Features of the Duckweed Lemna That Support Rapid Growth under Extremes of Light Intensity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.1.1. Controlled Conditions
2.1.2. Field Conditions
2.2. Growth Metrics
2.3. Photosynthesis and Respiration
2.4. Protein and Starch
2.5. Chlorophyll, Carotenoid, and α-Tocopherol Levels
2.6. Statistical Analyses
3. Results
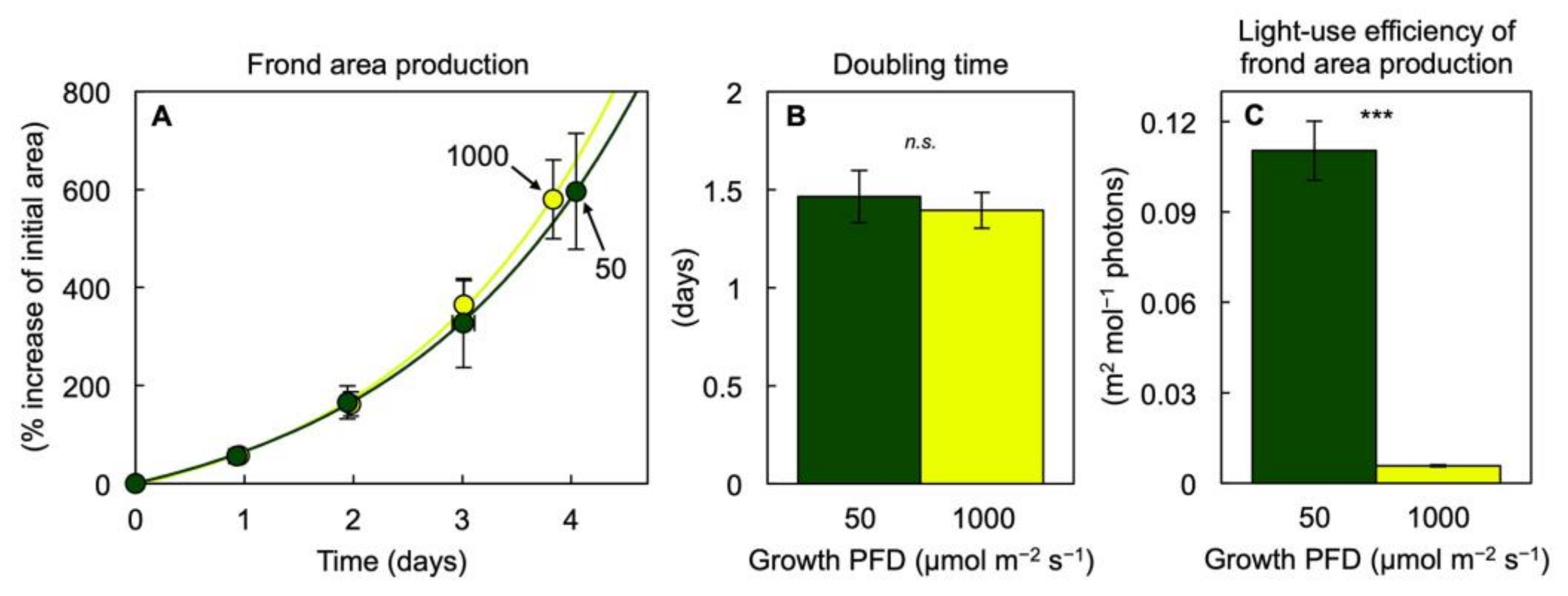
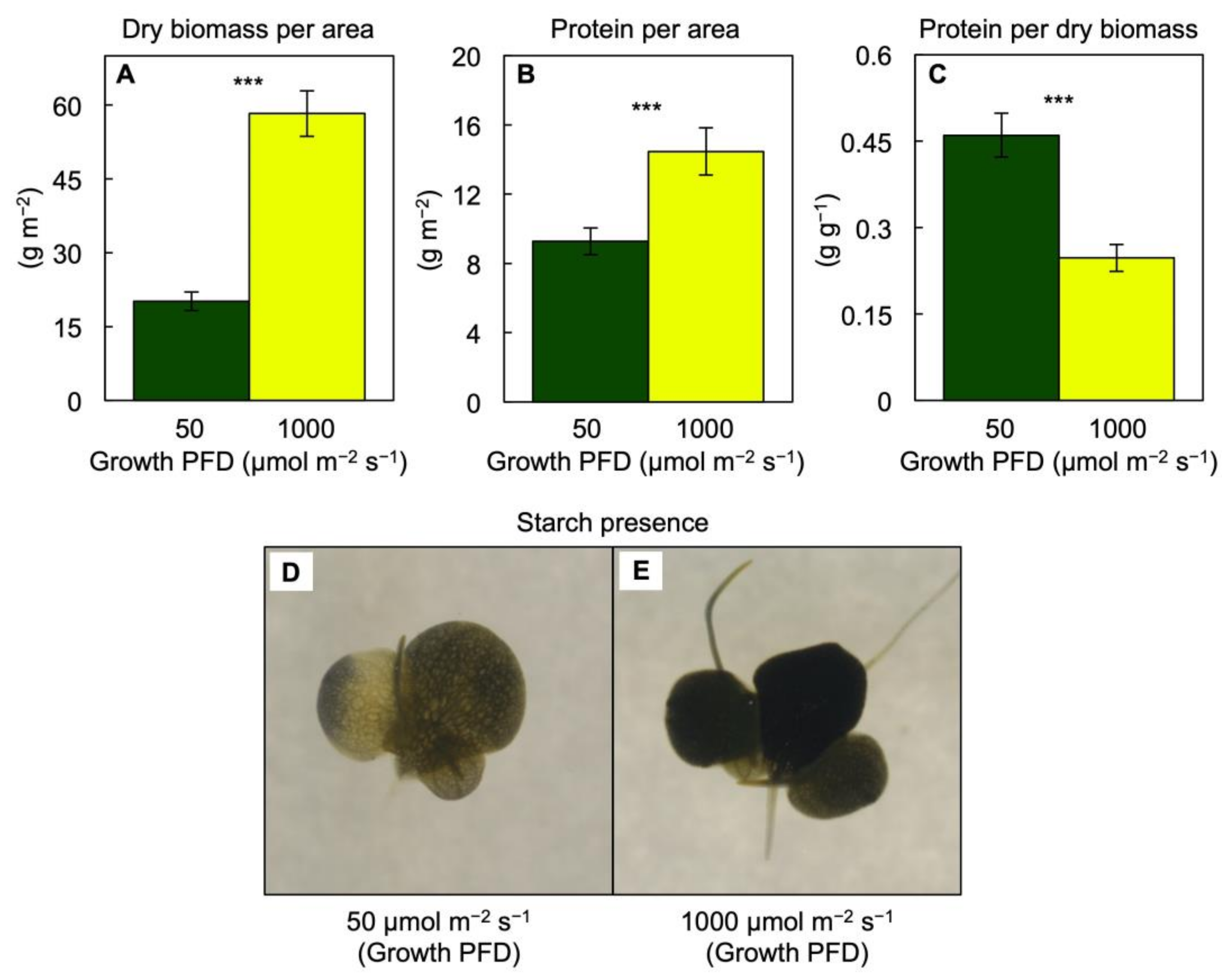
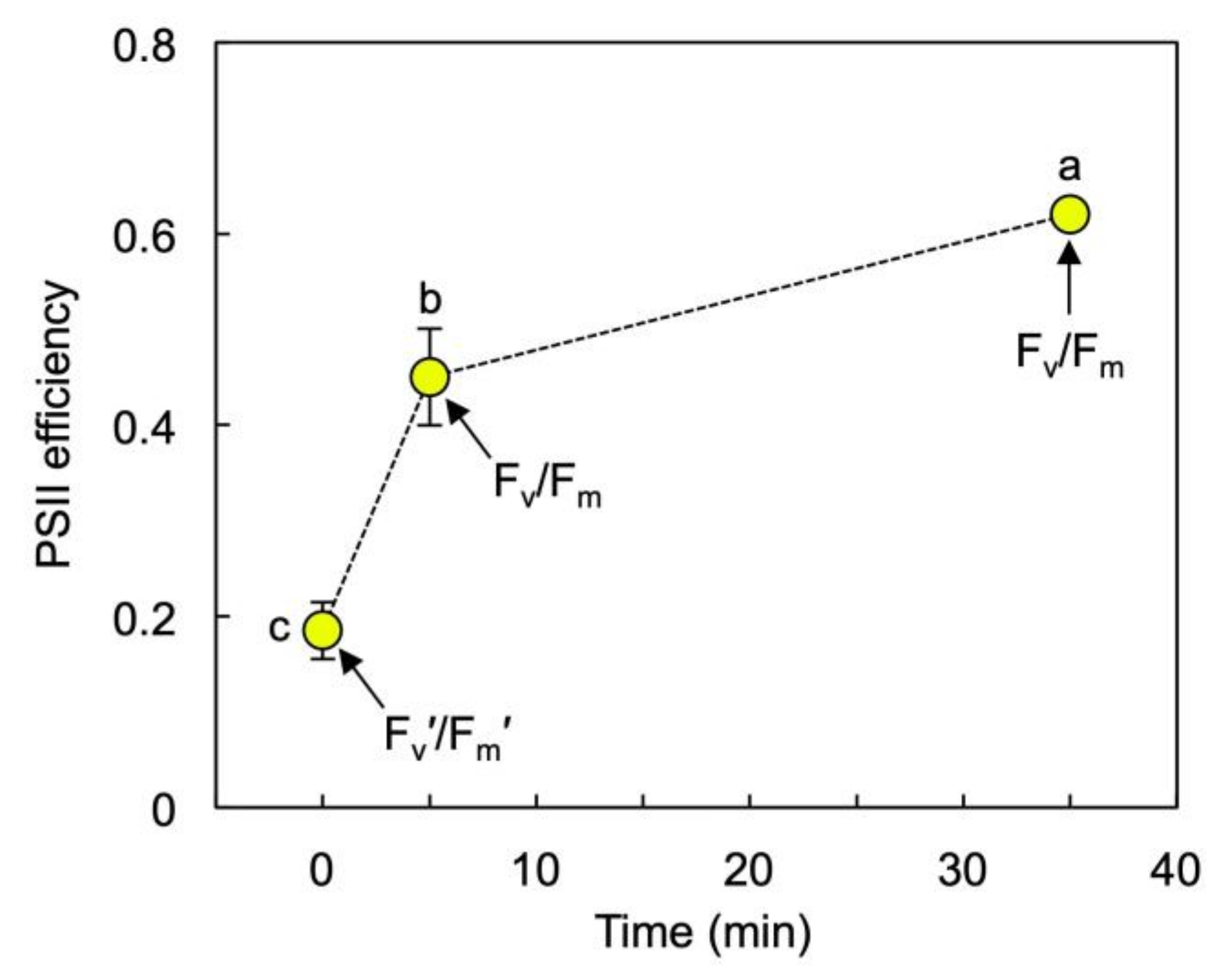
3.1. Growth and Photosynthesis of Lemna gibba under Extremes in Growth PFD
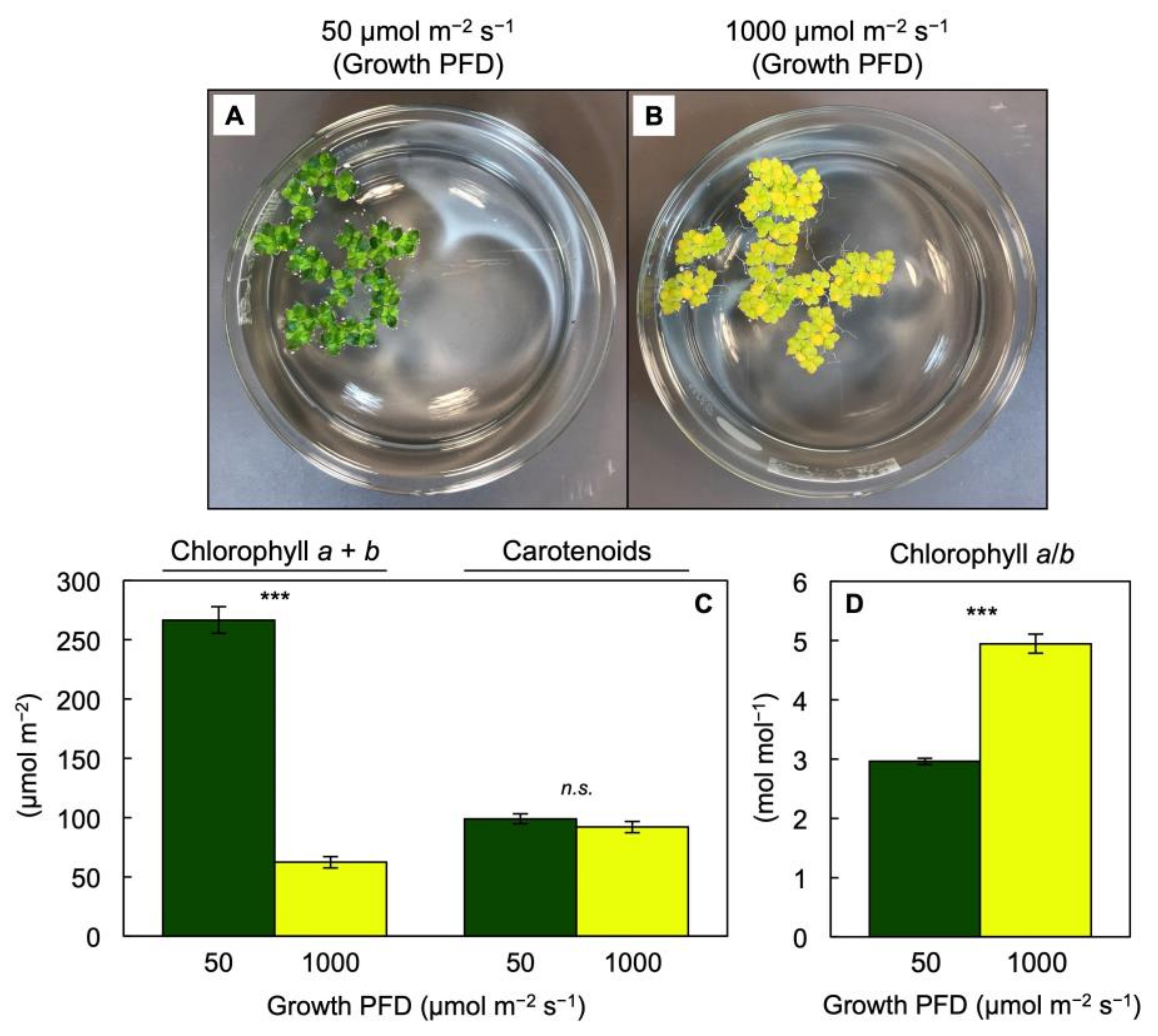
3.2. Pigment Composition, Light-Use Efficiency, and Photoprotection of Lemna gibba under Extremes in Growth PFD
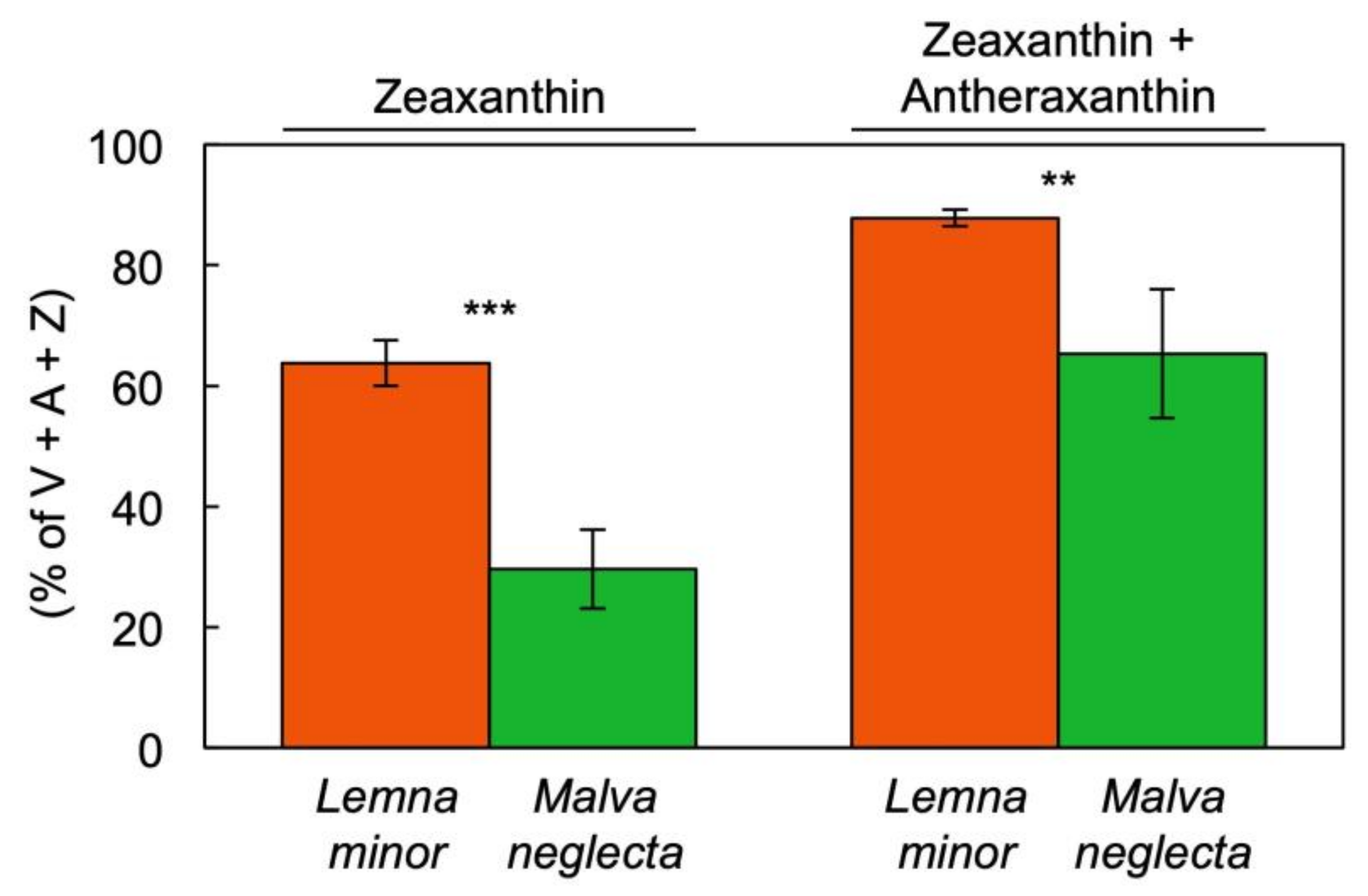
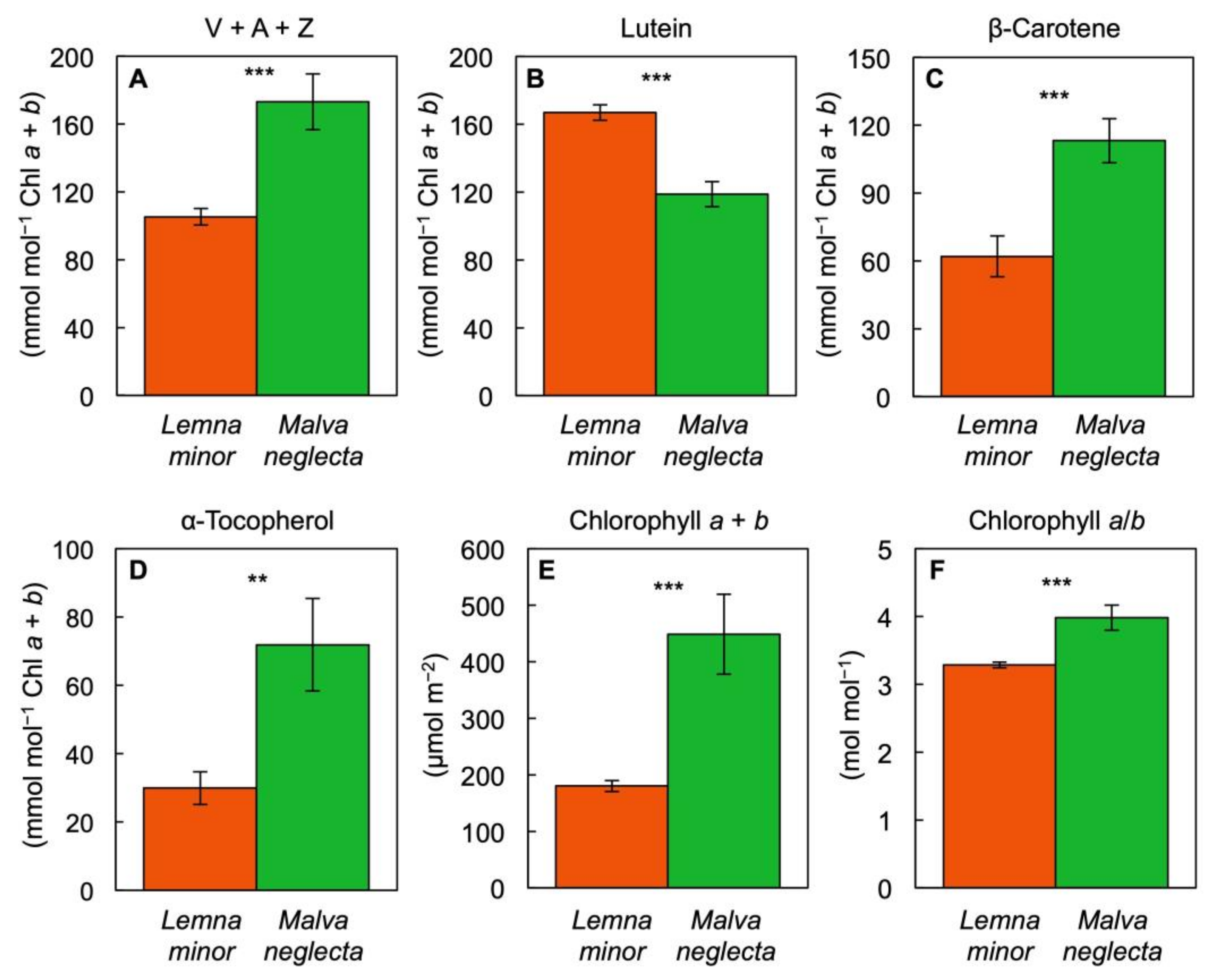
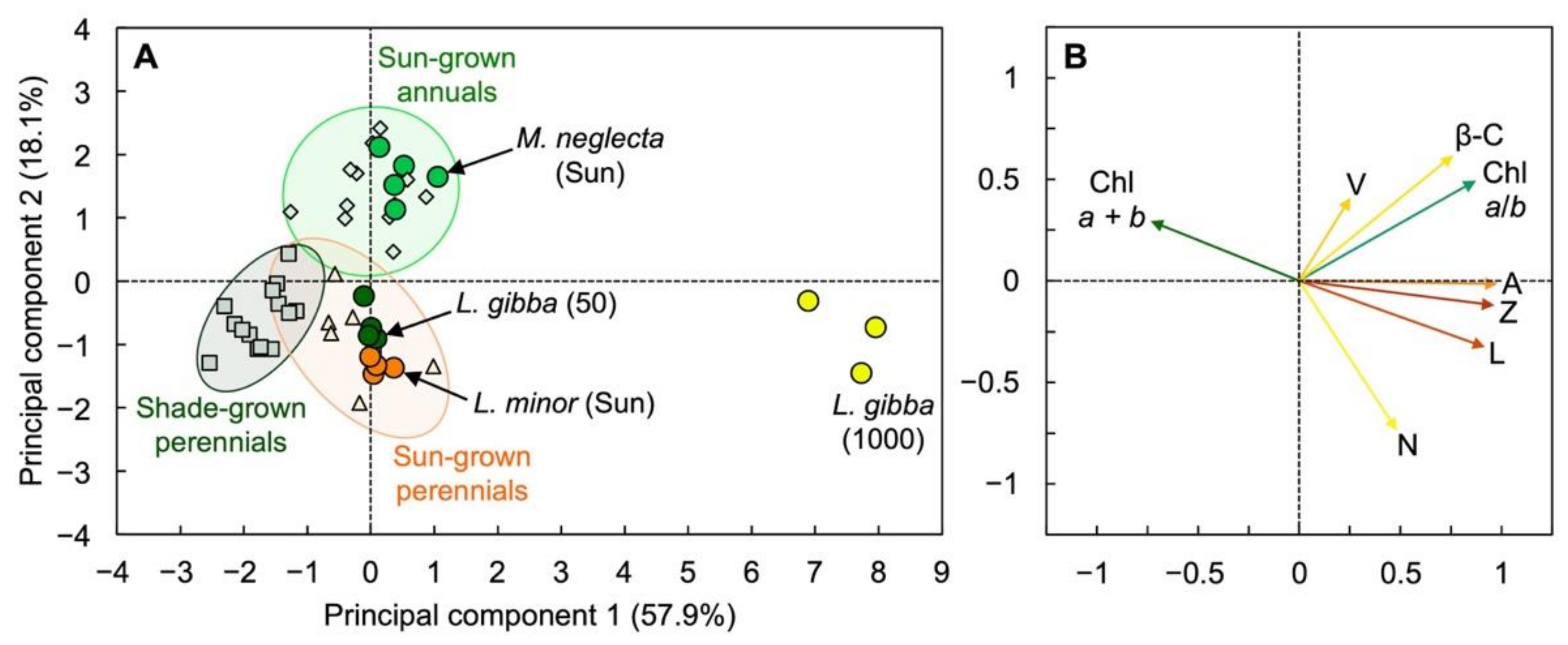
3.3. Unique Pigment Patterns in Lemna Compared to Other Species
4. Discussion
4.1. Interspecies Comparison
4.2. Specific Features That May Contribute to Duckweed’s High Shade Tolerance
4.3. Features That Likely Contribute to Duckweed’s Tolerance of High Light
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Appenroth, K.J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017, 15, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.; Adams, W.W., III; Escobar, C.M.; López-Pozo, M.; Demmig-Adams, B. Growth and essential carotenoid micronutrients in Lemna gibba as a function of growth light intensity. Front. Plant Sci. 2020, 11, 480. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W., III. Zeaxanthin, a molecule for photoprotection in many different environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; López-Pozo, M.; Stewart, J.J.; Adams, W.W., III. Zeaxanthin and lutein: Photoprotectors, anti-inflammatories, and brain food. Molecules 2020, 25, 3607. [Google Scholar] [CrossRef]
- Xu, J.; Shen, G. Growing duckweed in swine wastewater for nutrient recovery and biomass production. Bioresour. Technol. 2011, 102, 848–853. [Google Scholar] [CrossRef]
