The Influence of Mitochondrial Dynamics and Function on Retinal Ganglion Cell Susceptibility in Optic Nerve Disease
Abstract
:1. Cellular Function, Structure, and Activity of Mitochondria
2. Mitochondria in Cellular Pathology
3. Mitochondria in Normal Neuronal Activity and Neurodegeneration
4. Fuel Sources for Neurons
5. Mitochondrial Localization within Retinal Ganglion Cell Architecture
6. The Role of Mitochondria in Optic Nerve and RGC Pathology
7. A Bioenergetic Model of Glaucomatous Pathology
8. Therapies Targeting Mitochondria in RGCs
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, E.P.; Lehninger, A.L. Oxidation of fatty acids and tricarboxylic acid cycle intermediates by isolated rat liver mitochondria. J. Biol. Chem. 1949, 179, 957–972. [Google Scholar] [CrossRef]
- Prebble, J.N. The discovery of oxidative phosphorylation: A conceptual off-shoot from the study of glycolysis. Stud. Hist. Philos. Biol. Biomed. Sci. 2010, 41, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, L. The origin of mitochondria. J. Mol. Evol. 1975, 5, 167–175. [Google Scholar] [CrossRef]
- Maes, M.E.; Grosser, J.A.; Fehrman, R.L.; Schlamp, C.L.; Nickells, R.W. Completion of BAX recruitment correlates with mitochondrial fission during apoptosis. Sci. Rep. 2019, 9, 16565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glancy, B.; Hartnell, L.M.; Malide, D.; Yu, Z.X.; Combs, C.A.; Connelly, P.S.; Subramaniam, S.; Balaban, R.S. Mitochondrial reticulum for cellular energy distribution in muscle. Nature 2015, 523, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yapa, N.M.B.; Lisnyak, V.; Reljic, B.; Ryan, M.T. Mitochondrial dynamics in health and disease. FEBS Lett. 2021, 595, 1184–1204. [Google Scholar] [CrossRef] [PubMed]
- Elachouri, G.; Vidoni, S.; Zanna, C.; Pattyn, A.; Boukhaddaoui, H.; Gaget, K.; Yu-Wai-Man, P.; Gasparre, G.; Sarzi, E.; Delettre, C.; et al. OPA1 links human mitochondrial genome maintenance to mtDNA rerplication and distribution. Genome Res 2011, 21, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Yu-Wai-Man, P.; Stewart, J.D.; Hudson, G.; Andrews, R.M.; Griffiths, P.G.; Birch, M.K.; Chinnery, P.F. OPA1 increases the risk of normal but not high tension glaucoma. J. Med. Genet. 2010, 47, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Palau, F.; Estela, A.; Pla-Martin, D.; Sánchez-Piris, M. The role of mitochondrial network dynamics in the pathogenesis of Charcot-Marie-Tooth disease. Adv. Exp. Med. Biol. 2009, 652, 129–137. [Google Scholar]
- Hamedani, A.G.; Wilson, J.A.; Avery, R.A.; Scherer, S.S. Optic neuropathy in Charcot-Marie-Tooth disease. J. Neuro. Ophthalmol. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Berman, S.B.; Chen, Y.B.; Qi, B.; McCaffrey, J.M.; Rucker III, E.B.; Goebbels, S.; Nave, K.A.; Arnold, B.A.; Jonas, E.A.; Pineda, F.J.; et al. Bcl-XL increases mitochondrial fission, fusion, and biomass in neurons. J. Cell Biol. 2009, 184, 707–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppins, S.; Edlich, F.; Cleland, M.M.; Banerjee, S.; McCaffrey, J.M.; Youle, R.J.; Nunnari, J. The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol. Cell 2011, 41, 150–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleland, M.M.; Norris, K.L.; Karbowski, M.; Wang, C.; Suen, D.F.; Jiao, S.; George, N.M.; Luo, X.; Li, Z.; Youle, R.J. Bcl-2 family interaction with the mitochondrial morphogenesis machinery. Cell Death Differ. 2011, 18, 235–247. [Google Scholar] [CrossRef]
- Zhang, P.; Hinshaw, J.E. Three-dimensional reconstruction of dynamin in the constricted state. Nat. Cell Biol. 2001, 3, 922–926. [Google Scholar] [CrossRef]
- Basu, K.; Lajoie, D.; Aumentado-Armstrong, T.; Chen, J.; Koning, R.I.; Bossy, B.; Bostina, M.; Sik, A.; Bossy-Wetzel, E.; Rouiller, I. Molecular mechanism of DRP1 assembly studied in vitro by cryo-electron microscopy. PLoS ONE 2017, 12, e0179397. [Google Scholar] [CrossRef] [Green Version]
- Frank, S.; Gaume, B.; Bergmann-Leitner, E.S.; Leitner, W.W.; Robert, E.G.; Catez, F.; Smith, C.L.; Youle, R.J. The role of Dynamin-Related Protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 2001, 1, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Estaquier, J.; Arnoult, D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ. 2007, 14, 1086–1094. [Google Scholar] [CrossRef] [Green Version]
- Filichia, E.; Hoffer, B.; Qi, X.; Luo, Y. Inhibition of Drp1 mitochondrial translocation provides neural protection in dopaminergic system in a Parkinson’s disease model induced by MPTP. Sci. Rep. 2016, 6, 32656. [Google Scholar] [CrossRef] [Green Version]
- Joshi, A.U.; Saw, N.L.; Vogel, H.; Cunningham, A.D.; Shamloo, M.; Mochly-Rosen, D. Inhibition of Drp1/Fis1 interaction slows progression of amyotrophic lateral sclerosis. EMBO Mol. Med. 2018, 10, e8166. [Google Scholar] [CrossRef]
- Wu, J.H.; Zhang, S.H.; Gao, F.J.; Lei, Y.; Chen, X.Y.; Gao, F.; Zhang, S.J.; Sun, X. RNAi screening identifies GSK3beta as a regulator of DRP1 and the neuroprotection of lithium chloride against elevated pressure involved in downregulation of DRP1. Neurosci. Lett. 2013, 554, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Uo, T.; Dworzak, J.; Kinoshita, C.; Inman, D.M.; Kinoshita, Y.; Horner, P.J.; Morrison, R.S. Drp1 levels constitutively regulate mitochondrial dynamics and cell survivial in cortical neurons. Exp. Neurol. 2009, 218, 274–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, S.; Charif, M.; Chevrollier, A.; Chaumette, T.; Angebault, C.; Kane, M.S.; Paris, A.; Alban, J.; Quiles, M.; Delettre, C.; et al. Mutations ini DNM1L, as in OPA1, result in dominant optic atrophy despite opposite effects on mitochondrial fusion and fission. Brain 2017, 140, 2586–2596. [Google Scholar] [CrossRef]
- Greene, A.W.; Grenier, K.; Aguileta, M.A.; Muise, S.; Farazifard, R.; Haque, M.E.; McBride, H.M.; Park, D.S.; Fon, E.A. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep. 2012, 13, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, Parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, Y.C.; Holzbaur, E.L. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc. Natl. Acad. Sci. USA 2014, 111, E4439–E4448. [Google Scholar] [CrossRef] [Green Version]
- Richter, B.; Sliter, D.A.; Herhaus, L.; Stolz, A.; Wang, C.; Beli, P.; Zaffagnini, G.; Wild, P.; Martens, S.; Wagner, S.A.; et al. Phosphorylation of OPTN by TBK1 enhances its binding to Ub chains and promotes selective autophagy of damaged mitochondria. Proc. Natl. Acad. Sci. USA 2016, 113, 4039–4044. [Google Scholar] [CrossRef] [Green Version]
