NOX4 Signaling Mediates Cancer Development and Therapeutic Resistance through HER3 in Ovarian Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Transfection
2.2. Clinical Ovarian Cancer Specimens
2.3. Compounds and Plasmids
2.4. RNA Extraction and Quantitative Real-Time PCR
2.5. Western Blotting
2.6. Cell Viability Assays
2.7. Radiation and Clone Formation
2.8. Luciferase Reporter Assay
2.9. Database
2.10. Statistical Analysis
3. Results
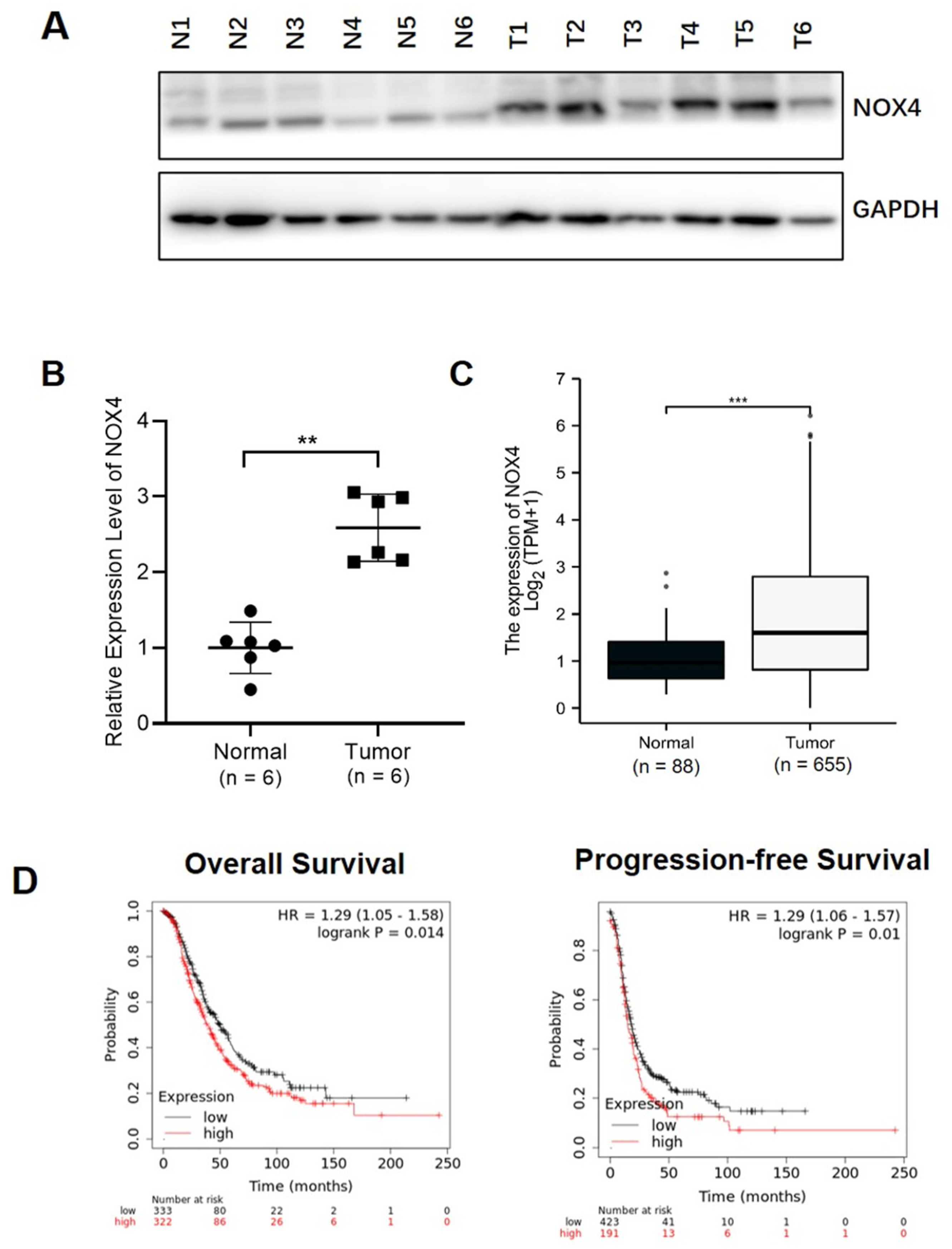
3.1. Higher NOX4 Levels Were Correlated with Ovarian Cancer Development and Poor Progression-Free Survival
3.2. Expression Levels of NOX4 Were Regulated by HIF-1α via Alternative Splicing in A2780 Cells
3.3. IGF-1 Treatment Increased the Expression Levels of HIF-1α and NOX4 in A2780 and OVCAR3 Cells
3.4. NOX4 Knockdown Increased the Sensitivity to Trastuzumab Treatment via Downregulating HER3 in Ovarian Cancer Cells
3.5. Knockdown of NOX4 and HIF-1α Increased Sensitivity to Radiation Therapy in Ovarian Cancer Cells
3.6. Knockdown of NOX4 Decreased the Transcriptional Activity and Expression Levels of NF-κB p65, and Increased Sensitivity to Afatinib Treatment in A2780 and OVCAR3 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Arend, R.; Martinez, A.; Szul, T.; Birrer, M.J. Biomarkers in ovarian cancer: To be or not to be. Cancer 2019, 125 (Suppl. 24), 4563–4572. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.W.; Kim, S. Overexpression of HER2/HER3 and clinical feature of ovarian cancer. J. Gynecol. Oncol. 2019, 30, e75. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Xia, C.; Meng, Q.; Liu, L.Z.; Rojanasakul, Y.; Wang, X.R.; Jiang, B.H. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res 2007, 67, 10823–10830. [Google Scholar] [CrossRef]
- Meden, H.; Kuhn, W. Overexpression of the oncogene c-erbB-2 (HER2/neu) in ovarian cancer: A new prognostic factor. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997, 71, 173–179. [Google Scholar] [CrossRef]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Bookman, M.A.; Darcy, K.M.; Clarke-Pearson, D.; Boothby, R.A.; Horowitz, I.R. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: A phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 2003, 21, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Oltean, S.; Bates, D.O. Hallmarks of alternative splicing in cancer. Oncogene 2014, 33, 5311–5318. [Google Scholar] [CrossRef]
- Goyal, P.; Weissmann, N.; Rose, F.; Grimminger, F.; Schafers, H.J.; Seeger, W.; Hanze, J. Identification of novel Nox4 splice variants with impact on ROS levels in A549 cells. Biochem. Biophys. Res. Commun. 2005, 329, 32–39. [Google Scholar] [CrossRef]
