Transcriptomic Profiling of Femoral Veins in Deep Vein Thrombosis in a Porcine Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Procedures in the Experimental and Control Animals
2.2. RNA Extraction, Library Construction and Sequencing
2.3. Transcriptome Assembly and Identification of Novel Transcripts
2.4. Real-Time PCR
3. Results
3.1. Transcriptomic Signatures of DVT
3.2. Transcript Assembly, Quantification and DE-TARs Analysis
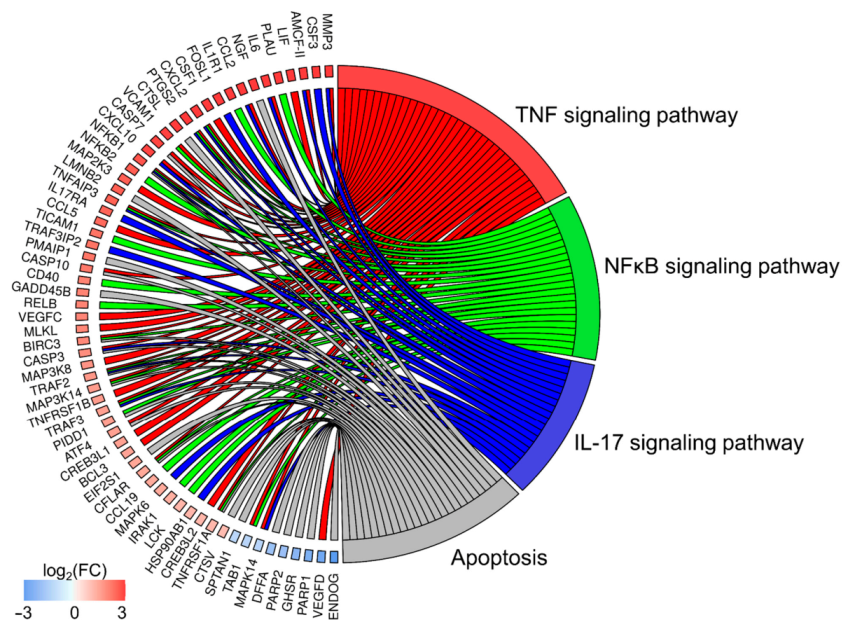
3.3. KEGGs Signaling Pathways Analysis
3.3.1. TNF Signaling Pathway
3.3.2. NF-κB Signaling Pathway
3.3.3. Apoptosis Signaling Pathway
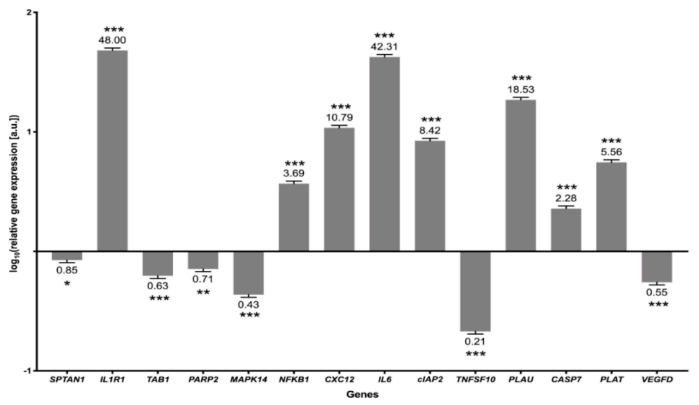
3.4. Validation of the Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VTE | venous thromboembolism |
| DVT | deep vein thrombosis |
| PE | pulmonary embolism |
| DEGs | differentially expressed genes |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| RNA-Seq | RNA Sequencing |
| CTPA | computed tomography pulmonary angiography |
| CTR | control |
| TARs | transcriptionally active regions |
| DE-TAR | differentially expressed TARs |
| BP | biological processes |
| MF | molecular functions |
| CC | cellular components |
| TNFR1 (TNFRSF1A) | TNF Receptor Superfamily Member 1A |
| TNFR2 (TNFRSF1B) | TNF Receptor Superfamily Member 1B |
| TRAF2 | TNF receptor associated factor 2 |
| TRAF3 | TNF receptor associated factor 3 |
| cIAP2 (BIRC3) | baculoviral IAP repeat-containing protein 2 |
| TAK1 (MAP3K7) | mitogen-activated protein kinase kinase kinase 7 |
| TAB1 (MAP3K7IP1) | TGF-beta activated kinase 1 (MAP3K7) binding protein 1 |
| NF-κB1(p50) | nuclear factor kappa B subunit 1 |
| NF-κB2 (p100; p52) | nuclear factor kappa B subunit 2 |
| MAPK14 (p38) | mitogen-activated protein kinase 14 |
| MKK3 (MAP2K3) | mitogen-activated protein kinase kinase 3 |
| CFLAR | CASP8 and FADD like apoptosis regulator |
| CASP | caspase; CCL2: chemokine (C-C motif) ligand 2 |
| CCL5 | C-C motif chemokine ligand 5 |
| CCL19 | C-C motif chemokine ligand 19 |
| CXCL2 | chemokine (C-X-C motif) ligand 2 |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| IL6 | interleukin 6 |
| LIF | interleukin 6 family cytokine |
| VEGFB | vascular endothelial growth factor B |
| VEGFD | vascular endothelial growth factor D |
| VEGFC | vascular endothelial growth factor C |
| IL-1β | interleukin 1 Beta |
| IL1R1 | interleukin 1 receptor type 1 |
| TNF | tumor necrosis factor |
| TNFα | tumor necrosis factor-Alpha |
| TNFAIP3 | TNF alpha induced protein 3 |
| CD40 | CD40 molecule |
| NIK (MAP3K14) | mitogen-activated protein kinase kinase kinase 14 |
| RELB | RELB proto-oncogene, NF-kB subunit |
| RELA | RELA proto-oncogene, NF-kB subunit |
| BCL3 | BCL3 transcription coactivator |
| PLAU | plasminogen activator, urokinase |
| PLAT | plasminogen activator, tissue type |
| COX2 (PTGS2) | prostaglandin-endoperoxide synthase 2 |
| VCAM1 | vascular cell adhesion molecule 1 |
| PIDD | p53-induced death domain protein 1 |