- Wild, P.; Farhan, H.; McEwan, D.G.; Wagner, S.; Rogov, V.V.; Brady, N.R.; Richter, B.; Korac, J.; Waidmann, O.; Choudhary, C.; et al. Phosphorylation of the autophagy receptor Optineurin restricts Salmonella growth. Science 2011, 333, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Alward, W.L.; Kwon, Y.H.; Kawase, K.; Craig, J.E.; Hayreh, S.S.; Johnson, A.T.; Khanna, C.L.; Yamamoto, T.; Mackey, D.A.; Roos, B.; et al. Evaluation of optineurin sequence variations in 1,048 patients with open-angle glaucoma. Am. J. Ophthalmol. 2003, 136, 904–910. [Google Scholar] [CrossRef]
- Leung, Y.F.; Fan, B.J.; Lam, D.S.; Lee, W.S.; Tam, P.O.; Chua, J.K.; Tham, C.C.; Lai, J.S.; Fan, D.S.; Pang, C.P. Different optineurin mutation pattern in primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3880–3884. [Google Scholar] [CrossRef] [Green Version]
- Rezaie, T.; Child, A.; Hitchings, R.; Brice, G.; Miller, L.; Coca-Prados, M.; Héon, E.; Krupin, T.; Ritch, R.; Kreutzer, D.; et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 2002, 295, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Fingert, J.H.; Robin, A.L.; Stone, J.L.; Roos, B.; Davis, L.K.; Scheetz, T.A.; Bennett, S.R.; Wassink, T.H.; Kwon, Y.H.; Alward, W.L.; et al. Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Hum. Mol. Genet. 2011, 20, 2482–2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawase, K.; Allingham, R.R.; Meguro, A.; Mizuki, N.; Roos, B.; Solivan-Timpe, F.M.; Robin, A.L.; Ritch, R.; Fingert, J.H. Confirmation of TBK1 duplication in normal tension glaucoma. Exp. Eye Res. 2012, 96, 178–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, C.H.O.; Marsh-Armstrong, N. Discovery and implications of transcellular mitophagy. Autophagy 2014, 10, 2383–2384. [Google Scholar] [CrossRef] [Green Version]
- Morales, I.; Sanchez, A.; Puertas-Avendano, R.; Rodriguez-Sabate, C.; Perez-Barreto, A.; Rodriguez, M. Neuorglial transmitophage and Parkinson’s disease. Glia 2020, 68, 2277–2299. [Google Scholar]
- Karbowski, M.; Lee, Y.J.; Gaume, B.; Jeong, S.Y.; Frank, S.; Nechushtan, A.; Santel, A.; Fuller, M.; Smith, C.L.; Youle, R.J. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 2002, 159, 931–938. [Google Scholar] [CrossRef] [Green Version]
- Karbowski, M.; Youle, R.J. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003, 10, 870–880. [Google Scholar] [CrossRef] [Green Version]
- Oetiinghaus, B.; D’Alonzo, D.; Barbieri, E.; Restelli, L.M.; Savoia, C.; Licci, M.; Tolnay, M.; Frank, S.; Scorrano, L. DRP1-dependent apoptotic mitochondrial fission occurs independently of BAX, BAK and APAF1 to amplify cell death by BID and oxidative stress. Biochim. Biophys. Acta 2016. epub ahead of print. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, F.; Zhang, Z.; Xing, D. Bax is essential for Drp1-mediated mitochondrial fission but not for mitochondrial outer membrane permeabilization caused by photodynamic therapy. J. Cell Physiol. 2011, 226, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Lackner, L.L.; West, M.; DiBennedetto, J.R.; Nunnari, J.; Voeltz, G.K. ER tubules mark sites of mitochondrial division. Science 2011, 334, 358–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [Green Version]
- Fadok, V.A.; Bratton, D.L.; Frasch, S.C.; Warner, M.L.; Henson, P.M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998, 5, 551–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lackner, L.L. Determining the shape and cellular distribution of mitochondria: The integrration of multiple activities. Curr. Opin. Cell Biol. 2013, 25, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. MIRO GTPases in mitochondrial transport, homeostasis and pathology. Cells 2015, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Schwarz, T.L. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell 2009, 136, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.H.; Agarwal, E.; Bryant, K.G.; Caino, M.C.; Kim, E.T.; Kossenkov, A.V.; Tang, H.Y.; Languino, L.R.; Gabrilovich, D.I.; Cohen, A.R.; et al. Syntaphilin ubiquitination regulates mitochondrial dynamics and tumor cell movements. Cancer Res. 2018, 78, 4215–4228. [Google Scholar] [CrossRef] [Green Version]
- Leal, N.S.; Martins, L.M. Mind the gap: Mitochondria and the endoplasmic reticulum in neurodegenerative diseases. Biomedicines 2021, 9, 227. [Google Scholar] [CrossRef]
- Loncke, J.; Kaasik, A.; Bezprozvanny, I.; Parys, J.B.; Kerkhofs, M.; Bultynck, G. Balancing ER-mitochondrial Ca2+ fluxes in health and disease. Trends Cell Biol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Prinz, W.A.; Toulmay, A.; Balla, T. The functional universe of membrane contact sites. Nat. Rev. Mol. Cell Biol. 2020, 21, 7–24. [Google Scholar] [CrossRef]
- Monaco, G.; Decrock, E.; Arbel, N.; van Vliet, A.R.; La Rovere, R.M.; De Smedt, H.; Parys, J.B.; Agostinis, P.; Leybaert, L.; Shoshan-Barmatz, V.; et al. The BH4 domain of anti-apoptotic Bcl-XL, but not that of Bcl-2, limits the voltage-dependent anion channel 1(VDAC1)-mediated transfer of pro-apoptotic Ca2+ signals to mitochondria. J. Biol. Chem. 2015, 290, 9150–9161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.; Hayashi, T.; Wolozny, D.; Yin, B.; Su, T.C.; Betenbaugh, M.J.; Su, T.P. The non-apoptotic action of BclxL: Regulating Ca2+ signaling and bioenergetics at the ER-mitochondrion interface. J. Bioenerg. Biomembr. 2016, 48, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Hayashi, T.; Su, T.P.; Betenbaugh, M.J. Bcl-2 family in inter-organelle modulation of calcium signaling; roles in bioienergetics and cell survival. J. Bioenerg. Biomembr. 2014, 46, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacAskill, A.F.; Kittler, J.T. Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 2010, 20, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Maday, S.; Twelvetrees, A.E.; Moughamian, A.J.; Holzbaur, E.L. Axonal transport: Cargo-specific mechanisms of motility and regulation. Neuron 2014, 84, 292–309. [Google Scholar] [CrossRef] [Green Version]
- López-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, A.; Madreiter-Sokolowski, C.; Stryeck, S.; Abdellatif, M. Targeting the mitochondria-proteostasis axis to delay aging. Front. Cell Dev. Biol. 2021, 9, 656201. [Google Scholar] [CrossRef]