- Mohedano, R.A.; Costa, R.H.R.; Tavares, F.A.; Filho, P.B. High nutrient removal rate from swine wastes and protein biomass production by full-scale duckweed ponds. Bioresour. Technol. 2012, 112, 98–104. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Yu, C.; Zhou, G. Multifaceted roles of duckweed in aquatic phytoremediation and bioproducts synthesis. GCB Bioenergy 2021, 13, 70–82. [Google Scholar] [CrossRef]
- Sun, H.; A, D.; Feng, Y.; Vithanage, M.; Mandal, S.; Shaheen, S.; Rinklebe, J.; Shi, W.; Wang, H. Floating duckweed mitigated ammonia volatilization and increased grain yield and nitrogen use efficiency of rice in biochar amended paddy soil. Chemosphere 2019, 237, 124532. [Google Scholar] [CrossRef]
- Demmig, B.; Winter, K.; Krüger, A.; Czygan, F.-C. Photoinhibition and zeaxanthin formation in intact leaves. A possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987, 84, 218–224. [Google Scholar] [CrossRef]
- Park, S.; Steen, C.J.; Lyska, D.; Fischer, A.L.; Endelman, B.; Iwai, M.; Niyogi, K.K.; Fleming, G.R. Chlorophyll–carotenoid excitation energy transfer and charge transfer in Nannochloropsis oceanica for the regulation of photosynthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 3385–3390. [Google Scholar] [CrossRef]
- Adams, W.W., III; Demmig-Adams, B. Operation of the xanthophyll cycle in higher plants in response to diurnal changes in incident sunlight. Planta 1992, 186, 390–398. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Muller, O.; Stewart, J.J.; Cohu, C.M.; Polutchko, S.K.; Demmig-Adams, B.; Adams, W.W., III. Leaf architectural, vascular and photosynthetic acclimation to temperature in two biennials. Physiol. Plant. 2014, 152, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Delieu, T.; Walker, D.A. Polarographic measurement of photosynthetic oxygen evolution by leaf discs. New Phytol. 1981, 89, 165–178. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Logan, B.A.; Adams, W.W., III; Demmig-Adams, B. Avoiding common pitfalls of chlorophyll fluorescence analysis in the field. Funct. Plant Biol. 2007, 34, 853–859. [Google Scholar] [CrossRef]
- Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Stewart, J.J.; Adams, W.W., III; Cohu, C.M.; Polutchko, S.K.; Lombardi, E.M.; Demmig-Adams, B. Differences in light-harvesting, acclimation to growth-light environment, and leaf structural development between Swedish and Italian ecotypes of Arabidopsis thaliana. Planta 2015, 242, 1277–1290. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Carotenoid composition in sun and shade leaves of plants with different life forms. Plant Cell Environ. 1992, 15, 411–419. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Cohu, C.M.; Muller, O.; Adams, W.W., III. Modulation of photosynthetic energy conversion efficiency in nature: From seconds to seasons. Photosynth. Res. 2012, 113, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, P.; Adelmann, K.; Zimmer, S.; Schmidt, C.; Appenroth, K.J. Relative in vitro growth rates of duckweeds (Lemnaceae)—The most rapidly growing higher plants. Plant Biol. 2015, 17, 33–41. [Google Scholar] [CrossRef]
- Bog, M.; Appenroth, K.-J.; Sree, K.S. Duckweed (Lemnaceae): Its molecular taxonomy. Front. Sustain. Food Syst. 2019, 3, 117. [Google Scholar] [CrossRef]
- Tippery, N.P.; Les, D.H. Tiny plants with enormous potential: Phylogeny and evolution of duckweeds. In The Duckweed Genomes. Compendium of Plant Genomes; Cao, X., Fourounjian, P., Wang, W., Eds.; Springer: Cham, Switzerland, 2020; pp. 19–38. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Capacity for energy dissipation in the pigment bed in leaves with different xanthophyll cycle pools. Aust. J. Plant Physiol. 1994, 21, 575–588. [Google Scholar] [CrossRef]
- Logan, B.A.; Barker, D.H.; Adams, W.W., III; Demmig-Adams, B. The response of xanthophyll cycle-dependent energy dissipation in Alocasia brisbanensis to sunflecks in a subtropical rainforest. Aust. J. Plant Physiol. 1997, 24, 27–33. [Google Scholar] [CrossRef]
- Skillman, J.B.; Garcia, M.; Virgo, A.; Winter, K. Growth irradiance effects on photosynthesis and growth in two co-occurring shade-tolerant neotropical perennials of contrasting photosynthetic pathways. Am. J. Bot. 2005, 92, 1811–1819. [Google Scholar] [CrossRef]