| PMAIP1 (NOXA) | phorbol-12-myristate-13-acetate-induced protein 1 |
| CTSL | cathepsin L |
| CTSV | cathepsin V |
| SPTAN1 | spectrin alpha, non-erythrocytic 1 |
| PARP1 | poly(ADP-ribose) polymerase 1 |
| PARP2 | poly(ADP-ribose) polymerase 2 |
| DFFA | DNA fragmentation factor subunit alpha |
| TNFSF10 (TRAIL) | TNF superfamily member 10 |
| TF | tissue factor |
| PAI-1 (Serpine 1) | plasminogen activator inhibitor-1 |
| TFPI | tissue factor pathway inhibitor |
| SELP | selectin P |
| IL-17A | interleukin 17A |
| IL17RA | interleukin 17 receptor A |
References
- Lopes, L.C.; Eikelboom, J.; Spencer, F.A.; Akl, E.A.; Kearon, C.; Neumann, I.; Schulman, S.; Bhatnagar, N.; Guyatt, G. Shorter or longer anticoagulation to prevent recurrent venous thromboembolism: Systematic review and meta-analysis. BMJ Open 2014, 4, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldhaber, S.Z.; Morrison, R.B. Pulmonary Embolism and Deep Vein Thrombosis. Circulation 2002, 106, 1436–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesieme, E.; Kesieme, C.; Jebbin, N.; Irekpita, E.; Dongo, A. Deep vein thrombosis: A clinical review. J. Blood Med. 2011, 2, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillippe, H.M. Overview of venous thromboembolism. Am. J. Manag. Care 2017, 23, S376–S382. [Google Scholar] [PubMed]
- Moheimani, F.; Jackson, D.E. Venous Thromboembolism: Classification, Risk Factors, Diagnosis, and Management. ISRN Hematol. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, S.R.; Comerota, A.J.; Cushman, M.; Evans, N.S.; Ginsberg, J.S.; Goldenberg, N.A.; Gupta, D.K.; Prandoni, P.; Vedantham, S.; Walsh, M.E.; et al. The postthrombotic syndrome: Evidence-based prevention, diagnosis, and treatment strategies: A scientific statement from the American heart association. Circulation 2014, 130, 1636–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.R.; Hanlin, E.R.; Glurich, I.; Mazza, J.J.; Yale, S.H. Virchow’s contribution to the understanding of thrombosis and cellular biology. Clin. Med. Res. 2010, 8, 168–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, J.; Hangge, P.; Albadawi, H.; Wallace, A.; Shamoun, F.; Knuttien, M.G.; Naidu, S.; Oklu, R. Deep vein thrombosis: Pathogenesis, diagnosis, and medical management. Cardiovasc. Diagn. Ther. 2017, 7, S276–S284. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.; Nikolopoulos, G.; Bagos, P.; Rapti, E.; Mantzios, G.; Kapsimali, V.; Travlou, A. Association between the plasminogen activator inhibitor-1 4G/5G polymorphism and venous thrombosis. Thromb. Haemost. 2007, 97, 907–913. [Google Scholar] [CrossRef]
- Budnik, I.; Brill, A. Immune Factors in Deep Vein Thrombosis Initiation. Trends Immunol. 2018, 39, 610–623. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Wang, Y.; Yang, Z.; Luo, J. Rivaroxaban attenuates thrombosis by targeting the NF-κB signaling pathway in a rat model of deep venous thrombus. Int. J. Mol. Med. 2017, 40, 1869–1880. [Google Scholar] [CrossRef] [Green Version]