- Qian, S.; Wang, W.; Yang, L.; Huang, H.W. Structure of transmembrane pore induced by Bax-derived peptide: Evidence for lipidic pores. Proc. Natl. Acad. Sci. USA 2008, 105, 17379–17383. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.P.; Zhai, D.; Kim, E.; Swift, M.; Reed, J.C.; Volkmann, N.; Hanein, D. Three dimensional structure of Bax-mediated pores in membrane bilayers. Cell Death Disease 2013, 4, e683. [Google Scholar] [CrossRef]
- Pena-Blanco, A.; Garcia-Sáez, A.J. Bax, Bak and beyond—Mitochondrial performance in apoptosis. FEBS J. 2017, 285, 416–431. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.A.; Cory, S. Apoptosomes: Engines for caspase activation. Curr. Opin. Cell Biol. 2002, 14, 715–720. [Google Scholar] [CrossRef]
- Bao, Q.; Shi, Y. Apoptosome: A platform for the activation of initiator caspases. Cell Death Differ. 2007, 14, 56–65. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, K.; Garcia-Saez, A.J. Bax and Bak pores: Are we closing the circle? Trends Cell Biol. 2017, 27, 266–275. [Google Scholar] [CrossRef]
- Große, L.; Wurm, C.A.; Brüser, C.; Neumann, D.; Jans, D.C.; Jakobs, S. Bax assembles into large ring-like structures remodeling the mitochondrial outer membrane in apoptosis. EMBO J. 2016, 35, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Gallego, R.; Mund, M.; Cosentino, K.; Schneider, J.; Unsay, J.; Schraermeyer, U.; Englehardt, J.; Ries, J.; Garcia-Saez, A.J. Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. EMBO J. 2016, 35, 389–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleicken, S.; Landeta, O.; Landajuela, A.; Basanez, G.; Garcia-Sáez, A.J. Proapoptotic Bax and Bak proteins form stable protein-permeable pores of tunable size. J. Biol. Chem. 2013, 288, 33241–33252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillies, L.A.; Du, H.; Peters, B.; Knudson, C.M.; Newmeyer, D.D.; Kuwana, T. Visual and functional demonstration of growing Bax-induced pores in mitochondrial outer membranes. Mol. Biol. Cell 2015, 26, 339–349. [Google Scholar] [CrossRef]
- Maes, M.E.; Schlamp, C.L.; Nickells, R.W. Live-cell imaging to measure BAX recruitment kinetics to mitochondria during apoptosis. PLoS ONE 2017, 12, e0184434. [Google Scholar] [CrossRef] [Green Version]
- McArthur, K.; Whitehead, L.W.; Heddleston, J.M.; Li, L.; Padman, B.S.; Oorschot, V.; Geoghegan, N.D.; Chappaz, S.; Davidson, S.; Chin, H.S.; et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 2018, 359. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPS cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Nuevo, A.; Zorzano, A. The sensing of mitochondrial DAMPs by non-immune cells. Cell Stress 2019, 3, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Kermer, P.; Ankerhold, R.; Klocker, N.; Krajewski, S.; Reed, J.C.; Bähr, M. Caspase-9 involvement in secondary death of axotomized rat retinal ganglion cells in vivo. Brain Res. Mol. Brain Res. 2000, 28, 144–150. [Google Scholar] [CrossRef]
- Kermer, P.; Klöcker, N.; Labes, M.; Bähr, M. Inhibition of CPP32-like proteases rescues axotomized retinal ganglion cells from secondary death in vivo. J. Neurosci. 1998, 18, 4656–4662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane, D.J.; Sarafian, T.A.; Anton, R.; Hahn, H.; Gralla, E.B.; Valentine, J.S.; Örd, T.; Bredesen, D.E. Bcl-2 inhibition of neural death: Decreased generation of reactive oxygen species. Science 1993, 262, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, R.A.; Franklin, J.L. Bax, reactive oxygen, and cytochrome c release in neuronal apoptosis. Antioxid. Redox Signal 2003, 5, 589–596. [Google Scholar] [CrossRef]
- Grosser, J.A.; Maes, M.E.; Nickells, R.W. Characteristics of intracellular propagation of mitochondrial BAX recruitment during apoptosis. Apoptosis 2021, 26, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Borutaite, V. There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion 2012, 12, 1–4. [Google Scholar] [CrossRef]
- Johri, A.; Beal, M.F. Mitochondrial dysfunction in neurodegenerative diseases. J. Pharm. Exp. Ther. 2012, 342, 619–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, V.K.; Singh, T.G.; Mehta, V. Stressed mitochondria: A target to intrude Alzheimer’s disease. Mitochondrion 2021, 59, 48–57. [Google Scholar] [CrossRef]
- Trinh, D.; Israwi, A.R.; Arathoon, L.R.; Gleave, J.A.; Nash, J.E. The multi-faceted role of mitochondria in the pathology of Parkinson’s disease. J. Neurochem. 2020, 156, 715–752. [Google Scholar] [CrossRef]
- Nicoletti, V.; Palmero, G.; Del Prete, E.; Mancuso, M.; Ceravolo, R. Understanding the multiple role of mitochondria in Parkinson’s disease and related disorders: Lessons from genetics and protein-interaction network. Front. Cell Dev. Biol. 2021, 9, 636506. [Google Scholar] [CrossRef]
- Bergaglio, T.; Luchicchi, A.; Schenk, G.J. Engine failure in axo-myelinic signaling: A potential key playere in the pathogenesis of multiple sclerosis. Front. Cell. Neurosci. 2021, 15, 619295. [Google Scholar] [CrossRef]
- Shi, P.; Gal, J.; Kwinter, D.M.; Liu, X.; Zhu, H. Mitochondrial dysfunction in amyotrophic lateral sclerosis. Biochim. Biophys. Acta 2010, 1802, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, P.H.; Mao, P.; Manczak, M. Mitochondrial structure and functional dynamics in Huntington’s disease. Brain Res. Rev. 2009, 61, 33–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zsurka, G.; Kunz, W.S. Mitochondrial dysfunction and seizures: The neuronal energy crisis. Lancet Neurol. 2015, 14, 956–966. [Google Scholar] [CrossRef]
- Lenaers, G.; Neutzner, A.; Le Dantec, Y.; Jüschke, C.; Xiao, T.; Decembrini, S.; Swirski, S.; Kieninger, S.; Agca, C.; Kim, U.S.; et al. Dominant optic atrophy: Culprit mitochondria in the optic nerve. Prog. Retin. Eye Res. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc. Natl. Acad. Sci. USA 2012, 109 (Suppl. 1), 10661–10668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attwell, D.; Laughlin, S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Met. 2001, 21, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic energy use and supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, K.A.; Sheng, Z.H. Mechanisms for the maintenance and regulation of axonal energy supply. J. Neurosci. Res. 2019, 97, 897–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiello, G.L.; Bach-y-Rita, P. The cost of an action potential. J. Neurosci. Methods 2000, 103, 145–149. [Google Scholar] [CrossRef]