- Sena, J.A.; Wang, L.; Pawlus, M.R.; Hu, C.J. HIFs enhance the transcriptional activation and splicing of adrenomedullin. Mol. Cancer Res. MCR 2014, 12, 728–741. [Google Scholar] [CrossRef]
- Liefers-Visser, J.A.L.; Meijering, R.A.M.; Reyners, A.K.L.; van der Zee, A.G.J.; de Jong, S. IGF system targeted therapy: Therapeutic opportunities for ovarian cancer. Cancer Treat. Rev. 2017, 60, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak-Baczmanska, D.; Malinowski, A.; Glowacka, E.; Wilczynski, M. Does IGF-1 play a role in the biology of ovarian cancer? Ginekol. Pol. 2018, 89, 13–19. [Google Scholar] [CrossRef]
- He, J.; Qian, X.; Carpenter, R.; Xu, Q.; Wang, L.; Qi, Y.; Wang, Z.X.; Liu, L.Z.; Jiang, B.H. Repression of miR-143 mediates Cr (VI)-induced tumor angiogenesis via IGF-IR/IRS1/ERK/IL-8 pathway. Toxicol. Sci. 2013, 134, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Bijman, M.N.; van Berkel, M.P.; Kok, M.; Janmaat, M.L.; Boven, E. Inhibition of functional HER family members increases the sensitivity to docetaxel in human ovarian cancer cell lines. Anti Cancer Drugs 2009, 20, 450–460. [Google Scholar] [CrossRef]
- Servidei, T.; Riccardi, A.; Mozzetti, S.; Ferlini, C.; Riccardi, R. Chemoresistant tumor cell lines display altered epidermal growth factor receptor and HER3 signaling and enhanced sensitivity to gefitinib. Int. J. Cancer 2008, 123, 2939–2949. [Google Scholar] [CrossRef] [PubMed]
- Teplinsky, E.; Muggia, F. Targeting HER2 in ovarian and uterine cancers: Challenges and future directions. Gynecol. Oncol. 2014, 135, 364–370. [Google Scholar] [CrossRef]
- Li, S.; Lv, M.; Qiu, S.; Meng, J.; Liu, W.; Zuo, J.; Yang, L. NF-kappaB p65 promotes ovarian cancer cell proliferation and migration via regulating mortalin. J. Cell Mol. Med. 2019, 23, 4338–4348. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, K.; Deng, L.; Liang, J.; Liang, H.; Feng, D.; Ling, B. Cyclooxygenase 2 Promotes Proliferation and Invasion in Ovarian Cancer Cells via the PGE2/NF-kappaB Pathway. Cell Transpl. 2019, 28, 1S–13S. [Google Scholar] [CrossRef]
- Yu, T.; Li, L.; Liu, W.; Ya, B.; Cheng, H.; Xin, Q. Silencing of NADPH Oxidase 4 Attenuates Hypoxia Resistance in Neuroblastoma Cells SH-SY5Y by Inhibiting PI3K/Akt-Dependent Glycolysis. Oncol. Res. 2019, 27, 525–532. [Google Scholar] [CrossRef]
- Diebold, I.; Petry, A.; Hess, J.; Gorlach, A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol. Biol. Cell 2010, 21, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Norouzi-Barough, L.; Sarookhani, M.R.; Sharifi, M. Molecular mechanisms of drug resistance in ovarian cancer. J. Cell. Physiol. 2018, 233, 4546–4562. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, Q.; Jing, Y.; Agani, F.; Qian, X.; Carpenter, R.; Li, Q.; Wang, X.R.; Peiper, S.S.; Lu, Z.; et al. Reactive oxygen species regulate ERBB2 and ERBB3 expression via miR-199a/125b and DNA methylation. EMBO Rep. 2012, 13, 1116–1122. [Google Scholar] [CrossRef]