- Byron, S.A.; Van Keuren-Jensen, K.R.; Engelthaler, D.M.; Carpten, J.D.; Craig, D.W. Translating RNA sequencing into clinical diagnostics: Opportunities and challenges. Nat. Rev. Genet. 2016, 17, 257–271. [Google Scholar] [CrossRef]
- Ming, Z.; Ding, W.; Yuan, R.; Jin, J.; Li, X. Differential co-expression analysis of venous thromboembolism based on gene expression profile data. Exp. Ther. Med. 2016, 11, 2193–2200. [Google Scholar] [CrossRef] [Green Version]
- Diaz, J.A.; Obi, A.T.; Myers, D.D.; Wrobleski, S.K.; Henke, P.K.; Mackman, N.; Wakefield, T.W. Critical Review of Mouse Models of Venous Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 556–562. [Google Scholar] [CrossRef] [Green Version]
- Albadawi, H.; Witting, A.A.; Pershad, Y.; Wallace, A.; Fleck, A.R.; Hoang, P.; Khademhosseini, A.; Oklu, R. Animal models of venous thrombosis. Cardiovasc. Diagn. Ther. 2017, 7, S197–S206. [Google Scholar] [CrossRef]
- Gromadziński, L.; Skowrońska, A.; Holak, P.; Smoliński, M.; Lepiarczyk, E.; Żurada, A.; Majewski, M.K.; Skowroński, M.T.; Majewska, M. A New Experimental Porcine Model of Venous Thromboembolism. J. Clin. Med. 2021, 10, 1862. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, X.; Xie, S.; Zhou, R. Transcriptome analysis of Klf15-mediated inhibitory functions in a mouse deep venous thrombosis model. Int. J. Mol. Med. 2020, 45, 1735–1752. [Google Scholar] [CrossRef] [Green Version]
- Siller-Matula, J.M.; Plasenzotti, R.; Spiel, A.; Quehenberger, P.; Jilma, B. Interspecies differences in coagulation profile. Thromb. Haemost. 2008, 100, 397–404. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Babraham Bioinform. 2010. Available online: www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 22 June 2021).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 22 June 2021).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Frazee, A.; Pertea, G.; Jaffe, A.; Langmead, B.; Salzberg, S.; Leek, J. Flexible analysis of transcriptome assemblies with Ballgown. bioRxiv 2014, 003665. [Google Scholar] [CrossRef] [Green Version]
- Reimand, J.; Arak, T.; Adler, P.; Kolberg, L.; Reisberg, S.; Peterson, H.; Vilo, J. g:Profiler—A web server for functional interpretation of gene lists. Nucleic Acids Res. 2016, 44, W83–W89. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [Green Version]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 45e. [Google Scholar] [CrossRef] [PubMed]
- Previtali, E.; Bucciarelli, P.; Passamonti, S.M.; Martinelli, I. Risk factors for venous and arterial thrombosis. Blood Transfus. 2011, 9, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.E.; Gores, G.J. Life and death by death receptors. FASEB J. 2009, 23, 1625–1637. [Google Scholar] [CrossRef] [Green Version]