- Vetter, P.; Roth, A.; Häusser, M. Action potential propagation in dendrites depends on dendritic morphology. J. Neurophysiol. 2001, 85, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Rangaraju, V.; Calloway, N.; Ryan, T.A. Activity-driven local ATP synthesis is required for synaptic function. Cell 2014, 156, 825–835. [Google Scholar] [CrossRef] [Green Version]
- MacAskill, A.F.; Rinholm, J.E.; Twelvetrees, A.E.; Arancibia-Carcamo, I.L.; Muir, J.; Fransson, A.; Aspenstrom, P.; Attwell, D.; Kittler, J.T. Miro1 is a calcium sensor for glutamate receptor-dependentt localization of mitochondria at synapses. Neuron 2009, 61, 541–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mar, F.M.; Simoes, A.R.; Leite, S.; Morgado, M.M.; Santos, T.E.; Rodrigo, I.S.; Teixeira, C.A.; Misgeld, T.; Sousa, M.M. CNS axons globally increase axonal transport after peripheral conditioning. J. Neurosci. 2014, 34, 5965–5970. [Google Scholar] [CrossRef] [Green Version]
- Han, S.M.; Baig, H.S.; Hammarlund, M. Mitochondria localize to injured axons to support regeneration. Neuron 2016, 92, 1308–1323. [Google Scholar] [CrossRef] [Green Version]
- Cartoni, R.; Norsworthy, M.W.; Bei, F.; Wang, C.; Li, S.; Zhang, Y.; Gabel, C.V.; Schwarz, T.L.; He, Z. The mammalian-specific protein ARMCX1 regulates mitohondrial transport during axon regeneration. Neuron 2016, 92, 1294–1307. [Google Scholar] [CrossRef] [Green Version]
- Cartoni, R.; Pekkurnaz, G.; Wang, C.; Schwarz, T.L.; He, Z. A high mitochondrial transport rate characterizes CNS neurons with high axonal regeneration capacity. PLoS ONE 2017, 12, e0184672. [Google Scholar] [CrossRef] [Green Version]
- Ferree, A.W.; Trudeau, K.; Zik, E.; Benador, I.Y.; Twig, G.; Gottlieb, R.A.; Shirihai, O.S. MitoTimer probe reveals the impact of autophagy, fusion, and motility on subcellular distribution of young and old mitochondrial protein and on relative mitochondrial protein age. Autophagy 2013, 9, 1887–1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.Y.; Cheng, X.T.; Tammineni, P.; Xie, Y.; Zhou, B.; Cai, Q.; Sheng, Z.H. Releasing syntaphilin removes stressed mitochondria from axons independent of mitophagy under pathophysiological conditions. Neuron 2017, 94, 595–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Zhang, X.; Wu, X.; Jiang, L.; Ahsan, A.; Ma, S.; Xiao, Z.; Han, F.; Qin, Z.H.; Hu, W.; et al. Somatic autophagyy of axonal mitochondria in ischemic neurons. J. Cell Biol. 2019, 218, 1891–1907. [Google Scholar] [CrossRef] [Green Version]
- Tekkök, S.B.; Brown, A.M.; Westenbroek, R.; Pellerin, L.; Ransom, B.R. Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J. Neurosci. Res. 2005, 81, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Ransom, B.R. Astrocyte glycogen and brain energy metabolism. Glia 2007, 55, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Alberini, C.M.; Cruz, E.; Descalzi, G.; Bessiérers, B.; Gao, V. Astrocyte glycogen and lactate: New insights into learning and memory mechanisms. Glia 2018, 66, 1244–1262. [Google Scholar] [CrossRef]
- Philips, T.; Mironova, Y.A.; Jouroukhin, Y.; Chew, J.; Vidensky, S.; Farah, M.H.; Pletnikov, M.V.; Bergles, D.E.; Morrison, B.M.; Rothstein, J.D. MCT1 deletion in oligodendrocyte lineage cells causes late-onset hypomyelination and axonal degeneration. Cell Rep. 2021, 34, 108610. [Google Scholar] [CrossRef]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.W.; et al. Oligodendroglia metabollically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Bak, L.K.; Walls, A.B.; Schousboe, A.; Waagepetersen, H.S. Astrocyte glycogen metabolism in the healthy and diseased brain. J. Biol. Chem. 2018, 293, 7108–7116. [Google Scholar] [CrossRef] [Green Version]
- Saab, A.S.; Tzvetavona, I.D.; Trevisiol, A.; Baltan, S.; Dibaj, P.; Kusch, K.; Möbius, W.; Goetze, B.; Jahn, H.M.; Huang, W.; et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 2016, 91, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Vohra, R.; Kolko, M. Lactate: More than merely a metabolic waste product in the inner retina. Mol. Neurobiol. 2020, 57, 2021–2037. [Google Scholar] [CrossRef]
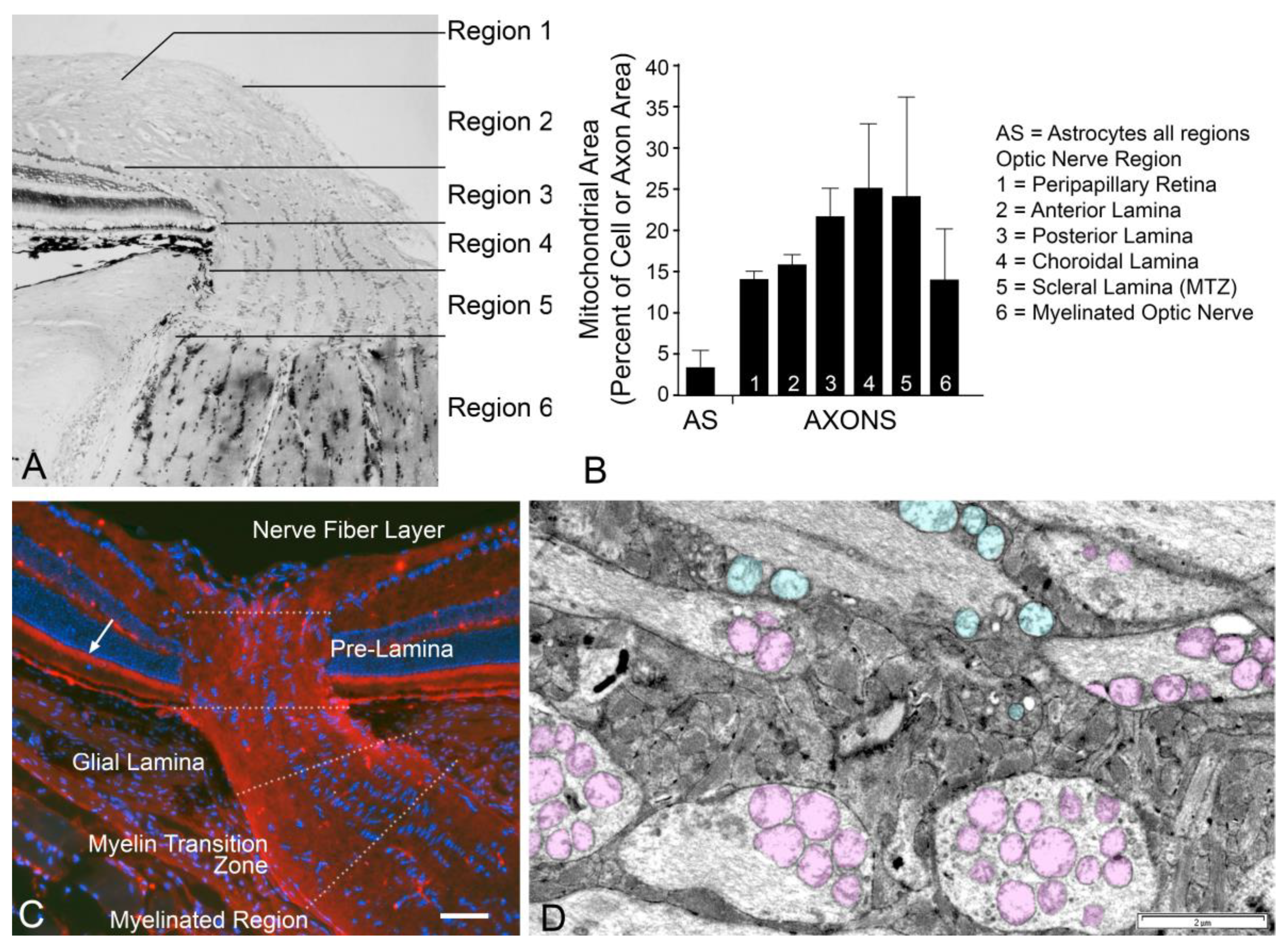
- Yu, D.Y.; Cringle, S.J.; Balaratnasingam, C.; Morgan, W.H.; Yu, P.K.; Su, E.N. Retinal ganglion cells: Energetics, compartmentalization, axonal transport, cytoskeletons, and vulnerability. Prog. Retin. Eye Res. 2013, 36, 217–236. [Google Scholar] [CrossRef]
- Wirtschafter, J.D. Optic nerve axons and acquired alterations in the appearance of the optic disc. Trans. Am. Ophthalmol. Soc. 1983, 81, 1034–1091. [Google Scholar]
- Wässle, H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 2004, 5, 747–757. [Google Scholar] [CrossRef]
- May, C.A.; Lütjen-Drecoll, E. Morphology of the murine optic nerve. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2206–2212. [Google Scholar]
- Schlamp, C.L.; Li, Y.; Dietz, J.A.; Janssen, K.T.; Nickells, R.W. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Howell, G.R.; Libby, R.T.; Jakobs, T.C.; Smith, R.S.; Phalan, F.C.; Barter, J.W.; Barbay, J.M.; Marchant, J.K.; Mahesh, N.; Porciatti, V.; et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 2007, 179, 1523–1537. [Google Scholar] [CrossRef] [Green Version]
- Morrison, J.C.; Farrell, S.K.; Johnson, E.C.; Deppmeier, L.M.H.; Moore, C.G.; Grossmann, E. Structure and composition of the rodent lamina cribrosa. Exp. Eye Res. 1995, 60, 127–135. [Google Scholar] [CrossRef]
- Elkington, A.R.; Inman, C.B.E.; Steart, P.V.; Weller, R.O. The structure of the lamina cribrosa of the human eye: An immunocytochemical and electron microscopical study. Eye 1990, 4, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.R.; Igoe, F.; Neufeld, A.H. Cell culture of the human lamina cribrosa. Investig. Ophthalmol. Vis. Sci. 1988, 29, 78–89. [Google Scholar]
- Quigley, H.A.; Addicks, E.M. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch. Ophthalmol. 1981, 99, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, K.; Teixeira, L.B.C.; Keikhosravi, A.; Eliceiri, K.W.; McLellan, G.J. Microstructure and resident cell-types of the feline optic nerve head resemble that of humans. Exp. Eye Res. 2021, 202, 108315. [Google Scholar] [CrossRef]
- Burgoyne, C.F.; Downs, J.C.; Bellezza, A.J.; Suh, J.K.; Hart, R.T. The optic nerve head as a biomechanical structure: A new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retin. Eye Res. 2005, 24, 39–73. [Google Scholar] [CrossRef]