- Sequist, L.V.; Yang, J.C.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.M.; Boyer, M.; et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Zhou, C.; Hu, C.P.; Feng, J.; Lu, S.; Huang, Y.; Li, W.; Hou, M.; Shi, J.H.; Lee, K.Y.; et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 213–222. [Google Scholar] [CrossRef]
- Suzawa, K.; Toyooka, S.; Sakaguchi, M.; Morita, M.; Yamamoto, H.; Tomida, S.; Ohtsuka, T.; Watanabe, M.; Hashida, S. Antitumor effect of afatinib, as a human epidermal growth factor receptor 2-targeted therapy, in lung cancers harboring HER2 oncogene alterations. Cancer Sci. 2016, 107, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Liu, S.T.; Zhao, B.X.; Yang, F.H.; Wang, Y.T.; Liang, Q.Y.; Sun, Y.B.; Liu, Y.; Song, Z.H.; Cai, Y.; et al. Afatinib reverses multidrug resistance in ovarian cancer via dually inhibiting ATP binding cassette subfamily B member 1. Oncotarget 2015, 6, 26142–26160. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Wu, C.P.; Lee, H.T.; Liang, J.A.; Yu, C.Y.; Lin, Y.J. NADPH oxidase subunit 4 mediates cycling hypoxia-promoted radiation resistance in glioblastoma multiforme. Free Radic. Biol. Med. 2012, 53, 649–658. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-J.; Huang, Y.-X.; Wang, W.; Zhang, Y.; Liu, B.-J.; Qiu, J.-G.; Jiang, B.-H.; Liu, L.-Z. NOX4 Signaling Mediates Cancer Development and Therapeutic Resistance through HER3 in Ovarian Cancer Cells. Cells 2021, 10, 1647. https://doi.org/10.3390/cells10071647
Liu W-J, Huang Y-X, Wang W, Zhang Y, Liu B-J, Qiu J-G, Jiang B-H, Liu L-Z. NOX4 Signaling Mediates Cancer Development and Therapeutic Resistance through HER3 in Ovarian Cancer Cells. Cells. 2021; 10(7):1647. https://doi.org/10.3390/cells10071647
Chicago/Turabian StyleLiu, Wen-Jing, Ying-Xue Huang, Wei Wang, Ye Zhang, Bing-Jie Liu, Jian-Ge Qiu, Bing-Hua Jiang, and Ling-Zhi Liu. 2021. "NOX4 Signaling Mediates Cancer Development and Therapeutic Resistance through HER3 in Ovarian Cancer Cells" Cells 10, no. 7: 1647. https://doi.org/10.3390/cells10071647
APA StyleLiu, W.-J., Huang, Y.-X., Wang, W., Zhang, Y., Liu, B.-J., Qiu, J.-G., Jiang, B.-H., & Liu, L.-Z. (2021). NOX4 Signaling Mediates Cancer Development and Therapeutic Resistance through HER3 in Ovarian Cancer Cells. Cells, 10(7), 1647. https://doi.org/10.3390/cells10071647