- Hou, B.; Eren, M.; Painter, C.A.; Covington, J.W.; Dixon, J.D.; Schoenhard, J.A.; Vaughan, D.E. Tumor Necrosis Factor α Activates the Human Plasminogen Activator Inhibitor-1 Gene through a Distal Nuclear Factor κB Site. J. Biol. Chem. 2004, 279, 18127–18136. [Google Scholar] [CrossRef] [Green Version]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Cabal-Hierro, L.; Rodríguez, M.; Artime, N.; Iglesias, J.; Ugarte, L.; Prado, M.A.; Lazo, P.S. TRAF-mediated modulation of NF-kB AND JNK Activation by TNFR2. Cell. Signal. 2014, 26, 2658–2666. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef] [Green Version]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kuninaka, Y.; Taruya, A.; Furuta, M.; Mukaida, N.; Kondo, T. Contribution of the TNF-α (tumor necrosis factor-α)-TNFrp55 (tumor necrosis factor receptor p55) axis in the resolution of venous thrombus. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2638–2650. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.; Zhang, L.; Zhang, M.; Yang, W.; Wang, K.; Zhang, J.; Otsu, K.; Huang, G.; Fan, X.; Liu, J. Platelet-specific p38α deficiency improved cardiac function after myocardial infarction in mice. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e185–e196. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lee, C.; Tang, Z.; Zhang, F.; Arjunan, P.; Li, Y.; Hou, X.; Kumar, A.; Dong, L. VEGF-B: A survival, or an angiogenic factor? Cell Adhes. Migr. 2009, 3, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Rissanen, T.T.; Markkanen, J.E.; Gruchala, M.; Heikura, T.; Puranen, A.; Kettunen, M.I.; Kholová, I.; Kauppinen, R.A.; Achen, M.G.; Stacker, S.A.; et al. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ. Res. 2003, 92, 1098–1106. [Google Scholar] [CrossRef] [Green Version]
- Lambert, M.P.; Sachais, B.S.; Kowalska, M.A. Chemokines and thrombogenicity. Thromb. Haemost. 2007, 97, 722–729. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Cao, Y.; Zhang, X.; Peng, C.; Tian, X.; Yan, C.; Liu, Y.; Liu, M.; Han, Y. Chemokine CC-motif ligand 2 participates in platelet function and arterial thrombosis by regulating PKCα-P38MAPK-HSP27 pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2901–2912. [Google Scholar] [CrossRef]
- Suffee, N.; Hlawaty, H.; Meddahi-Pelle, A.; Maillard, L.; Louedec, L.; Haddad, O.; Martin, L.; Laguillier, C.; Richard, B.; Oudar, O.; et al. RANTES/CCL5-induced pro-angiogenic effects depend on CCR1, CCR5 and glycosaminoglycans. Angiogenesis 2012, 15, 727–744. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, S.; Wang, Y.; Rahman, M.; Syk, I.; Zhang, E.; Thorlacius, H. Proinflammatory role of neutrophil extracellular traps in abdominal sepsis. Am. J. Physiol. Cell. Mol. Physiol. 2014, 307, L586–L596. [Google Scholar] [CrossRef]
- Guo, L.-Y.; Yang, F.; Peng, L.-J.; Li, Y.-B.; Wang, A.-P. CXCL2, a new critical factor and therapeutic target for cardiovascular diseases. Clin. Exp. Hypertens. 2020, 42, 428–437. [Google Scholar] [CrossRef]
- Hu, T.; Bach, R.R.; Horton, R.; Konigsberg, W.H.; Todd, M.B. Procoagulant activity in cancer cells is dependent on tissue factor expression. Oncol. Res. 1994, 6, 321–327. [Google Scholar]