- Bellezza, A.J.; Rintalan, C.J.; Thompson, H.W.; Downs, J.C.; Hart, R.T.; Burgoyne, C.F. Deformation of the lamina cribrosa and anterior scleral canal wall in early experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 623–637. [Google Scholar] [CrossRef]
- Pazos, M.; Yang, H.; Gardiner, S.K.; Cepurna, W.O.; Johnson, E.C.; Morrison, J.C.; Burgoyne, C.F. Expansions of the neurovascular scleral canal and contained optic nerve occur early in the hypertonic saline rat experimental glauocma model. Exp. Eye Res. 2016, 145, 173–186. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.D.; Grau, V.; Grimm, J.; Reynaud, J.; Bellezza, A.J.; Burgoyne, C.F.; Downs, J.C. Remodeling of the connective tissue microarchitecture of the lamina cribrosa in early experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 681–690. [Google Scholar] [CrossRef]
- Lye-Barthel, M.; Sun, D.; Jakobs, T.C. Morphology of astrocytes in a glaucomatous optic nerve. Investig. Ophthalmol. Vis. Sci. 2013, 54, 909–917. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Lye-Barthel, M.; Masland, R.H.; Jakobs, T.C. The morphology and spatial arrangement of astrocytes in the optic nerve head of the mouse. J. Comp. Neurol. 2009, 516, 1–19. [Google Scholar] [CrossRef]
- Sun, D.; Lye-Barthel, M.; Masland, R.H.; Jakobs, T.C. Structural remodeling of fibrous astrocytes after axonal injury. J. Neurosci. 2010, 30, 14008–14019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, C.; Khaw, P.T.; Yin, Z.Q.; Li, D.; Raisman, G.; Li, Y. Structural basis of glaucoma: The fortified astrocytes of the optic nerve head are the target of raised intraocular pressure. Glia 2012, 60, 13–28. [Google Scholar] [CrossRef]
- Hernandez, M.R. The optic nerve head in glaucoma: Role of astrocytes in tissue remodeling. Prog. Retin. Eye Res. 2000, 19, 297–321. [Google Scholar] [CrossRef]
- Quigley, H.A.; Addicks, E.M.; Green, W.R.; Maumenee, A.E. Optic nerve damage in human glaucoma: II. The site of injury and susceptibility to damage. Arch. Ophthalmol. 1981, 99, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Minckler, D.S.; Bunt, A.H.; Johanson, G.W. Orthograde and retrograde axoplasmic transport during acute ocular hyptertension in the monkey. Investig. Ophthalmol. Vis. Sci. 1977, 16, 426–441. [Google Scholar]
- Quigley, H.A.; Addicks, E.M. Chronic experimental glaucoma in primates II. Effect of extended intraocular pressure elevation on optic nerve head and axonal transport. Investig. Ophthalmol. Vis. Sci. 1980, 19, 137–152. [Google Scholar]
- Dandona, L.; Hendrickson, A.; Quigley, H.A. Selective effects of experimental glaucoma on axonal transport by retinal ganglion cells to the dorsal lateral geniculate nucleus. Investig. Ophthalmol. Vis. Sci. 1991, 32, 484–491. [Google Scholar]
- Quigley, H.A.; McKinnon, S.J.; Zack, D.J.; Pease, M.E.; Kerrigan-Baumrind, L.A.; Kerrigan, D.F.; Mitchell, R.S. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3460–3466. [Google Scholar]
- Pease, M.E.; McKinnon, S.J.; Quigley, H.A.; Kerrigan-Baumrind, L.A.; Zack, D.J. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2000, 41, 764–774. [Google Scholar]
- Minckler, D.S.; McLean, I.W.; Tso, M.O.M. Distribution of axonal and glial elements in the Rhesus optic nerve head studied by electron microscopy. Am. J. Ophthalmol. 1976, 82, 179–187. [Google Scholar] [CrossRef]
- Reilly, J.P.; Diamant, A.M. Spatial relationship iin electrostimulation: Application to electromagnetic field standards. IEEE Trans. Biomed. Eng. 2003, 50, 783–785. [Google Scholar] [CrossRef]
- Chidlow, G.; Ebneter, A.; Wood, J.P.M.; Casson, R.J. The optic nerve head is the site of axonal disruption, axonal cytoskeleton damage and putative axonal regeneration failure in a rat model of glaucoma. Acta Neuropathol. 2011, 121, 737–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahnenberger, R.W. Inhibition of fast anterograde axoplasmic transport by a pressure barrier. The effect of pressure gradient and maximal pressure. Acta Physiol. Scand. 1980, 109, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Balaratnasingam, C.; Pham, D.; Morgan, W.H.; Bass, L.; Cringle, S.J.; Yu, D.Y. Mitochondrial cytochrome c oxidase expression in the central nervous system is elevated at sites of pressure gradient elevation but not absolute pressure increase. J. Neurosci. Res. 2009, 87, 2973–2982. [Google Scholar] [CrossRef]
- Berdahl, J.P.; Allingham, R.R.; Johnson, D.H. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology 2008, 115, 763–768. [Google Scholar] [CrossRef]
- Jóhannesson, G.; Eklund, A.; Lindén, C. Intracranial and intraocular pressure at the lamina cribrosa: Gradient effects. Curr. Neurol. Neurosci. Rep. 2018, 18, 25. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Fu, J.; Hou, R.; Liu, K.; Jonas, J.B.; Wang, H.; Chen, W.; Li, Z.; Sang, J.; Zhang, Z.; et al. Optic neuropathy induced by experimentally reduced cerebrospinal fluid pressure in monkeys. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3067–3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
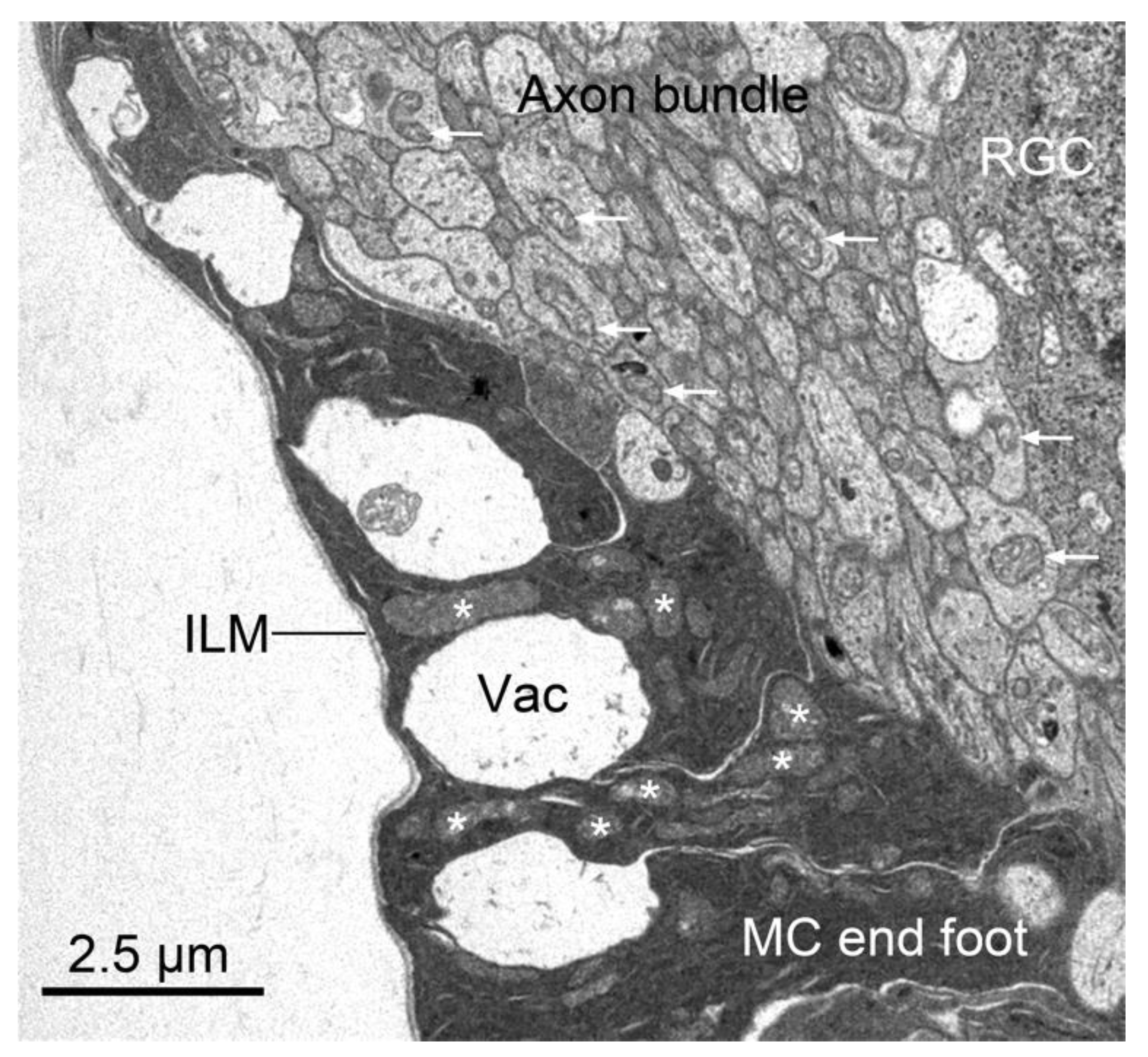
- Nguyen, J.V.; Soto, I.; Kim, K.-Y.; Bushong, E.A.; Oglesby, E.; Valiente-Soriano, F.J.; Yang, Z.; Davis, C.O.; Bedont, J.L.; Son, J.L.; et al. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc. Natl. Acad. Sci. USA 2011, 108, 1176–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, C.O.; Kim, K.Y.; Bushong, E.A.; Mills, E.A.; Boassa, D.; Shih, T.; Kinebuchi, M.; Phan, S.; Zhou, Y.; Bihlmeyer, N.A.; et al. Transcellular degradation of axonal mitochondria. Proc. Natl. Acad. Sci. USA 2014, 111, 9633–9638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, A.J.; Kaufman, P.L.; Hubbard, W.C. Morphology of single ganglion cells in the glaucomatous primate retina. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2304–2320. [Google Scholar]
- Weber, A.J.; Harman, C.D. BDNF preserves the dendritic morphology of a and b ganglion cells in the cat retina after optic nerve injury. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2456–2463. [Google Scholar] [CrossRef]
- Leung, C.K.; Weinreb, R.N.; Li, Z.W.; Lindsey, J.D.; Choi, N.; Cheung, C.Y.; Ye, C.; Qiu, K.; Chen, L.J.; Yung, W.H.; et al. Long-term in vivo imaging and measurement of dendritic shrinkage of retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1539–1547. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.A.; Howell, G.R.; Barbay, J.M.; Braine, C.E.; Sousa, G.L.; John, S.W.M.; Morgan, J.E. Retinal ganglion cell dendritic atrophy in DBA/2J glaucoma. PLoS ONE 2013, 8, e72282. [Google Scholar] [CrossRef]