- Tan, C.L.; Wong, N.H.; Tan, P.Y.; Wee, L.Y. Growth light provision for indoor greenery: A case study. Energy Build. 2017, 144, 207–217. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Ebbert, V.; Mellman, D.L.; Mueh, K.E.; Schaffer, L.; Funk, C.; Zarter, C.R.; Adamska, I.; Jansson, S.; Adams, W.W., III. Modulation of PsbS and flexible vs sustained energy dissipation by light environment in different species. Physiol. Plant. 2006, 127, 670–680. [Google Scholar] [CrossRef]
- Ray, T.S. Foraging behaviour in tropical herbaceous climbers (Araceae). J. Ecol. 1992, 80, 189–203. [Google Scholar] [CrossRef]
- Strong, D.R., Jr.; Ray, T.S., Jr. Host tree location behavior of a tropical vine (Monstera gigantea) by skototropism. Science 1975, 190, 804–806. [Google Scholar] [CrossRef]
- Mantovani, A.; Brito, C.; Mantuano, D. Does the same morphology mean the same physiology? Morphophysiological adjustments of Philodendron hederaceum (Jacq.) Schott, an isomorphic aroid, to groudcanopy transition. Theor. Exp. Plant Physiol. 2018, 30, 89–101. [Google Scholar] [CrossRef]
- Wang, W.; Haberer, G.; Gundlach, H.; Gläβer, C.; Nussbaumer, T.; Luo, M.C.; Lomsadze, A.; Borodovsky, M.; Kerstetter, R.A.; Shanklin, J.; et al. The Spirodela polyrhiza genome reveals insights into its neotenous reduction fast growth and aquatic lifestyle. Nat. Commun. 2013, 5, 3311. [Google Scholar] [CrossRef] [PubMed]
- Michael, T.P.; Ernst, E.; Hartwick, N.; Chu, P.; Bryant, D.; Gilbert, S.; Ortleb, S.; Baggs, E.L.; Sree, K.S.; Appenroth, K.J.; et al. Genome and time-of-day transcriptome of Wolffia australiana link morphological minimization with gene loss and less growth control. Genome Res. 2021, 31, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, T.C. Plant responses to water stress. Annu. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Paul, M.J.; Driscoll, S.P. Sugar repression of photosynthesis: The role of carbohydrates in signalling nitrogen deficiency through source: Sink imbalance. Plant Cell Environ. 1997, 20, 110–116. [Google Scholar] [CrossRef]
- Logan, B.A.; Demmig-Adams, B.; Rosenstiel, T.N.; Adams, W.W., III. Effect of nitrogen limitation on foliar antioxidants in relationship to other metabolic characteristics. Planta 1999, 209, 213–220. [Google Scholar] [CrossRef]