- Szotowski, B.; Antoniak, S.; Poller, W.; Schultheiss, H.P.; Rauch, U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ. Res. 2005, 96, 1233–1239. [Google Scholar] [CrossRef] [Green Version]
- Ou, M.; Zhang, Y.; Cui, S.; Zhao, S.; Tu, J. Upregulated MiR-9-5p Protects Against Inflammatory Response in Rats with Deep Vein Thrombosis via Inhibition of NF-κB p50. Inflammation 2019, 42, 1925–1938. [Google Scholar] [CrossRef]
- Kojok, K.; El-Kadiry, A.E.-H.; Merhi, Y. Role of NF-κB in Platelet Function. Int. J. Mol. Sci. 2019, 20, 4185. [Google Scholar] [CrossRef] [Green Version]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Malaponte, G.; Signorelli, S.S.; Bevelacqua, V.; Polesel, J.; Taborelli, M.; Guarneri, C.; Fenga, C.; Umezawa, K.; Libra, M. Increased levels of NF-κB-dependent markers in cancer-associated deep venous thrombosis. PLoS ONE 2015, 10, e0132496. [Google Scholar] [CrossRef] [Green Version]
- Ying, H.; Yue, B.Y.J.T. Cellular and Molecular Biology of Optineurin. Int. Rev. Cell. Mol. Biol. 2012, 294, 223–258. [Google Scholar] [PubMed] [Green Version]
- Sun, S.-C. The noncanonical NF-κB pathway. Immunol. Rev. 2012, 246, 125–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol. Sci. 2012, 33, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Novak, U.; Rinaldi, A.; Kwee, I.; Nandula, S.V.; Rancoita, P.M.V.; Compagno, M.; Cerri, M.; Rossi, D.; Murty, V.V.; Zucca, E.; et al. The NF-κB negative regulator TNFAIP3 (A20) is inactivated by somatic mutations and genomic deletions in marginal zone lymphomas. Blood 2009, 113, 4918–4921. [Google Scholar] [CrossRef]
- Seigner, J.; Basilio, J.; Resch, U.; de Martin, R. CD40L and TNF both activate the classical NF-κB pathway, which is not required for the CD40L induced alternative pathway in endothelial cells. Biochem. Biophys. Res. Commun. 2018, 495, 1389–1394. [Google Scholar] [CrossRef]
- Li, Y.D.; Ye, B.Q.; Zheng, S.X.; Wang, J.T.; Wang, J.G.; Chen, M.; Liu, J.G.; Pei, X.H.; Wang, L.J.; Lin, Z.L.; et al. NF-κB transcription factor p50 critically regulates tissue factor in deep vein thrombosis. J. Biol. Chem. 2009, 284, 4473–4483. [Google Scholar] [CrossRef] [Green Version]
- Tsantes, A.E.; Nikolopoulos, G.K.; Bagos, P.G.; Tsiara, C.G.; Kapsimali, V.; Travlou, A.; Vaiopoulos, G. Plasminogen activator inhibitor-1 4G/5G polymorphism and risk of ischemic stroke: A meta-analysis. Blood Coagul. Fibrinolysis 2007, 18, 497–504. [Google Scholar] [CrossRef]
- Wood, J.P.; Ellery, P.E.R.; Maroney, S.A.; Mast, A.E. Biology of tissue factor pathway inhibitor. Blood 2014, 123, 2934–2943. [Google Scholar] [CrossRef] [Green Version]
- Sidelmann, J.J.; Bladbjerg, E.M.; Gram, J.; Münster, A.M.B.; Jespersen, J. Tissue factor pathway inhibitor relates to fibrin degradation in patients with acute deep venous thrombosis. Blood Coagul. Fibrinolysis 2008, 19, 405–409. [Google Scholar] [CrossRef]