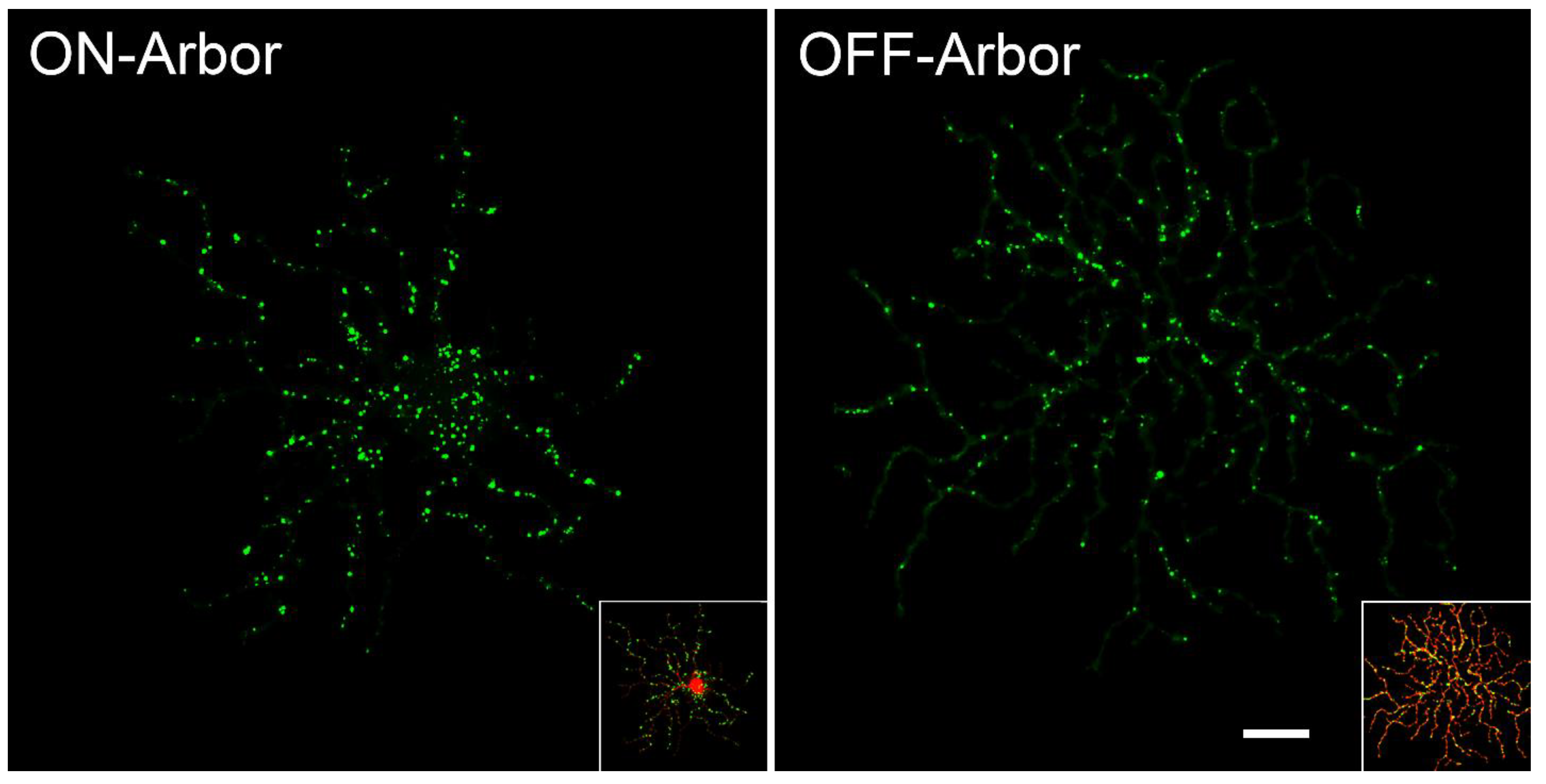
- Della Santina, L.; Inman, D.M.; Lupien, C.B.; Horner, P.J.; Wong, R.O.L. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J. Neurosci. 2013, 33, 17444–17457. [Google Scholar] [CrossRef]
- Agostinone, J.; Alarcon-Martinez, L.; Gamlin, C.; Yu, W.Q.; Wong, R.O.L.; Di Polo, A. Insulin signalling promotes dendrite and synapse regeneration and restores circuit function after axonal injury. Brain 2018, 141, 1963–1980. [Google Scholar] [CrossRef]
- El-Danaf, R.N.; Huberman, A.D. Characteristic patterns of dendritic remodeling in early-stage glaucoma: Evidence from genetically identified retinal ganglion cell types. J. Neurosci. 2015, 35, 2329–2343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, Y.; Jo, R.E.; Ullian, E.M.; Wong, R.O.L.; Della Santina, L. Selective vulnerability of specific retinal ganglion cell types and synapses after transient ocular hypertension. J. Neurosci. 2016, 36, 9240–9252. [Google Scholar] [CrossRef]
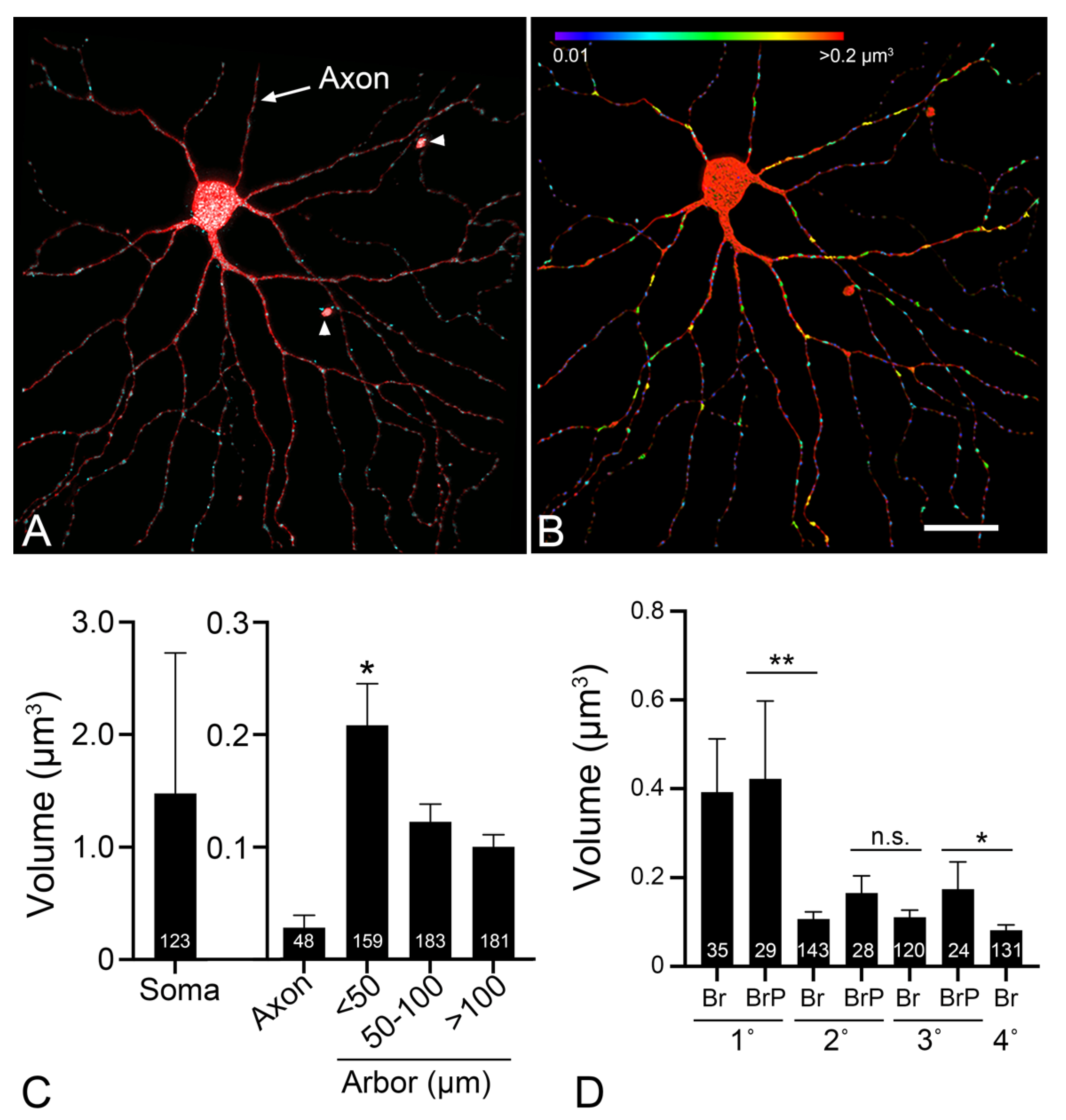
- Li, Z.; Okamoto, K.I.; Hayashi, Y.; Sheng, M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 2004, 119, 873–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Doménech, G.; Higgs, N.F.; Vaccaro, V.; Ros, H.; Arancibia-Carcamo, I.L.; MacAskill, A.F.; Kittler, J.T. Loss of dendritic complexity precedes neurodegeneration in a mouse model with disrupted mitochondrial distribution in mature dendrites. Cell Rep. 2016, 17, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuyama, T.; Tsubouchi, A.; Usui, T.; Imamura, H.; Uemura, T. Mitochondrial dysfunction induces dendritic loss via eIF2alpha phosphorylation. J. Cell Biol. 2017, 216, 815–834. [Google Scholar] [CrossRef] [Green Version]
- Carelli, V.; Ross-Cisneros, F.N.; Sadun, A.A. Mitochondrial dysfunction as a cause of optic neuropathies. Prog. Retin. Eye Res. 2004, 23, 53–89. [Google Scholar] [CrossRef]
- Williams, P.A.; Morgan, J.E.; Votruba, M. Opa1 deficiency in a mouse model of dominant optic atrophy leads to retinal ganglion cell dendropathy. Brain 2010, 133, 2942–2951. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.A.; Piechota, M.; von Ruhland, C.; Taylor, E.; Morgan, J.E.; Votruba, M. Opa1 is essential for retinal ganglion cell synaptic architecture and connectivity. Brain 2012, 135, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Davies, V.J.; Hollins, A.J.; Piechota, M.J.; Yip, W.; Davies, J.R.; White, K.E.; Nicols, P.P.; Boulton, M.E.; Vortruba, M. Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum. Mol. Genet. 2007, 16, 1307–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiduschka, P.; Schnichels, S.; Fuhrmann, N.; Hofmeister, S.; Schraermeyer, U.; Wissinger, B.; Alavi, M.V. Electrophysiological and histologic assessment of retinal ganglion cell fate in a mouse model of OPA1-associated autosomal dominant optic atrophy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1424–1431. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.H.; Higuchi, H.; Ikeda, S.; Macke, E.L.; Takimoto, T.; Pattnaik, B.R.; Liu, C.; Siepka, S.M.; Krentz, K.J.; Rubinstein, C.D.; et al. Mouse Tmem135 mutation reveals a mechanism involving mitochondrial dynamics that leads to age-dependent retinal pathologies. eLIFE 2016, 5, e19264. [Google Scholar] [CrossRef] [Green Version]
- Landowski, M.; Grindel, S.; Shahi, P.K.; Johnson, A.; Western, D.; Race, A.; Shi, F.; Benson, J.; Gao, M.; Santoirre, E.; et al. Modulation of Tmem135 leads to retinal pigmented epithelium pathologies in mice. Investig. Ophthalmol. Vis. Sci. 2020, 61, 16. [Google Scholar] [CrossRef]
- Lewis, S.A.; Takimoto, T.; Mehrvar, S.; Higuchi, H.; Doebley, A.L.; Stokes, G.; Sheibani, N.; Ikeda, S.; Ranji, M.; Ikeda, A. The effect of Tmem135 overexpression on the mouse heart. PLoS ONE 2018, 13, e0201986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golan, N.; Kartvelishvily, E.; Spiegel, I.; Salomon, D.; Sabanay, H.; Rechav, K.; Vainshtein, A.; Frechter, S.; Maik-Rachline, G.; Eshed-Eisenbach, Y.; et al. Genetic deletion of Cadm4 results in myelin abnormalities resembling Charcot-Marie-Tooth neuropathy. J. Neurosci. 2013, 33, 10950–10961. [Google Scholar] [CrossRef] [Green Version]
- Trevisiol, A.; Kusch, K.; Steyer, A.M.; Gregor, I.; Nardis, C.; Winkler, U.; Köhler, S.; Restrepo, A.; Möbius, W.; Werner, H.B.; et al. Structural myelin defects are associated with low axonal ATP levels but rapid recovery from energy deprivation in a mouse model of spastic paraplegia. PLoS Biol. 2020, 18, e3000943. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kim, K.Y.; Lindsey, J.D.; Dai, Y.; Heo, H.; Nguyen, D.H.; Ellisman, M.H.; Weinreb, R.N.; Ju, W.K. A selective inhibitor of Drp1, Mdivi-1, increases retinal ganglion cell survival in acute ischemic mouse retina. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2837–2843. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.Y.; Perkins, G.A.; Shim, M.S.; Bushong, E.; Alcasid, N.; Ju, S.; Ellisman, M.H.; Weinreb, R.N.; Ju, W.K. DRP1 inhibition rescues retinal ganglion cells and their axons by preserving mitochondrial integrity in a mouse model of glaucoma. Cell Death Dis. 2015, 6, e1839. [Google Scholar] [CrossRef] [PubMed]
- Slowicka, K.; Vereecke, L.; McGuire, C.; Sze, M.; Maelfait, J.; Kolpe, A.; Saelens, X.; Beyaert, R.; van Loo, G. Optineurin deficiency in mice is associated with increased sensitivity to Salmonella but does not affect proinflammatory NF-kappB signaling. Eur. J. Immunol. 2016, 46, 971–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, Z.L.; Akahori, M.; Obazawa, M.; Minami, M.; Noda, T.; Nakaya, N.; Tomarev, S.I.; Kawase, K.; Yamamoto, T.; Noda, S.; et al. Overexpression of optineurin E50K disrupts Rab8 interaction and leads to a progressive retinal degeneration in mice. Hum. Mol. Genet. 2010, 19, 2606–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, M.S.; Takihara, Y.; Kim, K.Y.; Iwata, T.; Yue, B.Y.J.T.; Inatani, M.; Weinreb, R.N.; Perkins, G.A.; Ju, W.K. Mitochondrial pathogenic mechanism and degradation in optineurin E50K mutation-mediated retinal ganglion cell degeneration. Sci. Rep. 2016, 6, 33830. [Google Scholar] [CrossRef]
