Two Sides of the Coin: Mast Cells as a Key Regulator of Allergy and Acute/Chronic Inflammation
Abstract
:1. Introduction
2. Origin and Heterogeneity of MCs
3. Pro-Allergic and Inflammatory Actions of MCs
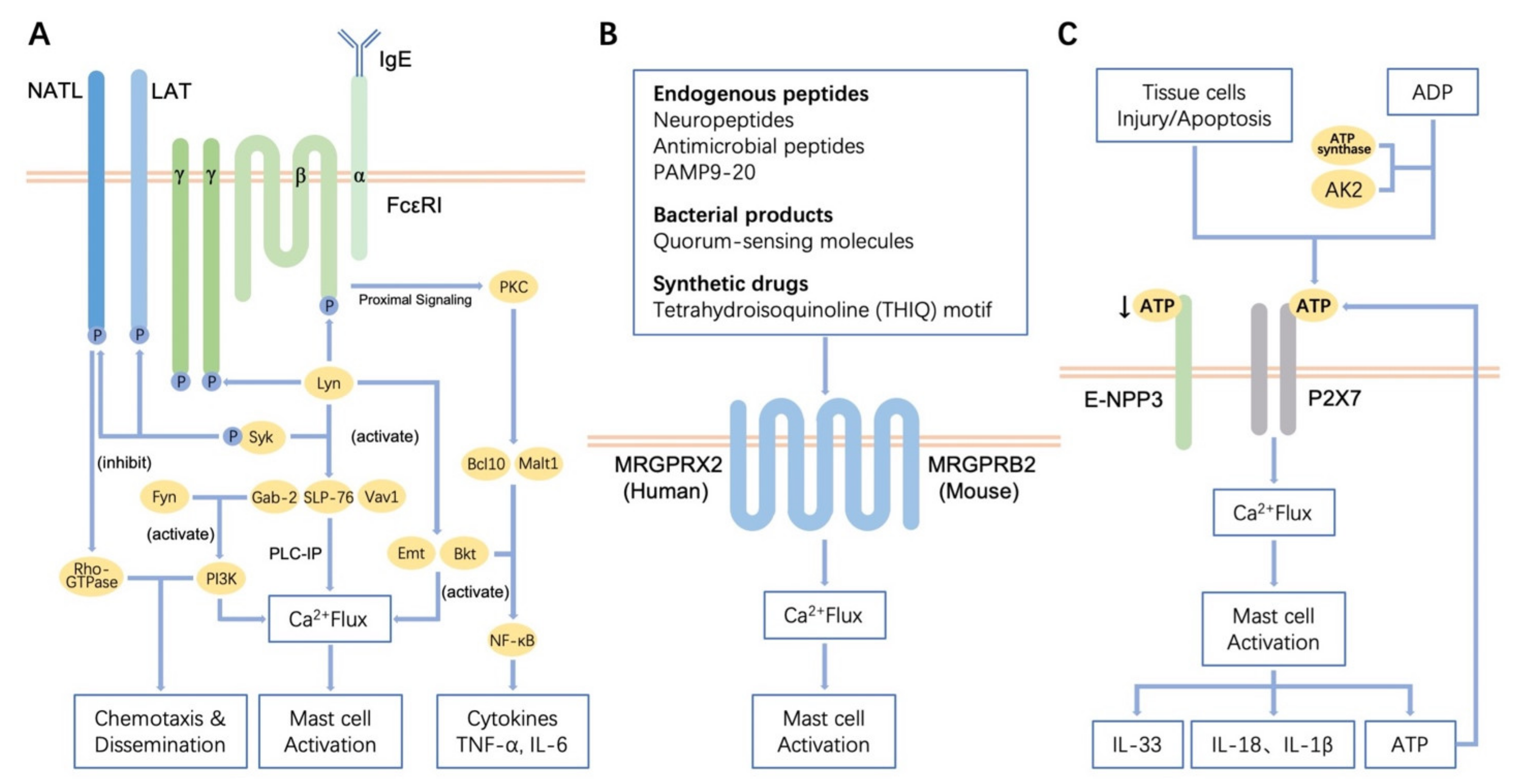
3.1. Regulation of Receptor-Mediated MCs Activation
3.2. Fibrogenic Actions of MCs
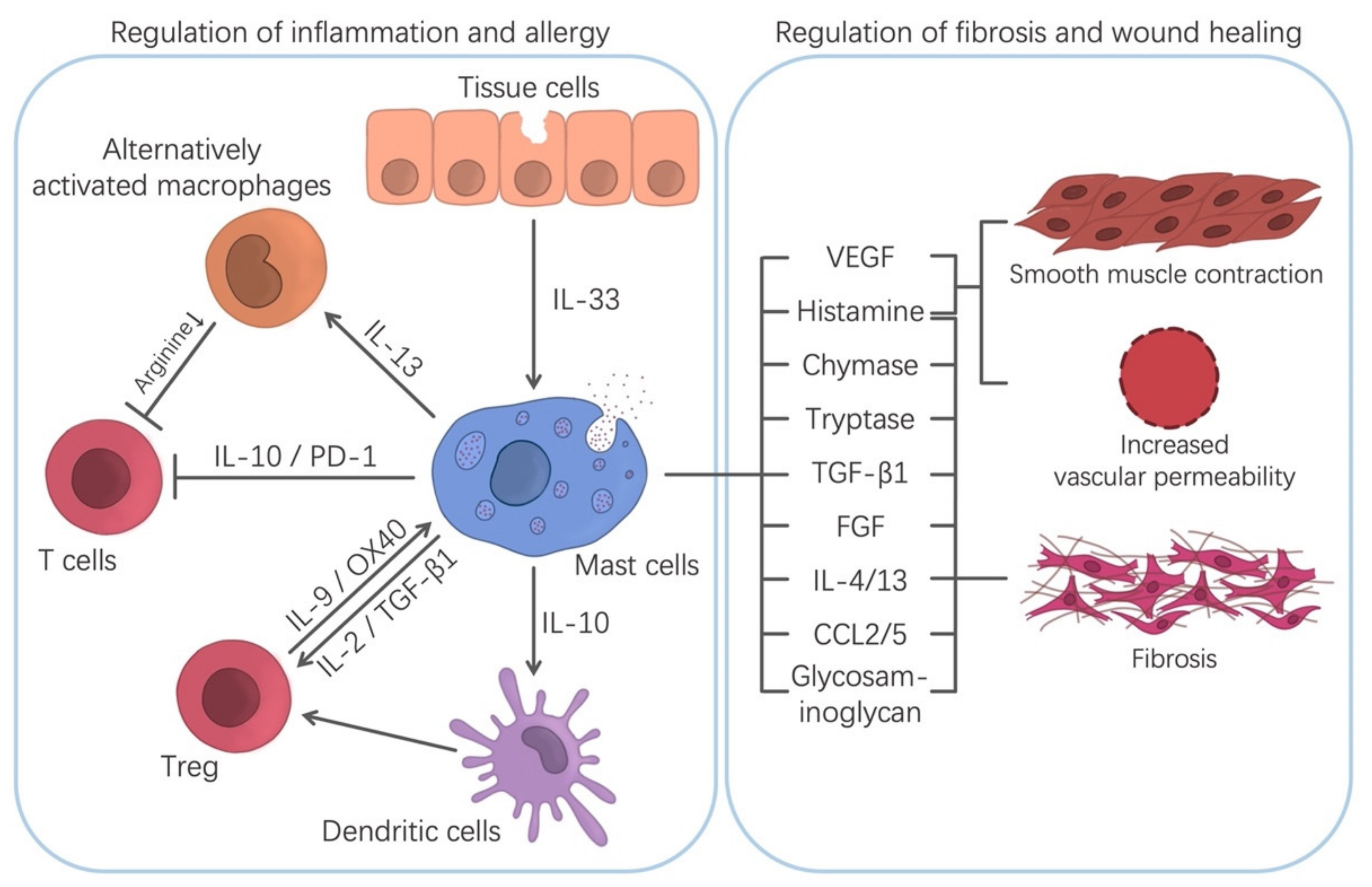
4. Regulatory-Type Actions of MCs in Allergy and Inflammation
4.1. Regulation of Chronic Inflammation/Fibrosis/Wound Healing
4.2. Regulation of Allergic and Inflammatory Diseases
5. Future Prospects in the Manipulation of the MCs Functions
5.1. Novel Prospective Mast Cell-Targeting Strategies
5.2. Current Problems and the Direction of Future Development
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collington, S.J.; Williams, T.J.; Weller, C.L. Mechanisms underlying the localization of mast cells in tissues. Trends Immunol. 2011, 32, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Gurish, M.F.; Tao, H.; Abonia, J.P.; Arya, A.; Friend, D.S.; Parker, C.M.; Austen, K.F. Intestinal mast cell progenitors require CD49dbeta7 (alpha4beta7 integrin) for tissue-specific homing. J. Exp. Med. 2001, 194, 1243–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallgren, J.; Gurish, M.F. Pathways of murine mast cell development and trafficking: Tracking the roots and routes of the mast cell. Immunol. Rev. 2007, 217, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Bulfone-Paus, S.; Nilsson, G.; Draber, P.; Blank, U.; Levi-Schaffer, F. Positive and negative signals in mast cell activation. Trends Immunol. 2017, 38, 657–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overed-Sayer, C.; Rapley, L.; Mustelin, T.; Clarke, D.L. Are mast cells instrumental for fibrotic diseases? Front. Pharmacol. 2013, 4, 174. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.F.; Lind, E.F.; Gondek, D.C.; Bennett, K.A.; Gleeson, M.W.; Pino-Lagos, K.; Scott, Z.A.; Coyle, A.J.; Reed, J.L.; Van Snick, J.; et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 2006, 442, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Song, J.; Hua, J.; Yang, M.; Ma, Y.; Yu, T.; Feng, J.; Liu, B.; Wang, X.; Li, Y.; et al. Mast cells are essential intermediaries in regulating IL-33/ST2 signaling for an immune network favorable to mucosal healing in experimentally inflamed colons. Cell Death Dis. 2018, 9, 1173. [Google Scholar] [CrossRef] [Green Version]
- Takasato, Y.; Kurashima, Y.; Kiuchi, M.; Hirahara, K.; Murasaki, S.; Arai, F.; Izawa, K.; Kaitani, A.; Shimada, K.; Saito, Y.; et al. Orally desensitized mast cells form a regulatory network with Treg cells for the control of food allergy. Mucosal Immunol. 2021, 14, 640–651. [Google Scholar] [CrossRef]
- Wulff, B.C.; Wilgus, T.A. Mast cell activity in the healing wound: More than meets the eye? Exp. Dermatol. 2013, 22, 507–510. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.A. Oral immunotherapy for food allergy. J. Investig. Allergol. Clin. Immunol. 2017, 27, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Cortes, S.; Jaqueti, P.; Arasi, S.; Machinena, A.; Alvaro-Lozano, M.; Fernandez-Rivas, M. Safety of food oral immunotherapy: What we know, and what we need to learn. Immunol. Allergy. Clin. N. Am. 2020, 40, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Maurer, M. Mast cells–key effector cells in immune responses. Trends Immunol. 2007, 28, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Ehara, T.; Shigematsu, H. Mast cells in the kidney. Nephrology 2003, 8, 130–138. [Google Scholar] [CrossRef]
- Farrell, D.J.; Hines, J.E.; Walls, A.F.; Kelly, P.J.; Burt, A.D. Intrahepatic mast cells in chronic liver diseases. Hepatology 2010, 22, 1175–1181. [Google Scholar]
- Weiskirchen, R.; Meurer, S.K.; Liedtke, C.; Huber, M. Mast cells in liver fibrogenesis. Cells 2019, 8, 1429. [Google Scholar] [CrossRef] [Green Version]
- Voehringer, D. Protective and pathological roles of mast cells and basophils. Nat. Rev. Immunol. 2013, 13, 362–375. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Wohrl, S.; Bielory, L. Mast cell biology at molecular level: A comprehensive review. Clin. Rev. Allergy. Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Bulle, M.J.; Msallam, R.; Gautier, G.; Launay, P.; Chen, J.; Ginhoux, F.; Bajenoff, M. Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity 2018, 48, 1160–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, G.; Dahlin, J.S. New insights into the origin of mast cells. Allergy 2019, 74, 844–845. [Google Scholar] [CrossRef]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurish, M.F.; Austen, K.F. Developmental origin and functional specialization of mast cell subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast cells in inflammation and disease: Recent progress and ongoing concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.F.; Barrett, N.A.; Austen, K.F. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat. Immunol. 2016, 17, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Kurashima, Y.; Amiya, T.; Fujisawa, K.; Shibata, N.; Suzuki, Y.; Kogure, Y.; Hashimoto, E.; Otsuka, A.; Kabashima, K.; Sato, S.; et al. The enzyme Cyp26b1 mediates inhibition of mast cell activation by fibroblasts to maintain skin-barrier homeostasis. Immunity 2014, 40, 530–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurashima, Y.; Amiya, T.; Nochi, T.; Fujisawa, K.; Haraguchi, T.; Iba, H.; Tsutsui, H.; Sato, S.; Nakajima, S.; Iijima, H.; et al. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat. Commun. 2012, 3, 1034. [Google Scholar] [CrossRef]
- Fujisawa, D.; Kashiwakura, J.; Kita, H.; Kikukawa, Y.; Fujitani, Y.; Sasaki-Sakamoto, T.; Kuroda, K.; Nunomura, S.; Hayama, K.; Terui, T.; et al. Expression of mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J. Allergy Clin. Immunol. 2014, 134, 622–633. [Google Scholar] [CrossRef]
- Xing, W.; Austen, K.F.; Gurish, M.F.; Jones, T.G. Protease phenotype of constitutive connective tissue and of induced mucosal mast cells in mice is regulated by the tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 14210–14215. [Google Scholar] [CrossRef] [Green Version]
- Andersson, C.K.; Mori, M.; Bjermer, L.; Lofdahl, C.G.; Erjefalt, J.S. Novel site-specific mast cell subpopulations in the human lung. Thorax 2009, 64, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Wang, Z.; Mascarenhas, N.; Eckmann, L.; Miyamoto, Y.; Sun, X.; Kawakami, T.; Di Nardo, A. Skin microbiome promotes mast cell maturation by triggering stem cell factor production in keratinocytes. J. Allergy Clin. Immunol. 2017, 139, 1205–1216. [Google Scholar] [CrossRef] [Green Version]
- Frossi, B.; Mion, F.; Sibilano, R.; Danelli, L.; Pucillo, C.E.M. Is it time for a new classification of mast cells? What do we know about mast cell heterogeneity? Immunol. Rev. 2018, 282, 35–46. [Google Scholar] [CrossRef]
- Pritchard, D.I.; Falcone, F.H.; Mitchell, P.D. The evolution of IgE-mediated type I hypersensitivity and its immunological value. Allergy 2021, 76, 1024–1040. [Google Scholar] [CrossRef]
- Cauvi, D.M.; Tian, X.; von Loehneysen, K.; Robertson, M.W. Transport of the IgE receptor alpha-chain is controlled by a multicomponent intracellular retention signal. J. Biol. Chem. 2006, 281, 10448–10460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitoh, S.; Arudchandran, R.; Manetz, T.S.; Zhang, W.; Sommers, C.L.; Love, P.E.; Rivera, J.; Samelson, L.E. LAT is essential for FcεRI-mediated mast cell activation. Immunity 2000, 12, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Potuckova, L.; Draberova, L.; Halova, I.; Paulenda, T.; Draber, P. Positive and negative regulatory roles of C-terminal Src Kinase (CSK) in FcepsilonRI-mediated mast cell activation, independent of the transmembrane adaptor PAG/CSK-binding protein. Front. Immunol. 2018, 9, 1771. [Google Scholar] [CrossRef]
- Iva, P.; Lubica, D.; Michal, S.; Petr, D. Multiple regulatory roles of the mouse transmembrane adaptor protein NTAL in gene transcription and mast cell physiology. PLoS ONE 2014, 9, e105539. [Google Scholar]
- Shin, J.; Zhang, P.; Wang, S.; Wu, J.; Guan, Z.; Zhong, X.P. Negative control of mast cell degranulation and the anaphylactic response by the phosphatase lipin1. Eur. J. Immunol. 2013, 43, 240–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanashima, K.; Chieosilapatham, P.; Yoshimoto, E.; Okumura, K.; Ogawa, H.; Niyonsaba, F. Innate defense regulator IDR-1018 activates human mast cells through G protein-, phospholipase C-, MAPK- and NF-kB-sensitive pathways. Immunol. Res. 2017, 65, 920–931. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, D.Y.; Xu, H.; Zhou, H.; Yang, Q.Y.; Liu, F.; Zhou, G.P. Down-regulation of microRNA-223 promotes degranulation via the PI3K/Akt pathway by targeting IGF-1R in mast cells. PLoS ONE 2015, 10, e0123575. [Google Scholar] [CrossRef]
- Klemm, S.; Gutermuth, J.; Hultner, L.; Sparwasser, T.; Behrendt, H.; Peschel, C.; Mak, T.W.; Jakob, T.; Ruland, J. The Bcl10-Malt1 complex segregates Fc epsilon RI-mediated nuclear factor kappa B activation and cytokine production from mast cell degranulation. J. Exp. Med. 2006, 203, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Parravicini, V.; Gadina, M.; Kovarova, M.; Odom, S.; Gonzalez-Espinosa, C.; Furumoto, Y.; Saitoh, S.; Samelson, L.E.; O’Shea, J.J.; Rivera, J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 2002, 3, 741–748. [Google Scholar] [CrossRef]
- Samayawardhena, L.A.; Kapur, R.; Craig, A.W. Involvement of Fyn kinase in Kit and integrin-mediated Rac activation, cytoskeletal reorganization, and chemotaxis of mast cells. Blood 2007, 109, 3679–3686. [Google Scholar] [CrossRef]