- Memon, A.A.; Sundquist, K.; PirouziFard, M.; Elf, J.L.; Strandberg, K.; Svensson, P.J.; Sundquist, J.; Zöller, B. Identification of novel diagnostic biomarkers for deep venous thrombosis. Br. J. Haematol. 2018, 181, 378–385. [Google Scholar] [CrossRef]
- Halter, F.; Tarnawski, A.S.; Schmassmann, A.; Peskar, B.M. Cyclooxygenase 2-implications on maintenance of gastric mucosal integrity and ulcer healing: Controversial issues and perspectives. Gut 2001, 49, 443–453. [Google Scholar] [CrossRef]
- Bolli, R.; Shinmura, K.; Tang, X.L.; Kodani, E.; Xuan, Y.T.; Guo, Y.; Dawn, B. Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc. Res. 2002, 55, 506–519. [Google Scholar] [CrossRef]
- Cipollone, F.; Prontera, C.; Pini, B.; Marini, M.; Fazia, M.; De Cesare, D.; Iezzi, A.; Ucchino, S.; Boccoli, G.; Saba, V.; et al. Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E2-dependent plaque instability. Circulation 2001, 104, 921–927. [Google Scholar] [CrossRef] [Green Version]
- Minami, T.; Abid, M.R.; Zhang, J.; King, G.; Kodama, T.; Aird, W.C. Thrombin Stimulation of Vascular Adhesion Molecule-1 in Endothelial Cells Is Mediated by Protein Kinase C (PKC)-δ-NF-κB and PKC-ζ-GATA Signaling Pathways. J. Biol. Chem. 2003, 278, 6976–6984. [Google Scholar] [CrossRef] [Green Version]
- Abid, M.R.; Shih, S.C.; Otu, H.H.; Spokes, K.C.; Okada, Y.; Curiel, D.T.; Minami, T.; Aird, W.C. A novel class of vascular endothelial growth factor-responsive genes that require forkhead activity for expression. J. Biol. Chem. 2006, 281, 35544–35553. [Google Scholar] [CrossRef] [Green Version]
- Peschen, M.; Lahaye, T.; Hennig, B.; Weyl, A.; Simon, J.C.; Vanscheidt, W. Expression of the adhesion molecules ICAM-1, VCAM-1, LFA-1 and VLA-4 in the skin is modulated in progressing stages of chronic venous insufficiency. Acta Derm. Venereol. 1999, 79, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Golay, J.; Manganini, M.; Facchinetti, V.; Gramigna, R.; Broady, R.; Borleri, G.; Rambaldi, A.; Introna, M. The concentrations of soluble vascular cell adhesion molecule-1 and lipids are independently associated with venous thromboembolism. Haematologica 2003, 88, 1002–1012. [Google Scholar]
- Najem, M.Y.; Couturaud, F.; Lemarié, C.A. Cytokine and chemokine regulation of venous thromboembolism. J. Thromb. Haemost. 2020, 18, 1009–1019. [Google Scholar] [CrossRef]
- Maione, F.; Cicala, C.; Liverani, E.; Mascolo, N.; Perretti, M.; D’Acquisto, F. IL-17A increases ADP-induced platelet aggregation. Biochem. Biophys. Res. Commun. 2011, 408, 658–662. [Google Scholar] [CrossRef] [Green Version]
- Ding, P.; Zhang, S.; Yu, M.; Feng, Y.; Long, Q.; Yang, H.; Li, J.; Wang, M. IL-17A promotes the formation of deep vein thrombosis in a mouse model. Int. Immunopharmacol. 2018, 57, 132–138. [Google Scholar] [CrossRef]
- Rukoyatkina, N.; Mindukshev, I.; Walter, U.; Gambaryan, S. Dual role of the p38 MAPK/cPLA2 pathway in the regulation of platelet apoptosis induced by ABT-737 and strong platelet agonists. Cell Death Dis. 2013, 4, e931. [Google Scholar] [CrossRef] [Green Version]
