The Vascular Circadian Clock in Chronic Kidney Disease
Abstract
:1. Introduction
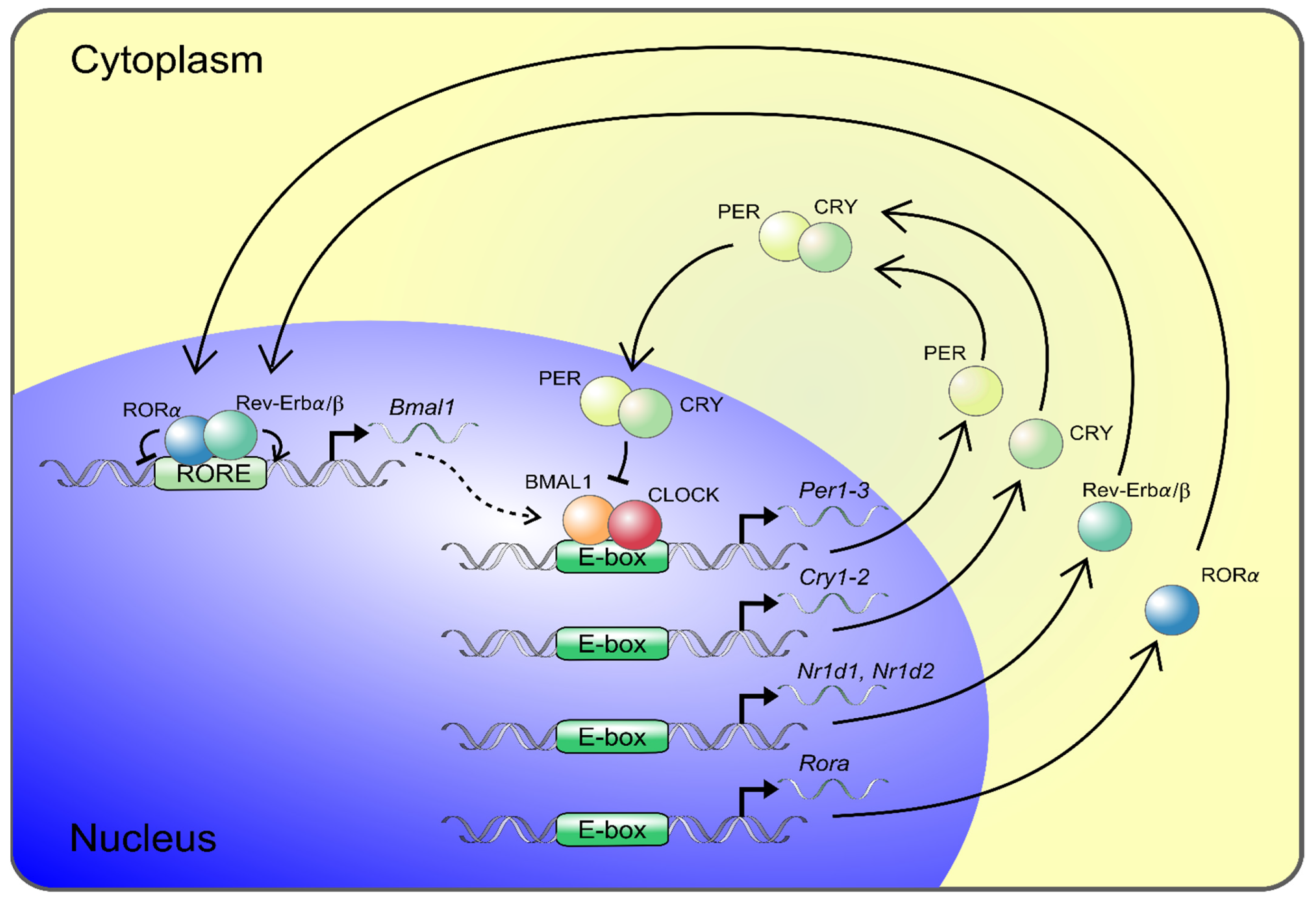
2. The Molecular Circadian Clock
3. The Internal Vascular Circadian Clock and Its Role in Vascular Pathology
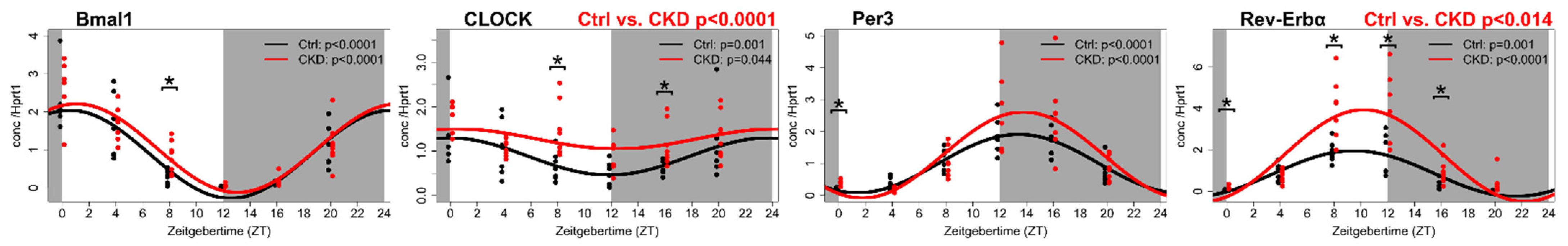
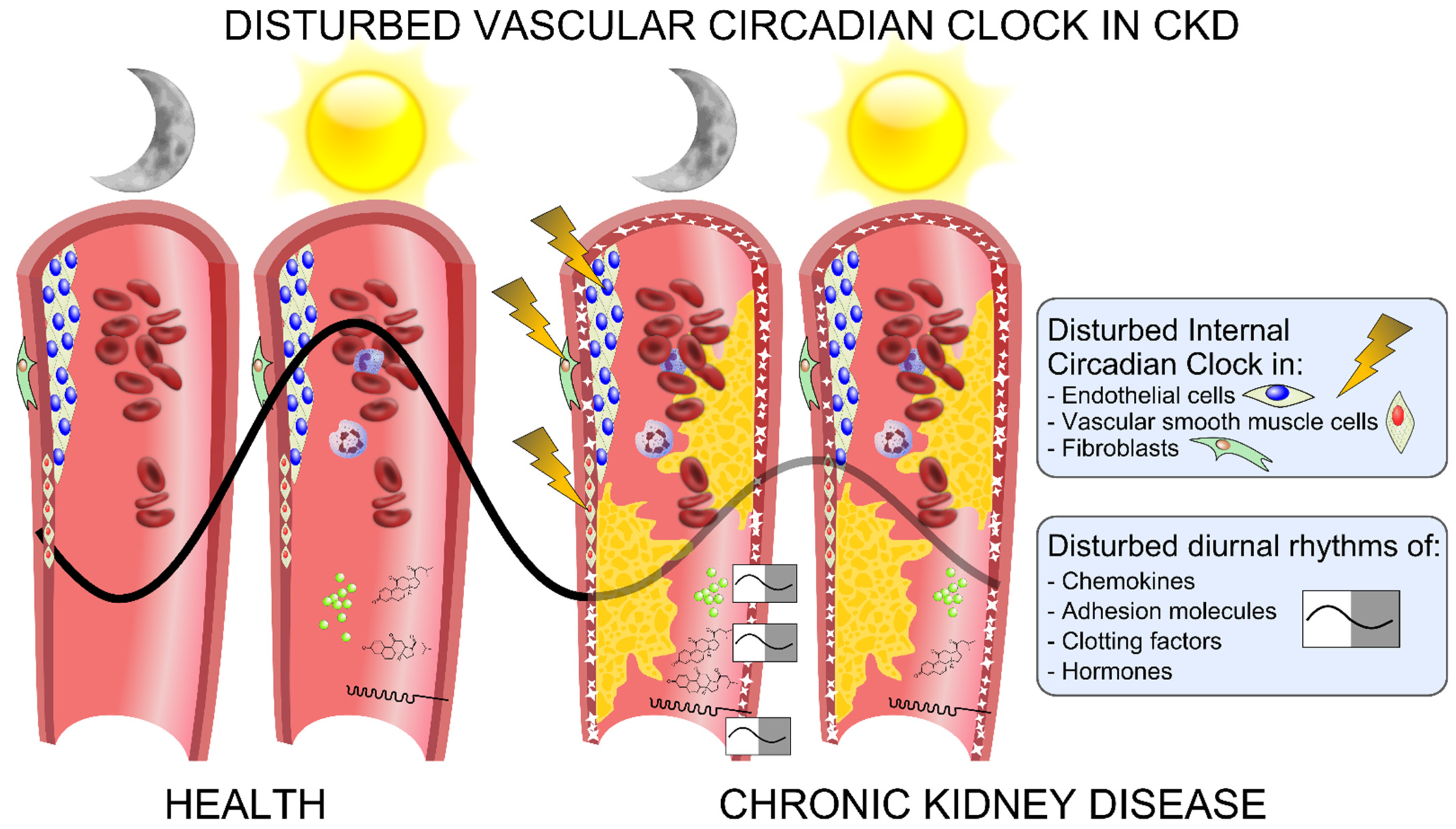
4. The Vascular Circadian Clock Is Disrupted in CKD
5. The Circadian Clock and the Clock-Controlled Genes in the Calcified Aorta of a CKD Rat Model
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farshadi, E.; van der Horst, G.T.J.; Chaves, I. Molecular Links between the Circadian Clock and the Cell Cycle. J. Mol. Biol. 2020, 432, 3515–3524. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Panda, S. The circadian coordination of cell biology. J. Cell. Biol. 2016, 215, 15–25. [Google Scholar] [CrossRef]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokkan, K.A.; Yamazaki, S.; Tei, H.; Sakaki, Y.; Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 2001, 291, 490–493. [Google Scholar] [CrossRef] [Green Version]