- Cedergreen, N.; Madsen, T.V. Nitrogen uptake by the floating macrophyte Lemna minor. New Phytol. 2002, 155, 285–292. [Google Scholar] [CrossRef]
- Yamamoto, H.Y.; Bassi, R. Carotenoids: Localization and function. In Oxygenic Photosynthesis: The Light Reactions. Advances in Photosynthesis and Respiration; Ort, D.R., Yocum, C.F., Heichel, I.F., Eds.; Springer: Dordrecht, The Netherlands, 1996; Volume 4, pp. 539–563. [Google Scholar] [CrossRef]
- Adams, W.W., III; Demmig-Adams, B.; Logan, B.A.; Barker, D.H.; Osmond, C.B. Rapid changes in xanthophyll cycle-dependent energy dissipation and photosystem II efficiency in two vines, Stephania japonica and Smilax australis, growing in the understory of an open Eucalyptus forest. Plant Cell Environ. 1999, 22, 125–136. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Muller, O.; Stewart, J.J.; Cohu, C.M.; Adams, W.W., III. Chloroplast thylakoid structure in evergreen leaves employing strong thermal energy dissipation. J. Photochem. Photobiol. B 2015, 152, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Moeller, D.L.; Logan, B.A.; Adams, W.W., III. Positive correlation between levels of retained zeaxanthin plus antheraxanthin and degree of photoinhibition in shade leaves of Schefflera arboricola (Hayata) Merrill. Planta 1998, 205, 367–374. [Google Scholar] [CrossRef]
- Martindale, W.; Bowes, G. The effects of irradiance and CO2 on the activity and activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in the aquatic plant Spirodela polyrhiza. J. Exp. Bot. 1996, 47, 781–784. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III; Ebbert, V.; Logan, B.A. Ecophysiology of the xanthophyll cycle. In The Photochemistry of Carotenoids. Advances in Photosynthesis and Respiration; Frank, H.A., Young, A.J., Britton, G., Cogdell, R.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; Volume 8, pp. 245–269. [Google Scholar] [CrossRef]
- Havaux, M.; García-Plazaola, J.I. Beyond non-photochemical fluorescence quenching: The overlapping antioxidant functions of zeaxanthin and tocopherols. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, G.H., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 583–603. [Google Scholar] [CrossRef]