- Tumova, M.; Koffer, A.; Simicek, M.; Draberova, L.; Draber, P. The transmembrane adaptor protein NTAL signals to mast cell cytoskeleton via the small GTPase Rho. Eur. J. Immunol. 2010, 40, 3235–3245. [Google Scholar] [CrossRef] [PubMed]
- Halova, I.; Draberova, L.; Draber, P. Mast cell chemotaxis-chemoattractants and signaling pathways. Front. Immunol. 2012, 3, 119. [Google Scholar] [CrossRef] [Green Version]
- Gomez, G.; Gonzalez-Espinosa, C.; Odom, S.; Baez, G.; Cid, M.E.; Ryan, J.J.; Rivera, J. Impaired FcepsilonRI-dependent gene expression and defective eicosanoid and cytokine production as a consequence of Fyn deficiency in mast cells. J. Immunol. 2005, 175, 7602–7610. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Renga, G.; Oikonomou, V.; Galosi, C.; Pariano, M.; Iannitti, R.G.; Borghi, M.; Puccetti, M.; De Zuani, M.; Pucillo, C.E.; et al. A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. Nat. Commun 2017, 8, 14017. [Google Scholar] [CrossRef] [PubMed]
- Feuser, K.; Feilhauer, K.; Staib, L.; Bischoff, S.C.; Lorentz, A. Akt cross-links IL-4 priming, stem cell factor signaling, and IgE-dependent activation in mature human mast cells. Mol. Immunol. 2011, 48, 546–552. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, L.; Lin, Q. Release of IL-4 from mast cells induced by IL-12 relates to AKT and ERK signaling pathways. Jiangsu Med. J. 2008, 34, 1262–1265. [Google Scholar]
- Masuda, A.; Matsuguchi, T.; Yamaki, K.; Hayakawa, T.; Kubo, M.; LaRochelle, W.J.; Yoshikai, Y. Interleukin-15 induces rapid tyrosine phosphorylation of STAT6 and the expression of interleukin-4 in mouse mast cells. J. Biol. Chem. 2000, 275, 29331–29337. [Google Scholar] [CrossRef] [Green Version]
- Silver, M.R.; Margulis, A.; Wood, N.; Goldman, S.J.; Kasaian, M.; Chaudhary, D. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflamm. Res. 2010, 59, 207–218. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Nolin, J.D.; Lai, Y.; Ogden, H.L.; Manicone, A.M.; Murphy, R.C.; An, D.; Frevert, C.W.; Ghomashchi, F.; Naika, G.S.; Gelb, M.H.; et al. Secreted PLA2 group X orchestrates innate and adaptive immune responses to inhaled allergen. JCI Insight 2017, 2, e94929. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Eckart, M.R.; Morgan, A.A.; Mukai, K.; Butte, A.J.; Tsai, M.; Galli, S.J. Identification of an IFN-gamma/mast cell axis in a mouse model of chronic asthma. J. Clin. Investig. 2011, 121, 3133–3143. [Google Scholar] [CrossRef] [Green Version]
- Lunderius-Andersson, C.; Enoksson, M.; Nilsson, G. Mast Cells Respond to Cell Injury through the Recognition of IL-33. Front. Immunol. 2012, 3, 82. [Google Scholar] [CrossRef] [Green Version]
- Hung, L.Y.; Lewkowich, I.P.; Dawson, L.A.; Downey, J.; Yang, Y.; Smith, D.E.; Herbert, D.R. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc. Natl. Acad. Sci. USA 2013, 110, 282–287. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Yin, H.; Yuan, B.; Liu, T.; Luo, L.; Huang, P.; Dai, L.; Zeng, K. IL-33 improves wound healing through enhanced M2 macrophage polarization in diabetic mice. Mol. Immunol. 2017, 90, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Castillo, J.M.; Das, M.; Artru, E.; Yoon, J.; Galand, C.; Geha, R.S. Mast cell-derived IL-13 downregulates IL-12 production by skin dendritic cells to inhibit the TH1 cell response to cutaneous antigen exposure. J. Allergy Clin. Immunol. 2021, 147, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.O.; Plewes, M.R.; Pullen, N.A. Soluble transforming growth factor beta-1 enhances murine mast cell release of Interleukin 6 in IgE-independent and Interleukin 13 in IgE-dependent settings in vitro. PLoS ONE 2018, 13, e0207704. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.; Takeishi, T.; Thompson, H.; Langley, K.E.; Zsebo, K.M.; Metcalfe, D.D.; Geissler, E.N.; Galli, S.J. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc. Natl. Acad. Sci. USA 1991, 88, 6382–6386. [Google Scholar] [CrossRef] [Green Version]
- Lipitsa, T.; Naukkarinen, A.; Laitala, J.; Harvima, I.T. Complement C3 is expressed by mast cells in cutaneous vasculitis and is degraded by chymase. Arch. Dermatol. Res. 2016, 308, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Subramanian, H.; Gupta, K.; Ali, H. Regulation of C3a receptor signaling in human mast cells by G protein coupled receptor kinases. PLoS ONE 2011, 6, e22559. [Google Scholar] [CrossRef] [Green Version]