- Tseng, H.C.; Riday, T.T.; McKee, C.; Braine, C.E.; Bomze, H.; Barak, I.; Marean-Reardon, C.; John, S.W.M.; Philpot, B.D.; Ehlers, M.D. Visual impairment in an optineurin mouse model of primary open angle glaucoma. Neurobiol. Aging 2015, 36, 2201–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernyshova, K.; Inoue, K.; Yamashita, S.I.; Fukuchi, T.; Kanki, T. Glaucoma-associated mutations in the optineurin gene have limited impact on Parkin-dependent mitophagy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3625–3635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayyad, Z.; Sirohi, K.; Radha, V.; Swarup, G. 661W is a retinal ganglion precursor-like cell line in which glaucoma-associated optineurin mutants induce cell death selectively. Sci. Rep. 2017, 7, 16855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ao, X.; Zou, L.; Wu, Y. Regulation of autophagy by the Rab GTPase network. Cell Death Differ. 2014, 21, 348–358. [Google Scholar] [CrossRef] [Green Version]
- Chalasani, M.L.S.; Kumari, A.; Radha, V.; Swarup, G. E50K-OPTN-induced retinal cell death involves the Rab GTPase-activating protein, TBC1D17 mediated block in autophagy. PLoS ONE 2014, 9, e95758. [Google Scholar] [CrossRef]
- Oakes, J.A.; Davies, M.C.; Collins, M.O. TBK1: A new player in ALS linking autophagy and neuroinflammation. Mol. Brain 2017, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Tucker, B.A.; Solivan-Timpe, F.M.; Roos, B.R.; Anfinson, K.R.; Robin, A.L.; Wiley, L.A.; Mullins, R.F.; Fingert, J.H. Duplication of TBK1 stimulates autophagy in iPSC-derived retinal cells from a patient with normal tension glaucoma. J. Stem Cell Res. Ther. 2014, 3, 161. [Google Scholar] [CrossRef]
- Fingert, J.H.; Miller, K.; Hedberg-Buenz, A.; Roos, B.R.; Lewis, C.J.; Mullins, R.F.; Anderson, M.G. Transgenic TBK1 mice have features of normal tension glaucoma. Hum. Mol. Genet. 2017, 26, 124–132. [Google Scholar]
- Manickam, A.H.; Michael, M.J.; Ramasamy, S. Mitochondrial genetics and therapeutic overview of Leber’s hereditary optic neuropathy. Indian J. Ophthalmol. 2017, 65, 1087–1092. [Google Scholar]
- Wallace, D.C.; Lott, M.T. Leber hereditary optic neuropathy: Exemplar of an mtDNA disease. Handb. Exp. Pharmacol. 2017, 240, 339–376. [Google Scholar] [PubMed]
- Sundaramurthy, S.; SelvaKumar, A.; Ching, J.; Dharani, V.; Sarangapani, S.; Yu-Wai-Man, P. Leber hereditary optic neuropathy—New insights and old challenges. Graefes Arch. Clin. Exp. Ophthalmol. 2020. online ahead of print. [Google Scholar] [CrossRef]
- Stenton, S.L.; Sheremet, N.L.; Catarino, C.B.; Andreeva, N.A.; Assouline, Z.; Barboni, P.; Barel, O.; Berutti, R.; Bychkov, I.; Caporali, L.; et al. Impaired complex I repair causes recessive Leber’s hereditary optic neuropathy. J. Clin. Investig. 2021, 131, e138267. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.N.; Loomis, S.J.; Kang, J.H.; Allingham, R.R.; Gharahkhani, P.; Khor, C.C.; Burdon, K.P.; Aschard, H.; Chasman, D.I.; Igo, R.P., Jr.; et al. Genome-wide association analysis identifies TXNRD2, ATXN2, and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat. Genet. 2016, 48, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibbing, D.; Pfeufer, A.; Perisic, T.; Mannes, A.M.; Fritz-Wolf, K.; Unwin, S.; Sinner, M.F.; Gieger, C.; Gloeckner, C.J.; Wichmann, H.E.; et al. Mutations in the mitochondrial thioredoxin reductase gene TXNRD2 cause dilated cardiomyopathy. Eur. Heart J. 2011, 32, 1121–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khawaja, A.P.; Cooke Bailey, J.N.; Kang, J.H.; Allingham, R.R.; Hauser, M.A.; Brilliant, M.; Budenz, D.L.; Christen, W.G.; Fingert, J.H.; Gaasterland, D.; et al. Assessing the association of mitochondrial genetic variation with primary open-angle glaucoma using gene-set analysis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5046–5052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, W.K.; Kim, K.Y.; Lindsey, J.D.; Angert, M.; Duong-Polk, K.; Scott, R.T.; Kim, J.J.; Kukhmazov, I.; Ellisman, M.H.; Perkins, G.A.; et al. Intraocular pressure elevation induces mitochondrial fission and triggers OPA1 release in glaucomatous optic nerve. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4903–4911. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Perkins, G.A.; Kim, K.Y.; Kong, Y.; Lee, Y.; Choi, S.H.; Liu, Y.; Skowronska-Krawczyk, D.; Weinreb, R.N.; Zangwill, L.; et al. Loss of AKAP1 triggers Drp1 dephosphorylation-mediated mitochodnrial fission and loss in retinal ganglion cells. Cell Death Dis. 2020, 11, 254. [Google Scholar] [CrossRef] [Green Version]
- Flippo, K.H.; Gnanasekaran, A.; Perkins, G.A.; Ajmal, A.; Merrill, R.A.; Dickey, A.S.; Taylor, S.S.; McKnight, G.S.; Chauhan, A.K.; Usachev, Y.M.; et al. AKAP1 protects from cerebral ischemic stroke by inhibiting DRP1-dependent mitochondrial fission. J. Neurosci. 2018, 38, 8233–8242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Amero, K.K.; Morales, J.; Bosley, T.M. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2533–2541. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Bosley, T.M.; Morales, J. Analysis of nuclear and mitochondrial genes in patients with pseudoexfoliation glaucoma. Mol. Vis. 2008, 14, 29–36. [Google Scholar]
- Abu-Amero, K.K.; Morales, J.; Osman, M.N.; Bosley, T.M. Nuclear and mitochondrial analysis of patients with primary angle-closure glaucoma. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5591–5596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jassim, A.H.; Coughlin, L.; Harun-Or-Rashid, M.; Kang, P.T.; Chen, Y.R.; Inman, D.M. Higher reliance on glycolysis limits glycolytic responsiveness in degenerating glaucomatous optic nerve. Mol. Neurobiol. 2019, 56, 7097–7112. [Google Scholar] [CrossRef] [Green Version]
- Downs, J.C. Optic nerve head biomechanics in aging and disease. Exp. Eye Res. 2015, 133, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Moore, S.; Jakobs, T.C. Optic nerve astrocyte reactivity protects function in experimental glaucoma and other nerve injuries. J. Exp. Med. 2017, 214, 1411–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, M.L.; Collyer, J.W.; Calkins, D.J. Astrocyte remodeling without gliosis precedes optic nerve axonopathy. Acta Neuropathol. Comm. 2018, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.L.; Pasini, S.; Lambert, W.S.; D’Alessandro, K.B.; Yao, V.; Risner, M.L.; Calkins, D.J. Redistribution of metabolic resources through astrocyte networks mitigates neurodegenerative stress. Proc. Natl. Acad. Sci. USA 2020, 117, 18810–18821. [Google Scholar] [CrossRef]