- Huang, M.; Shan, S.; Zhou, X.; Chen, J.; Cao, F.; Jiang, L.; Zou, Y. Leaf photosynthetic performance related to higher radiation use efficiency and grain yield in hybrid rice. Field Crops Res. 2016, 193, 87–93. [Google Scholar] [CrossRef]
- Garbulsky, M.F.; Peñuelas, J.; Gamon, J.; Inoue, Y.; Filella, I. The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies a review and meta-analysis. Remote Sens. Environ. 2011, 115, 281–297. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; van der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef] [PubMed]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.W., III; Demmig-Adams, B.; Winter, K.; Schreiber, U. The ratio of variable to maximum chlorophyll fluorescence from photosystem II, measured in leaves at ambient temperature and at 77 K, as an indicator of the photon yield of photosynthesis. Planta 1990, 180, 166–174. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Sipka, G.; Magyar, M.; Mezzetti, A.; Akhtar, P.; Zhu, Q.; Xiao, Y.; Han, G.; Santabarbara, S.; Shen, J.-R.; Lambrev, P.H.; et al. Light-adapted charge-separated state of photosystem II: Structural and functional dynamics of the closed reaction center. Plant Cell 2021, 33, 1286–1302. [Google Scholar] [CrossRef]
- Walker, B.J.; Kramer, D.M.; Fisher, N.; Fu, X. Flexibility in the energy balancing network of photosynthesis enables safe operation under changing environmental conditions. Plants 2020, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.A.; Demmig-Adams, B.; Adams, W.W., III. Acclimation of photosynthesis to the environment. In Concepts in Plant Photobiology: Photosynthesis and Photomorphogenesis; Singhal, G.S., Renger, G., Sopory, S.K., Irrgang, K.-D., Govindjee, G.H., Eds.; Narosa Publishing House: New Dehli, India, 1999; pp. 477–512. [Google Scholar] [CrossRef]
- Logan, B.A.; Demmig-Adams, B.; Adams, W.W., III; Bilger, W. Context, quantification, and measurement guide for non-photochemical quenching of chlorophyll fluorescence. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, G.H., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 187–201. [Google Scholar] [CrossRef]