- Khodoun, M.; Strait, R.; Orekov, T.; Hogan, S.; Karasuyama, H.; Herbert, D.R.; Kohl, J.; Finkelman, F.D. Peanuts can contribute to anaphylactic shock by activating complement. J. Allergy Clin. Immunol. 2009, 123, 342–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, H. Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a. Immunol. Lett. 2010, 128, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.P.; Limjunyawong, N.; Gour, N.; Pundir, P.; Dong, X. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron 2019, 101, 412–420.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, J.; Ge, S.; Zeng, Y.; Wang, N.; Wu, Y. Roxithromycin inhibits compound 48/80-induced pseudo-allergy via the MrgprX2 pathway both in vitro and in vivo. Cell. Immunol. 2020, 358, 104239. [Google Scholar] [CrossRef]
- Wolf, K.; Kuhn, H.; Boehm, F.; Gebhardt, L.; Glaudo, M.; Agelopoulos, K.; Stander, S.; Ectors, P.; Zahn, D.; Riedel, Y.K.; et al. A group of cationic amphiphilic drugs activates MRGPRX2 and induces scratching behavior in mice. J. Allergy Clin. Immunol. 2021, in press. [Google Scholar] [CrossRef]
- Chen, E.; Chuang, L.S.; Giri, M.; Villaverde, N.; Hsu, N.Y.; Sabic, K.; Joshowitz, S.; Gettler, K.; Nayar, S.; Chai, Z.; et al. Inflamed ulcerative colitis regions associated with MRGPRX2-mediated mast cell degranulation and cell activation modules, defining a new therapeutic target. Gastroenterology 2021, 160, 1709–1724. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Guo, Q.; Price, R.; Ali, H. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: Resistance to receptor phosphorylation, desensitization, and internalization. J. Biol. Chem. 2011, 286, 44739–44749. [Google Scholar] [CrossRef] [Green Version]
- Ali, H. Emerging roles for MAS-related G protein-coupled receptor-X2 in host defense peptide, opioid, and neuropeptide-mediated inflammatory reactions. Adv. Immunol. 2017, 136, 123–162. [Google Scholar]
- Wilhelm, K.; Ganesan, J.; Muller, T.; Durr, C.; Grimm, M.; Beilhack, A.; Krempl, C.D.; Sorichter, S.; Gerlach, U.V.; Juttner, E.; et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat. Med. 2010, 16, 1434–1438. [Google Scholar] [CrossRef]
- Shimokawa, C.; Kanaya, T.; Hachisuka, M.; Ishiwata, K.; Hisaeda, H.; Kurashima, Y.; Kiyono, H.; Yoshimoto, T.; Kaisho, T.; Ohno, H. Mast cells are crucial for induction of group 2 innate lymphoid cells and clearance of helminth infections. Immunity 2017, 46, 863–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, S.H.; Kinoshita, M.; Kusu, T.; Kayama, H.; Okumura, R.; Ikeda, K.; Shimada, Y.; Takeda, A.; Yoshikawa, S.; Obata-Ninomiya, K.; et al. The ectoenzyme E-NPP3 negatively regulates ATP-dependent chronic allergic responses by basophils and mast cells. Immunity 2015, 42, 279–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yegutkin, G.G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 2008, 1783, 673–694. [Google Scholar] [CrossRef] [Green Version]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Yang, M.Q.; Yu, T.Y.; Yin, Y.Y.; Liu, Y.; Wang, X.D.; He, Z.G.; Yin, L.; Chen, C.Q.; Li, J.Y. Mast cell tryptase promotes inflammatory bowel disease-induced intestinal fibrosis. Inflamm. Bowel Dis. 2021, 27, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cells and collagen fibrillogenesis. Histochem. Cell Biol. 2020, 154, 21–40. [Google Scholar] [CrossRef]
- Miyazawa, S.; Hotta, O.; Doi, N.; Natori, Y.; Nishikawa, K.; Natori, Y. Role of mast cells in the development of renal fibrosis: Use of mast cell-deficient rats. Kidney Int. 2004, 65, 2228–2237. [Google Scholar] [CrossRef] [Green Version]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci 2014, 71, 549–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimbori, C.; Upagupta, C.; Bellaye, P.S.; Ayaub, E.A.; Sato, S.; Yanagihara, T.; Zhou, Q.; Ognjanovic, A.; Ask, K.; Gauldie, J.; et al. Mechanical stress-induced mast cell degranulation activates TGF-beta1 signalling pathway in pulmonary fibrosis. Thorax 2019, 74, 455–465. [Google Scholar] [CrossRef]
- Hargrove, L.; Kennedy, L.; Demieville, J.; Jones, H.; Meng, F.; DeMorrow, S.; Karstens, W.; Madeka, T.; Greene, J., Jr.; Francis, H. Bile duct ligation-induced biliary hyperplasia, hepatic injury, and fibrosis are reduced in mast cell-deficient Kit(W-sh) mice. Hepatology 2017, 65, 1991–2004. [Google Scholar] [CrossRef] [Green Version]
- Moyer, K.E.; Saggers, G.C.; Ehrlich, H.P. Mast cells promote fibroblast populated collagen lattice contraction through gap junction intercellular communication. Wound Repair Regen. 2004, 12, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, Y.; Yang, G.; Zhang, Q.; Huang, X.; Yu, L.; Dong, X. Mast cell chymase promotes hypertrophic scar fibroblast proliferation and collagen synthesis by activating TGF-beta1/Smads signaling pathway. Exp. Ther. Med. 2017, 14, 4438–4442. [Google Scholar] [PubMed]
- Yin, M.; Wu, L. Effect of Mast Cell Chymase on Activation, Proliferation and Transdifferentiation of Hepatic Stellate Cells. Hepato-Gastroenterology 2015, 62, 1007–1010. [Google Scholar] [PubMed]