- Janus, P.; Toma-Jonik, A.; Vydra, N.; Mrowiec, K.; Korfanty, J.; Chadalski, M.; Widłak, P.; Dudek, K.; Paszek, A.; Rusin, M.; et al. Pro-death signaling of cytoprotective heat shock factor 1: Upregulation of NOXA leading to apoptosis in heat-sensitive cells. Cell Death Differ. 2020, 27, 2280–2292. [Google Scholar] [CrossRef] [Green Version]




| RNA-Seq Libraries | Number of Raw Reads | Number of Processed Reads | Number of Uniquely Mapped Reads | Uniquely Mapped Reads (%) | Number of Multimapped Reads |
|---|---|---|---|---|---|
| FV_C1 | 31,683,264 | 29,014,357 | 27,462,945 | 94.65 | 764,528 |
| FV_C2 | 36,724,405 | 33,348,453 | 31,094,279 | 93.24 | 1,740,840 |
| FV_C3 | 39,861,429 | 36,179,831 | 34,714,702 | 95.95 | 850,949 |
| FV_C4 | 40,514,407 | 35,976,149 | 34,248,079 | 95.20 | 928,229 |
| FV_C5 | 32,653,642 | 29,745,640 | 28,547,359 | 95.97 | 617,930 |
| FV_T1 | 41,965,137 | 38,446,156 | 36,212,924 | 94.19 | 868,794 |
| FV_T2 | 41,704,895 | 38,432,218 | 36,876,819 | 95.95 | 926,073 |
| FV_T3 | 32,831,286 | 29,868,416 | 28,793,908 | 96.40 | 590,702 |
| FV_T4 | 38,083,555 | 34,833,923 | 33,551,704 | 96.32 | 756,735 |
| FV_T5 | 41,165,295 | 37,191,583 | 35,210,456 | 94.67 | 1,236,692 |
| FV_T6 | 33,899,526 | 30,601,833 | 29,534,470 | 96.51 | 637,436 |
| RNA-Seq Libraries | Reads Mapped on Codding Regions (%) | Reads Mapped on UTR Regions (%) | Reads Mapped on Intronic Regions (%) | Reads Mapped on Intergenic Regions (%) |
|---|---|---|---|---|
| FV_C1 | 45.30 | 28.31 | 10.91 | 15.47 |
| FV_C2 | 46.08 | 27.59 | 11.65 | 14.68 |
| FV_C3 | 47.10 | 29.98 | 9.05 | 13.87 |
| FV_C4 | 65.44 | 16.07 | 6.65 | 11.84 |
| FV_C5 | 46.41 | 28.41 | 10.18 | 15.00 |
| FV_T1 | 45.11 | 29.44 | 11.02 | 14.42 |
| FV_T2 | 45.78 | 30.39 | 10.21 | 13.62 |
| FV_T3 | 44.20 | 30.73 | 10.16 | 14.91 |
| FV_T4 | 42.98 | 30.79 | 11.94 | 14.29 |
| FV_T5 | 47.00 | 30.92 | 8.48 | 13.60 |
| FV_T6 | 46.11 | 32.17 | 8.39 | 13.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gromadziński, L.; Paukszto, Ł.; Skowrońska, A.; Holak, P.; Smoliński, M.; Łopieńska-Biernat, E.; Lepiarczyk, E.; Lipka, A.; Jastrzębski, J.P.; Majewska, M. Transcriptomic Profiling of Femoral Veins in Deep Vein Thrombosis in a Porcine Model. Cells 2021, 10, 1576. https://doi.org/10.3390/cells10071576
Gromadziński L, Paukszto Ł, Skowrońska A, Holak P, Smoliński M, Łopieńska-Biernat E, Lepiarczyk E, Lipka A, Jastrzębski JP, Majewska M. Transcriptomic Profiling of Femoral Veins in Deep Vein Thrombosis in a Porcine Model. Cells. 2021; 10(7):1576. https://doi.org/10.3390/cells10071576
Chicago/Turabian StyleGromadziński, Leszek, Łukasz Paukszto, Agnieszka Skowrońska, Piotr Holak, Michał Smoliński, Elżbieta Łopieńska-Biernat, Ewa Lepiarczyk, Aleksandra Lipka, Jan Paweł Jastrzębski, and Marta Majewska. 2021. "Transcriptomic Profiling of Femoral Veins in Deep Vein Thrombosis in a Porcine Model" Cells 10, no. 7: 1576. https://doi.org/10.3390/cells10071576