- Treves, H.; Raanan, H.; Kedem, I.; Murik, O.; Keren, N.; Zer, H.; Berkowicz, S.M.; Giordano, M.; Norici, A.; Shotland, Y.; et al. The mechanisms whereby the green alga Chlorella ohadii, isolated from desert soil crust, exhibits unparalleled photodamage resistance. New Phytol. 2016, 210, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Dall’Osto, L.; Lico, C.; Alric, J.; Giuliano, G.; Havaux, M.; Bassi, R. Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol. 2006, 6, 32. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Li, Z.; Niyogi, K.K.; Bassi, R.; Dall’Osto, L. The Arabidopsis szl1 mutant reveals a critical role of β-carotene in photosystem I photoprotection. Plant Physiol. 2012, 159, 1745–1758. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Cohu, C.M.; Lombardi, E.; Adams, W.W., III; Demmig-Adams, B. Increased nutritional quality of plants for long-duration space missions through choice of plant variety and manipulation of growth conditions. Acta Astronaut. 2014, 94, 799–806. [Google Scholar] [CrossRef]



| Units | Compound(s) | Growth PFD (µmol m−2 s−1) | p | |
|---|---|---|---|---|
| 50 | 1000 | |||
| (µmol m−2) | Zeaxanthin | 1 ± 0 | 35 ± 2 | *** |
| Antheraxanthin | 1 ± 0 | 15 ± 1 | *** | |
| Violaxanthin | 22 ± 1 | 3 ± 0 | *** | |
| Lutein | 43 ± 2 | 26 ± 2 | *** | |
| Neoxanthin | 13 ± 0 | 3 ± 0 | *** | |
| β-Carotene | 19 ± 3 | 9 ± 1 | ** | |
| Z + A | 2 ± 1 | 50 ± 3 | *** | |
| V + A + Z | 24 ± 1 | 54 ± 3 | *** | |
| α-Tocopherol | 5 ± 1 | 5 ± 1 | n.s. | |
| (mmol g−1 DM) | Zeaxanthin | 30 ± 12 | 843 ± 60 | *** |
| Antheraxanthin | 57 ± 26 | 362 ± 23 | *** | |
| Violaxanthin | 1156 ± 56 | 77 ± 11 | *** | |
| Lutein | 2214 ± 124 | 627 ± 37 | *** | |
| Neoxanthin | 692 ± 20 | 78 ± 10 | *** | |
| β-Carotene | 995 ± 157 | 212 ± 13 | *** | |
| Z + A | 88 ± 38 | 1205 ± 64 | *** | |
| V + A + Z | 1255 ± 55 | 1282 ± 62 | n.s. | |
| Carotenoids | 5192 ± 220 | 2198 ± 113 | *** | |
| α-Tocopherol | 241 ± 38 | 113 ± 18 | ** | |
| Units | Compound(s) | Growth PFD (µmol m−2 s−1) | p | |
|---|---|---|---|---|
| 50 | 1000 | |||
| (mmol mol−1 Chl a + b) | Lutein | 160 ± 8 | 421 ± 25 | *** |
| Neoxanthin | 50 ± 2 | 52 ± 3 | n.s. | |