- Jing, J.; Dou, T.T.; Yang, J.Q.; Chen, X.B.; Cao, H.L.; Min, M.; Cai, S.Q.; Zheng, M.; Man, X.Y. Role of endothelin-1 in the skin fibrosis of systemic sclerosis. Eur. Cytokine Netw. 2015, 26, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Furubayashi, K.; Takai, S.; Jin, D.; Miyazaki, M.; Katsumata, T.; Inagaki, S.; Kimura, M.; Tanaka, K.; Nishimoto, M.; Fukumoto, H. Chymase activates promatrix metalloproteinase-9 in human abdominal aortic aneurysm. Clin. Chim. Acta 2008, 388, 214–216. [Google Scholar] [CrossRef]
- Bagher, M.; Larsson-Callerfelt, A.K.; Rosmark, O.; Hallgren, O.; Bjermer, L.; Westergren-Thorsson, G. Mast cells and mast cell tryptase enhance migration of human lung fibroblasts through protease-activated receptor 2. Cell. Commun. Signal 2018, 16, 59. [Google Scholar] [CrossRef]
- Knight, V.; Tchongue, J.; Lourensz, D.; Tipping, P.; Sievert, W. Protease-activated receptor 2 promotes experimental liver fibrosis in mice and activates human hepatic stellate cells. Hepatology 2012, 55, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Chu, S.G.; Patino-Jaramillo, N.G.; Wilder, J.; Villalba, J.; Doyle-Eisele, M.; McDonald, J.; Liu, X.; El-Chemaly, S.; Perrella, M.A.; et al. Syndecan-2 Attenuates Radiation-induced Pulmonary Fibrosis and Inhibits Fibroblast Activation by Regulating PI3K/Akt/ROCK Pathway via CD148. Am. J. Respir. Cell. Mol. Biol. 2018, 58, 208–215. [Google Scholar] [CrossRef]
- Tan, H.; Chen, Z.; Chen, F.; Yao, Y.; Lai, Y.; Xu, W.; Liu, X. Tryptase promotes the profibrotic phenotype transfer of atrial fibroblasts by PAR2 and PPARgamma pathway. Arch. Med. Res. 2018, 49, 568–575. [Google Scholar] [CrossRef]
- Blank, U.; Madera-Salcedo, I.K.; Danelli, L.; Claver, J.; Tiwari, N.; Sanchez-Miranda, E.; Vazquez-Victorio, G.; Ramirez-Valadez, K.A.; Macias-Silva, M.; Gonzalez-Espinosa, C. Vesicular trafficking and signaling for cytokine and chemokine secretion in mast cells. Front. Immunol. 2014, 5, 453. [Google Scholar] [CrossRef] [Green Version]
- Wolak, M.; Bojanowska, E.; Staszewska, T.; Piera, L.; Szymanski, J.; Drobnik, J. Histamine augments collagen content via H1 receptor stimulation in cultures of myofibroblasts taken from wound granulation tissue. Mol. Cell. Biochem. 2021, 476, 1083–1092. [Google Scholar] [CrossRef]
- Kennedy, L.; Meadows, V.; Demieville, J.; Hargrove, L.; Virani, S.; Glaser, S.; Zhou, T.; Rinehart, E.; Jaeger, V.; Kyritsi, K.; et al. Biliary damage and liver fibrosis are ameliorated in a novel mouse model lacking l-histidine decarboxylase/histamine signaling. Lab. Investig. 2020, 100, 837–848. [Google Scholar] [CrossRef]
- Jordana, M.; Befus, A.D.; Newhouse, M.T.; Bienenstock, J.; Gauldie, J. Effect of histamine on proliferation of normal human adult lung fibroblasts. Thorax 1988, 43, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Wilgus, T.A.; Ud-Din, S.; Bayat, A. A review of the evidence for and against a role for mast cells in cutaneous scarring and fibrosis. Int. J. Mol. Sci. 2020, 21, 9673. [Google Scholar] [CrossRef]
- Lin, L.; Yamagata, K.; Nakayamada, S.; Sawamukai, N.; Yamaoka, K.; Sakata, K.; Nakano, K.; Tanaka, Y. Histamine inhibits differentiation of skin fibroblasts into myofibroblasts. Biochem. Biophys. Res. Commun. 2015, 463, 434–439. [Google Scholar] [CrossRef]
- Brown, M. O’Reilly, S. The immunopathogenesis of fibrosis in systemic sclerosis. Clin. Exp. Immunol. 2019, 195, 310–321. [Google Scholar] [PubMed] [Green Version]
- Norozian, F.; Kashyap, M.; Ramirez, C.D.; Patel, N.; Kepley, C.L.; Barnstein, B.O.; Ryan, J.J. TGFbeta1 induces mast cell apoptosis. Exp. Hematol. 2006, 34, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Oriente, A.; Fedarko, N.S.; Pacocha, S.E.; Huang, S.K.; Lichtenstein, L.M.; Essayan, D.M. Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J. Pharmacol. Exp. Ther. 2000, 292, 988–994. [Google Scholar] [PubMed]
- Ingram, J.L.; Rice, A.B.; Geisenhoffer, K.; Madtes, D.K.; Bonner, J.C. IL-13 and IL-1beta promote lung fibroblast growth through coordinated up-regulation of PDGF-AA and PDGF-Ralpha. FASEB J. 2004, 18, 1132–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manuyakorn, W.; Howarth, P.H.; Holgate, S.T. Airway remodelling in asthma and novel therapy. Asian Pac. J. Allergy Immunol. 2013, 31, 3–10. [Google Scholar]
- Kulke, M.; Geist, N.; Friedrichs, W.; Langel, W. Molecular dynamics simulations on networks of heparin and collagen. Proteins 2017, 85, 1119–1130. [Google Scholar] [CrossRef]