- Calkins, D.J. Adaptive responses to neurodegenerative stress in glaucoma. Prog. Retin. Eye Res. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Davis, B.M.; Tian, K.; Pahlitzsch, M.; Brenton, J.; Ravindran, N.; Butt, G.; Malaguarnera, G.; Normando, E.M.; Guo, L.; Cordeiro, M.F. Topical coenzyme Q10 demonstrates mitochondrial-mediated neuroprotection in a rodent model of ocular hypertension. Mitochondrion 2017, 36, 114–123. [Google Scholar] [CrossRef]
- Arranz-Romera, A.; Davis, B.M.; Bravo-Osuna, I.; Esteban-Pérez, S.; Molina-Martínez, I.T.; Shamsher, E.; Ravindran, N.; Guo, L.; Cordeiro, M.F.; Herrero-Vanrell, R. Simultaneous co-delivery of neuroprotective drugs from multi-loaded PLGA microspheres for the treatment of glaucoma. J. Control. Release 2019, 297, 26–38. [Google Scholar] [CrossRef]
- Edwards, G.; Lee, Y.; Kim, M.; Bhanvadia, S.; Kim, K.Y.; Ju, W.K. Effect of ubiquinol on glaucomatous neurodegeneration and oxidative stress: Studies for retinal ganglion cell survival and/or visiual function. Antioxidants 2020, 9, 952. [Google Scholar] [CrossRef]
- Quaranta, L.; Riva, I.; Biagioli, E.; Rulli, E.; Rulli, E.; Poli, D.; Legramandi, L.; Group, C.S. Evaluating the effects of an ophthalmic solution of coenzyme Q10 and vitamin E in open-angle glaucoma patients: A study protocol. Adv. Ther. 2019, 36, 2506–2514. [Google Scholar] [CrossRef]
- Williams, P.A.; Harder, J.M.; Foxworth, N.E.; Cochran, K.E.; Philip, V.M.; Porciatti, V.; Smithies, O.; John, S.W.M. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 2017, 355, 756–760. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.A.; Harder, J.M.; John, S.W.M. Glaucoma as a metabolic optic neuropathy: Making the case for nicotinamide treatment in glaucoma. J. Glaucoma 2017, 26, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Crane, F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Gerdts, J.; Brace, E.J.; Sasaki, Y.; DiAntonio, A.; Milbrandt, J. SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 2015, 348, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Loreto, A.; Di Stefano, M.; Gering, M.; Conforti, L. Wallerian degeneration is executed by an NMN-SARM1-dependent late Ca2+ influx but only modestly influenced by mitochondria. Cell Rep. 2015, 13, 2539–2552. [Google Scholar] [CrossRef] [Green Version]
- Gerdts, J.; Summers, D.W.; Milbrandt, J.; DiAntonio, A. Axon self-destruction: Newe links among SARM1, MAPKs, and NAD+ metabolism. Neuron 2016, 89, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Coleman, M.P.; Höke, A. Programmed axon degeneration: From mouse to mechanism to medicine. Nat. Rev. Neurosci. 2020, 21, 183–196. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, T.; Lee, C.H.; Chang, Q.; Yang, J.; Zhang, Z. The NAD+-mediated self-inhibition mechanisms of pro-neurodegenerative SARM1. Nature 2020, 588, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Szeto, H.H.; Liu, S. Cardiolipin-targeted peptides rejuvenate mitochondrial function, remodel mitochodria, and promote tissue regeneration during aging. Arch. Biochem. Biophys. 2018, 660, 137–148. [Google Scholar] [CrossRef]
- Szeto, H.H.; Liu, S.; Seshan, S.V.; Cohen-Gould, L.; Manichev, V.; Feldman, L.C.; Gustafsson, T. Mitochondria protection after acute ischemia prevents prolonged upregulation of IL-1beta and IL-18 and arrests CKD. J. Am. Soc. Nephrol. 2017, 28, 1437–1449. [Google Scholar] [CrossRef] [Green Version]
- Grosser, J.A.; Fehrman, R.L.; Keefe, D.; Redmon, M.; Nickells, R.W. The effects of a mitochondrial targeted peptide (elamipretide/SS31) on BAX recruitment and activation during apoptosis. BMC Res. Notes 2021, 14, 198. [Google Scholar] [CrossRef]
- Wu, X.; Pang, Y.; Zhang, Z.; Li, X.; Wang, C.; Lei, Y.; Li, A.; Yu, L.; Ye, J. Mitochondria-targeted antioxidant peptide SS-31 mediates neuroprotection in a rat experimental glaucoma model. Acta Biochim. Biophys. Sin. 2019, 51, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Tse, B.C.; Dvoriantchikova, G.; Tao, W.; Gallo, R.A.; Lee, J.Y.; Ivanov, D.; Tse, D.T.; Pelaez, D. Mitochondrial targeted therapy with elamipretide (MTP-131) as an adjunct to tumor necrosis factor inhibition for traumatic optic neuropathy in the acute setting. Exp. Eye Res. 2020, 199, 108178. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Yin, X.L.; Reddy, A.P. Synergistic protective effects of mitochondrial division inhibitor 1 and mitochondria-targeted small peptide SS31 in Alzheimer’s disease. J. Alzheimers Dis. 2018, 62, 1549–1565. [Google Scholar] [CrossRef] [Green Version]
- Bertero, E.; Maack, C.; O’Rourke, B. Mitochondrial transplantation in humans: “magical” cure or cause for concern? J. Clin. Investig. 2018, 128, 5191–5194. [Google Scholar] [CrossRef] [PubMed]
- Lightowlers, R.N.; Chrzanowska-Lightowlers, Z.M.A.; Russell, O.M. Mitochondrial transplantation—A possible therapeutic for mitochondrial dysfunction? EMBO Rep. 2020, 21, e50964. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhao, M.; Fu, C.; Fu, A. Intravenous administration of mitochondria for treating experimental Parkinson’s disease. Mitochondrion 2017, 34, 91–100. [Google Scholar] [CrossRef]
- Nascimento-dos-Santos, G.; de-Souza-Ferreira, E.; Lani, R.; Faria, C.C.; Araújo, V.G.; Teixeira-Pinheiro, L.C.; Vasconcelos, T.; Gonçalo, T.; Santiago, M.F.; Linden, R.; et al. Neuroprotection from optic nerve injury and modulation of oxidative metabolism by transplantation of active mitochondria to the retina. BBA Mol. Basis Dis. 2020, 1866, 165686. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muench, N.A.; Patel, S.; Maes, M.E.; Donahue, R.J.; Ikeda, A.; Nickells, R.W. The Influence of Mitochondrial Dynamics and Function on Retinal Ganglion Cell Susceptibility in Optic Nerve Disease. Cells 2021, 10, 1593. https://doi.org/10.3390/cells10071593
Muench NA, Patel S, Maes ME, Donahue RJ, Ikeda A, Nickells RW. The Influence of Mitochondrial Dynamics and Function on Retinal Ganglion Cell Susceptibility in Optic Nerve Disease. Cells. 2021; 10(7):1593. https://doi.org/10.3390/cells10071593
Chicago/Turabian StyleMuench, Nicole A., Sonia Patel, Margaret E. Maes, Ryan J. Donahue, Akihiro Ikeda, and Robert W. Nickells. 2021. "The Influence of Mitochondrial Dynamics and Function on Retinal Ganglion Cell Susceptibility in Optic Nerve Disease" Cells 10, no. 7: 1593. https://doi.org/10.3390/cells10071593