| β-Carotene | 72 ± 11 | 142 ± 5 | *** | |
| Z + A | 6 ± 3 | 811 ± 57 | ** | |
| V + A + Z | 90 ± 5 | 862 ± 52 | *** | |
| Carotenoids | 371 ± 14 | 1478 ± 75 | ** | |
| α-Tocopherol | 17 ± 3 | 73 ± 12 | ** | |
| (mmol mol−1 Chl b) | Zeaxanthin | 9 ± 4 | 3372 ± 301 | ** |
| Antheraxanthin | 17 ± 8 | 1445 ± 61 | *** | |
| Violaxanthin | 330 ± 14 | 307 ± 44 | n.s. | |
| Lutein | 632 ± 24 | 2504 ± 125 | *** | |
| Neoxanthin | 198 ± 10 | 308 ± 12 | *** | |
| β-Carotene | 284 ± 47 | 847 ± 52 | *** | |
| Z + A | 25 ± 12 | 4818 ± 306 | ** | |
| V + A + Z | 356 ± 20 | 5125 ± 289 | *** | |
| Carotenoids | 1470 ± 61 | 8784 ± 416 | *** | |
| α-Tocopherol | 69 ± 11 | 446 ± 72 | *** | |
| (mol mol−1) | Xanthophylls/β-Carotene | 4.25 ± 0.71 | 9.39 ± 0.67 | *** |
| Zeaxanthin/Lutein | 0.01 ± 0.01 | 1.35 ± 0.05 | *** | |
| (V + A + Z)/Lutein | 0.56 ± 0.03 | 2.05 ± 0.02 | *** | |
| (% of V + A + Z) | Zeaxanthin | 2 ± 1 | 66 ± 3 | *** |
| Z + A | 7 ± 3 | 94 ± 1 | *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, J.J.; Adams, W.W., III; López-Pozo, M.; Doherty Garcia, N.; McNamara, M.; Escobar, C.M.; Demmig-Adams, B. Features of the Duckweed Lemna That Support Rapid Growth under Extremes of Light Intensity. Cells 2021, 10, 1481. https://doi.org/10.3390/cells10061481
Stewart JJ, Adams WW III, López-Pozo M, Doherty Garcia N, McNamara M, Escobar CM, Demmig-Adams B. Features of the Duckweed Lemna That Support Rapid Growth under Extremes of Light Intensity. Cells. 2021; 10(6):1481. https://doi.org/10.3390/cells10061481
Chicago/Turabian StyleStewart, Jared J., William W. Adams, III, Marina López-Pozo, Naiara Doherty Garcia, Maureen McNamara, Christine M. Escobar, and Barbara Demmig-Adams. 2021. "Features of the Duckweed Lemna That Support Rapid Growth under Extremes of Light Intensity" Cells 10, no. 6: 1481. https://doi.org/10.3390/cells10061481
APA StyleStewart, J. J., Adams, W. W., III, López-Pozo, M., Doherty Garcia, N., McNamara, M., Escobar, C. M., & Demmig-Adams, B. (2021). Features of the Duckweed Lemna That Support Rapid Growth under Extremes of Light Intensity. Cells, 10(6), 1481. https://doi.org/10.3390/cells10061481