- Roy, A.; Ganesh, G.; Sippola, H.; Bolin, S.; Sawesi, O.; Dagalv, A.; Schlenner, S.M.; Feyerabend, T.; Rodewald, H.R.; Kjellen, L.; et al. Mast cell chymase degrades the alarmins heat shock protein 70, biglycan, HMGB1, and interleukin-33 (IL-33) and limits danger-induced inflammation. J. Biol. Chem. 2014, 289, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Atiakshin, D.; Buchwalow, I.; Horny, P.; Tiemann, M. Protease profile of normal and neoplastic mast cells in the human bone marrow with special emphasis on systemic mastocytosis. Histochem. Cell Biol. 2021, 155, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cell chymase: Morphofunctional characteristics. Histochem. Cell Biol. 2019, 152, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Tryptase as a polyfunctional component of mast cells. Histochem. Cell Biol. 2018, 149, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Chien, S.; Chehelcheraghi, F. Co- localization of Flt1 and tryptase of mast cells in skin wound of rats with type I diabetes: Initial studies. Acta Histochem. 2021, 123, 151680. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gao, B.; Zhang, Y.; Lu, H.; Li, X.; Pan, L.; Yin, L.; Zhi, X. PAR2 promotes M1 macrophage polarization and inflammation via FOXO1 pathway. J. Cell. Biochem. 2019, 120, 9799–9809. [Google Scholar] [CrossRef]
- Wang, Q.; Lepus, C.M.; Raghu, H.; Reber, L.L.; Tsai, M.M.; Wong, H.H.; von Kaeppler, E.; Lingampalli, N.; Bloom, M.S.; Hu, N.; et al. IgE-mediated mast cell activation promotes inflammation and cartilage destruction in osteoarthritis. eLife 2019, 8, e39905. [Google Scholar] [CrossRef] [PubMed]
- Pejler, G. The emerging role of mast cell proteases in asthma. Eur. Respir. J. 2019, 54, 1900685. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Altemeier, W.A.; Vandree, J.; Piliponsky, A.M.; Johnson, B.; Appel, C.L.; Frevert, C.W.; Hyde, D.M.; Ziegler, S.F.; Smith, D.E.; et al. Increased density of intraepithelial mast cells in patients with exercise-induced bronchoconstriction regulated through epithelially derived thymic stromal lymphopoietin and IL-33. J. Allergy Clin. Immunol. 2014, 133, 1448–1455. [Google Scholar] [CrossRef] [Green Version]
- Takabayashi, T.; Kato, A.; Peters, A.T.; Suh, L.A.; Carter, R.; Norton, J.; Grammer, L.C.; Tan, B.K.; Chandra, R.K.; Conley, D.B.; et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2012, 130, 410–420.e415. [Google Scholar] [CrossRef] [Green Version]
- Younan, G.J.; Heit, Y.I.; Dastouri, P.; Kekhia, H.; Xing, W.; Gurish, M.F.; Orgill, D.P. Mast cells are required in the proliferation and remodeling phases of microdeformational wound therapy. Plast. Reconstr. Surg. 2011, 128, 649e–658e. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, A.; Toksoy, A.; Engelhardt, E.; Gillitzer, R. Mast cell involvement in normal human skin wound healing: Expression of monocyte chemoattractant protein-1 is correlated with recruitment of mast cells which synthesize interleukin-4 in vivo. J. Pathol. 2000, 190, 100–106. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A review of the contribution of mast cells in wound healing: Involved molecular and cellular mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Tellechea, A.; Leal, E.C.; Kafanas, A.; Auster, M.E.; Kuchibhotla, S.; Ostrovsky, Y.; Tecilazich, F.; Baltzis, D.; Zheng, Y.; Carvalho, E.; et al. Mast cells regulate wound healing in diabetes. Diabetes 2016, 65, 2006–2019. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Chen, L.; Zhang, Y.; Jayaswal, N.; Mezghani, I.; Zhang, W.; Veves, A. Mast cells in diabetes and diabetic wound healing. Adv. Ther. 2020, 37, 4519–4537. [Google Scholar] [CrossRef] [PubMed]
- Nishikori, Y.; Shiota, N.; Okunishi, H. The role of mast cells in cutaneous wound healing in streptozotocin-induced diabetic mice. Arch. Dermatol. Res. 2014, 306, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.C.; Grova, M.; Montoro, D.T.; Zimmermann, A.; Tsai, M.; Gurtner, G.C.; Galli, S.J.; Longaker, M.T. Evidence that mast cells are not required for healing of splinted cutaneous excisional wounds in mice. PLoS ONE 2013, 8, e59167. [Google Scholar] [CrossRef] [PubMed]
- Willenborg, S.; Eckes, B.; Brinckmann, J.; Krieg, T.; Waisman, A.; Hartmann, K.; Roers, A.; Eming, S.A. Genetic ablation of mast cells redefines the role of mast cells in skin wound healing and bleomycin-induced fibrosis. J. Investig. Dermatol. 2014, 134, 2005–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ud-Din, S.; Wilgus, T.A.; Bayat, A. Mast cells in skin scarring: A review of animal and human research. Front. Immunol. 2020, 11, 552205. [Google Scholar] [CrossRef]
- Nishida, K.; Hasegawa, A.; Yamasaki, S.; Uchida, R.; Ohashi, W.; Kurashima, Y.; Kunisawa, J.; Kimura, S.; Iwanaga, T.; Watarai, H.; et al. Mast cells play role in wound healing through the ZnT2/GPR39/IL-6 axis. Sci. Rep. 2019, 9, 10842. [Google Scholar] [CrossRef]
