affect ovarian reserve? Insights from IVF cycles. Hum. Reprod. 2003, 18, 2450–2453. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: A committee opinion. Fertil. Steril. 2012, 98, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Garavaglia, E.; Sala, C.; Taccagni, G.; Traglia, M.; Barbieri, C.; Ferrari, S.; Candiani, M.; Panina-Bordignon, P.; Toniolo, D. Fertility Preservation in Endometriosis Patients: Anti-Müllerian Hormone Is a Reliable Marker of the Ovarian Follicle Density. Front. Surg 2017, 4, 40. [Google Scholar] [CrossRef]
- Chapron, C.; Pietin-Vialle, C.; Borghese, B.; Davy, C.; Foulot, H.; Chopin, N. Associated ovarian endometrioma is a marker for greater severity of deeply infiltrating endometriosis. Fertil. Steril. 2009, 92, 453–457. [Google Scholar] [CrossRef]
- Lafay Pillet, M.C.; Huchon, C.; Santulli, P.; Borghese, B.; Chapron, C.; Fauconnier, A. A clinical score can predict associated deep infiltrating endometriosis before surgery for an endometrioma. Hum. Reprod. 2014, 29, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W. The Pathogenesis of Adenomyosis vis-à-vis Endometriosis. J. Clin. Med. 2020, 9, 485. [Google Scholar] [CrossRef]
- Kunz, G.; Beil, D.; Huppert, P.; Noe, M.; Kissler, S.; Leyendecker, G. Adenomyosis in endometriosis–prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum. Reprod. 2005, 20, 2309–2316. [Google Scholar] [CrossRef]
- Leyendecker, G. Evolutionäre Aspekte in der Pathogenese und Pathophysiologie von Adenomyose und Endmemtriose. J. Gynäkologische Endokrinol. Österr. 2009, 29, 110–121. [Google Scholar] [CrossRef]
- Leyendecker, G.; Wildt, L.; Mall, G. The pathophysiology of endometriosis and adenomyosis: Tissue injury and repair. Arch. Gynecol. Obstet. 2009, 280, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Kissler, S.; Hamscho, N.; Zangos, S.; Wiegratz, I.; Schlichter, S.; Menzel, C.; Doebert, N.; Gruenwald, F.; Vogl, T.J.; Gaetje, R.; et al. Uterotubal transport disorder in adenomyosis and endometriosis—A cause for infertility. BJOG 2006, 113, 902–908. [Google Scholar] [CrossRef]
- Attar, E.; Tokunaga, H.; Imir, G.; Yilmaz, M.B.; Redwine, D.; Putman, M.; Gurates, B.; Attar, R.; Yaegashi, N.; Hales, D.B.; et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J. Clin. Endocrinol. Metab. 2009, 94, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Harlow, C.R.; Cahill, D.J.; Maile, L.A.; Talbot, W.M.; Mears, J.; Wardle, P.G.; Hull, M.G. Reduced preovulatory granulosa cell steroidogenesis in women with endometriosis. J. Clin. Endocrinol. Metab. 1996, 81, 426–429. [Google Scholar]
- Campo, S.; Campo, V.; Benagiano, G. Adenomyosis and infertility. Reprod. Biomed. Online 2012, 24, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Conejero, J.A.; Morgan, M.; Montesinos, M.; Fortuño, S.; Meseguer, M.; Simón, C.; Horcajadas, J.A.; Pellicer, A. Adenomyosis does not affect implantation, but is associated with miscarriage in patients undergoing oocyte donation. Fertil. Steril. 2011, 96, 943–950. [Google Scholar] [CrossRef]
- Ota, H.; Igarashi, S.; Hatazawa, J.; Tanaka, T. Is adenomyosis an immune disease? Hum. Reprod. Update 1998, 4, 360–367. [Google Scholar] [CrossRef]
- Youm, H.S.; Choi, Y.S.; Han, H.D. In vitro fertilization and embryo transfer outcomes in relation to myometrial thickness. J. Assist. Reprod. Genet. 2011, 28, 1135–1140. [Google Scholar] [CrossRef]
- Dunselman, G.A.J.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef]
- Kuznetsov, L.; Dworzynski, K.; Davies, M.; Overton, C. Diagnosis and management of endometriosis: Summary of NICE guidance. BMJ 2017, 358, j3935. [Google Scholar] [CrossRef]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Simopoulou, M.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Tsioulou, P.; Maziotis, E.; Tzanakaki, D.; Triantafyllidou, O.; Kalampokas, T.; Siristatidis, C.; et al. Getting to Know Endometriosis-Related Infertility Better: A Review on How Endometriosis Affects Oocyte Quality and Embryo Development. Biomedicines 2021, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Dückelmann, A.M.; Taube, E.; Abesadze, E.; Chiantera, V.; Sehouli, J.; Mechsner, S. When and how should peritoneal endometriosis be operated on in order to improve fertility rates and symptoms? The experience and outcomes of nearly 100 cases. Arch. Gynecol. Obstet. 2021. [Google Scholar] [CrossRef] [PubMed]
- Abesadze, E.; Sehouli, J.; Mechsner, S.; Chiantera, V. Possible Role of the Posterior Compartment Peritonectomy, as a Part of the Complex Surgery, Regarding Recurrence Rate, Improvement of Symptoms and Fertility Rate in Patients with Endometriosis, Long-Term Follow-Up. J. Minim. Invasive Gynecol. 2020, 27, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- de Ziegler, D.; Pirtea, P.; Carbonnel, M.; Poulain, M.; Cicinelli, E.; Bulletti, C.; Kostaras, K.; Kontopoulos, G.; Keefe, D.; Ayoubi, J.M. Assisted reproduction in endometriosis. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 47–59. [Google Scholar] [CrossRef]
- Nicolaus, K.; Bräuer, D.; Sczesny, R.; Jimenez-Cruz, J.; Bühler, K.; Hoppe, I.; Runnebaum, I.B. Endometriosis reduces ovarian response in controlled ovarian hyperstimulation independent of AMH, AFC, and women’s age measured by follicular output rate (FORT) and number of oocytes retrieved. Arch. Gynecol. Obstet. 2019, 300, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Mizrachi, Y.; Horowitz, E.; Farhi, J.; Raziel, A.; Weissman, A. Ovarian stimulation for freeze-all IVF cycles: A systematic review. Hum. Reprod. Update 2020, 26, 118–135. [Google Scholar] [CrossRef]
- Coccia, M.E.; Rizzello, F.; Gianfranco, S. Does controlled ovarian hyperstimulation in women with a history of endometriosis influence recurrence rate? J. Womens Health 2010, 19, 2063–2069. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruber, T.M.; Mechsner, S. Pathogenesis of Endometriosis: The Origin of Pain and Subfertility. Cells 2021, 10, 1381. https://doi.org/10.3390/cells10061381
Gruber TM, Mechsner S. Pathogenesis of Endometriosis: The Origin of Pain and Subfertility. Cells. 2021; 10(6):1381. https://doi.org/10.3390/cells10061381
Chicago/Turabian StyleGruber, Teresa Mira, and Sylvia Mechsner. 2021. "Pathogenesis of Endometriosis: The Origin of Pain and Subfertility" Cells 10, no. 6: 1381. https://doi.org/10.3390/cells10061381
APA StyleGruber, T. M., & Mechsner, S. (2021). Pathogenesis of Endometriosis: The Origin of Pain and Subfertility. Cells, 10(6), 1381. https://doi.org/10.3390/cells10061381