- Kashyap, M.; Thornton, A.M.; Norton, S.K.; Barnstein, B.; Macey, M.; Brenzovich, J.; Shevach, E.; Leonard, W.J.; Ryan, J.J. Cutting edge: CD4 T cell-mast cell interactions alter IgE receptor expression and signaling. J. Immunol. 2008, 180, 2039–2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorczynski, R.M.; Chen, Z.; Khatri, I.; Yu, K. Graft-infiltrating cells expressing a CD200 transgene prolong allogeneic skin graft survival in association with local increases in Foxp3(+)Treg and mast cells. Transpl. Immunol. 2011, 25, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Gri, G.; Piconese, S.; Frossi, B.; Manfroi, V.; Merluzzi, S.; Tripodo, C.; Viola, A.; Odom, S.; Rivera, J.; Colombo, M.P.; et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity 2008, 29, 771–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leveson-Gower, D.B.; Sega, E.I.; Kalesnikoff, J.; Florek, M.; Pan, Y.; Pierini, A.; Galli, S.J.; Negrin, R.S. Mast cells suppress murine GVHD in a mechanism independent of CD4+CD25+ regulatory T cells. Blood 2013, 122, 3659–3665. [Google Scholar] [CrossRef] [Green Version]
- Kushnir-Sukhov, N.M.; Brown, J.M.; Wu, Y.; Kirshenbaum, A.; Metcalfe, D.D. Human mast cells are capable of serotonin synthesis and release. J. Allergy Clin. Immunol. 2007, 119, 498–499. [Google Scholar] [CrossRef]
- Lee, G.K.; Park, H.J.; Macleod, M.; Chandler, P.; Munn, D.H.; Mellor, A.L. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 2002, 107, 452–460. [Google Scholar] [CrossRef]
- Chichlowski, M.; Westwood, G.S.; Abraham, S.N.; Hale, L.P. Role of mast cells in inflammatory bowel disease and inflammation-associated colorectal neoplasia in IL-10-deficient mice. PLoS ONE 2010, 5, e12220. [Google Scholar] [CrossRef]
- Ohkura, N.; Kitagawa, Y.; Sakaguchi, S. Development and maintenance of regulatory T cells. Immunity 2013, 38, 414–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, B.; Vigneron, J.; Levacher, B.; Vazquez, T.; Pitoiset, F.; Brimaud, F.; Churlaud, G.; Klatzmann, D.; Bellier, B. Low-Dose IL-2 Induces Regulatory T Cell-Mediated Control of Experimental Food Allergy. J. Immunol. 2016, 197, 188–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevach, E.M. Application of IL-2 therapy to target T regulatory cell function. Trends. Immunol. 2012, 33, 626–632. [Google Scholar] [CrossRef] [Green Version]
- Morita, H.; Arae, K.; Unno, H.; Miyauchi, K.; Toyama, S.; Nambu, A.; Oboki, K.; Ohno, T.; Motomura, K.; Matsuda, A.; et al. An interleukin-33-mast cell-interleukin-2 axis suppresses papain-induced allergic inflammation by promoting regulatory T cell numbers. Immunity 2015, 43, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatzioannou, A.; Banos, A.; Sakelaropoulos, T.; Fedonidis, C.; Vidali, M.S.; Kohne, M.; Handler, K.; Boon, L.; Henriques, A.; Koliaraki, V.; et al. An intrinsic role of IL-33 in Treg cell-mediated tumor immunoevasion. Nat. Immunol. 2020, 21, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesce, J.T.; Ramalingam, T.R.; Mentink-Kane, M.M.; Wilson, M.S.; El Kasmi, K.C.; Smith, A.M.; Thompson, R.W.; Cheever, A.W.; Murray, P.J.; Wynn, T.A. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009, 5, e1000371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finlay, C.M.; Cunningham, K.T.; Doyle, B.; Mills, K.H.G. IL-33-Stimulated Murine Mast Cells Polarize Alternatively Activated Macrophages, Which Suppress T Cells That Mediate Experimental Autoimmune Encephalomyelitis. J. Immunol. 2020, 205, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Carlos, D.; Yaochite, J.N.; Rocha, F.A.; Toso, V.D.; Malmegrim, K.C.; Ramos, S.G.; Jamur, M.C.; Oliver, C.; Camara, N.O.; Andrade, M.V.; et al. Mast cells control insulitis and increase Treg cells to confer protection against STZ-induced type 1 diabetes in mice. Eur. J. Immunol. 2015, 45, 2873–2885. [Google Scholar] [CrossRef] [Green Version]
- Hirano, T.; Honda, T.; Kanameishi, S.; Honda, Y.; Egawa, G.; Kitoh, A.; Nakajima, S.; Otsuka, A.; Nomura, T.; Dainichi, T.; et al. PD-L1 on mast cells suppresses effector CD8(+) T-cell activation in the skin in murine contact hypersensitivity. J. Allergy Clin. Immunol. 2021, in press. [Google Scholar] [CrossRef]
- Peavy, R.D.; Metcalfe, D.D. Understanding the mechanisms of anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lester, P.; Builder, S.; Shire, S.J. Characterization of complex formation by humanized anti-IgE monoclonal antibody and monoclonal human IgE. Biochemistry 1995, 34, 10474–10482. [Google Scholar] [CrossRef]
- Leung, D.Y.; Sampson, H.A.; Yunginger, J.W.; Burks, A.W., Jr.; Schneider, L.C.; Wortel, C.H.; Davis, F.M.; Hyun, J.D.; Shanahan, W.R., Jr. Effect of anti-IgE therapy in patients with peanut allergy. N. Engl. J. Med. 2003, 348, 986–993. [Google Scholar] [CrossRef] [Green Version]
- Gasser, P.; Tarchevskaya, S.S.; Guntern, P.; Brigger, D.; Ruppli, R.; Zbaren, N.; Kleinboelting, S.; Heusser, C.; Jardetzky, T.S.; Eggel, A. The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat. Commun. 2020, 11, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauvreau, G.M.; Harris, J.M.; Boulet, L.P.; Scheerens, H.; Fitzgerald, J.M.; Putnam, W.S.; Cockcroft, D.W.; Davis, B.E.; Leigh, R.; Zheng, Y.; et al. Targeting membrane-expressed IgE B cell receptor with an antibody to the M1 prime epitope reduces IgE production. Sci. Transl. Med. 2014, 6, 243ra285. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, E.; Schwickart, M.; Li, J.; Kim, K.; Crouch, S.; Parveen, S.; Kell, C.; Birrell, C. Pharmacokinetics, pharmacodynamics, and safety of MEDI4212, an anti-IgE monoclonal antibody, in subjects with atopy: A phase I study. Adv. Ther. 2016, 33, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Burton, O.T.; Noval Rivas, M.; Zhou, J.S.; Logsdon, S.L.; Darling, A.R.; Koleoglou, K.J.; Roers, A.; Houshyar, H.; Crackower, M.A.; Chatila, T.A.; et al. Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity 2014, 41, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez Molina, C.; Falkencrone, S.; Skov, P.S.; Hooper-Greenhill, E.; Barker, M.; Dickson, M.C. GSK2646264, a spleen tyrosine kinase inhibitor, attenuates the release of histamine in ex vivo human skin. Br. J. Pharmacol. 2019, 176, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.H.; Kim, D.K.; Kim, H.S.; Lee, D.; Lee, M.B.; Min, K.Y.; Jo, M.G.; Lee, J.E.; Kim, Y.M.; Choi, W.S. WZ3146 inhibits mast cell Lyn and Fyn to reduce IgE-mediated allergic responses in vitro and in vivo. Toxicol. Appl. Pharmacol. 2019, 383, 114763. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, D.K.; Kim, H.W.; Kim, H.S.; Lee, D.; Lee, M.B.; Min, K.Y.; Koo, J.; Kim, S.J.; Kang, C.; et al. Repositioning of anti-cancer drug candidate, AZD7762, to an anti-allergic drug suppressing IgE-mediated mast cells and allergic responses via the inhibition of Lyn and Fyn. Biochem. Pharmacol. 2018, 154, 270–277. [Google Scholar] [CrossRef]
- Kikly, K.K.; Bochner, B.S.; Freeman, S.D.; Tan, K.B.; Gallagher, K.T.; D’Alessio, K.J.; Holmes, S.D.; Abrahamson, J.A.; Erickson-Miller, C.L.; Murdock, P.R.; et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J. Allergy Clin. Immunol. 2000, 105, 1093–1100. [Google Scholar] [CrossRef]
- Floyd, H.; Ni, J.; Cornish, A.L.; Zeng, Z.; Liu, D.; Carter, K.C.; Steel, J.; Crocker, P.R. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J. Biol. Chem. 2000, 275, 861–866. [Google Scholar] [CrossRef] [Green Version]
- Dellon, E.S.; Peterson, K.A.; Murray, J.A.; Falk, G.W.; Gonsalves, N.; Chehade, M.; Genta, R.M.; Leung, J.; Khoury, P.; Klion, A.D.; et al. Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N. Engl. J. Med. 2020, 383, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
| Target | Name | Mechanism of Action | Characteristics | Reference |
|---|---|---|---|---|
| IgE-FcεRI | Omalizumab | Prevents free IgE from binding to FcεRI | Dose not effective for all patients | [140] |
| Talizumab | Prevents free IgE from binding to FcεRI | Still in clinical trials | [141] | |
| Ligelizumab | Prevents free IgE from binding to FcεRI, may reduce B cell production of IgE | Combines with IgE more efficiently than Omalizumab | [142] | |
| Quilizumab | Depletes IgE-producing B cells | Does not seem to reduce allergic reactions | [143] | |
| MEDI4212 | Prevents free/bound IgE from binding to FcεRI; depletes IgE-producing B cells | Probably better than omalizumab when IgE level is high | [144] | |
| Kinases | Fostamatinib | Inhibits Syk | The effect is fast, but the safety is poor | [145] |
| GSK2646264 | Inhibits Syk | Has potential toxicity | [146] | |
| WZ3146 | Inhibits Lyn/Fyn | Has no clinical data | [147] | |
| AZD7762 | Inhibits Lyn/Fyn | Has cardiotoxicity | [148] | |
| Siglec-8 | Lirentelimab | Inhibits FcεRI signaling | The most promising target | [151] |
| Tregs | - | Induced by low doses of IL-2 | Has great potential against food allergy | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Kurashima, Y. Two Sides of the Coin: Mast Cells as a Key Regulator of Allergy and Acute/Chronic Inflammation. Cells 2021, 10, 1615. https://doi.org/10.3390/cells10071615
Zhang Z, Kurashima Y. Two Sides of the Coin: Mast Cells as a Key Regulator of Allergy and Acute/Chronic Inflammation. Cells. 2021; 10(7):1615. https://doi.org/10.3390/cells10071615
Chicago/Turabian StyleZhang, Zhongwei, and Yosuke Kurashima. 2021. "Two Sides of the Coin: Mast Cells as a Key Regulator of Allergy and Acute/Chronic Inflammation" Cells 10, no. 7: 1615. https://doi.org/10.3390/cells10071615
APA StyleZhang, Z., & Kurashima, Y. (2021). Two Sides of the Coin: Mast Cells as a Key Regulator of Allergy and Acute/Chronic Inflammation. Cells, 10(7), 1615. https://doi.org/10.3390/cells10071615