- Damiola, F.; Le Minh, N.; Preitner, N.; Kornmann, B.; Fleury-Olela, F.; Schibler, U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef] [Green Version]
- Balsalobre, A.; Brown, S.A.; Marcacci, L.; Tronche, F.; Kellendonk, C.; Reichardt, H.M.; Schutz, G.; Schibler, U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 2000, 289, 2344–2347. [Google Scholar] [CrossRef] [Green Version]
- Crosby, P.; Hamnett, R.; Putker, M.; Hoyle, N.P.; Reed, M.; Karam, C.J.; Maywood, E.S.; Stangherlin, A.; Chesham, J.E.; Hayter, E.A.; et al. Insulin/IGF-1 Drives PERIOD Synthesis to Entrain Circadian Rhythms with Feeding Time. Cell 2019. [Google Scholar] [CrossRef] [Green Version]
- Weaver, D.R. The suprachiasmatic nucleus: A 25-year retrospective. J. Biol. Rhythms 1998, 13, 100–112. [Google Scholar] [CrossRef]
- McAlpine, C.S.; Swirski, F.K. Circadian Influence on Metabolism and Inflammation in Atherosclerosis. Circ. Res. 2016, 119, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalby, M.C.; Davidson, S.J.; Burman, J.F.; Davies, S.W. Diurnal variation in platelet aggregation iwth the PFA-100 platelet function analyser. Platelets 2000, 11, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Soulban, G.; Labrecque, G. Circadian rhythms of blood clotting time and coagulation factors II, VII, IX and X in rats. Life Sci. 1989, 45, 2485–2489. [Google Scholar] [CrossRef]
- Bremner, W.F.; Sothern, R.B.; Kanabrocki, E.L.; Ryan, M.; McCormick, J.B.; Dawson, S.; Connors, E.S.; Rothschild, R.; Third, J.L.; Vahed, S.; et al. Relation between circadian patterns in levels of circulating lipoprotein(a), fibrinogen, platelets, and related lipid variables in men. Am. Heart J. 2000, 139, 164–173. [Google Scholar] [CrossRef]
- Kitchen, G.B.; Cunningham, P.S.; Poolman, T.M.; Iqbal, M.; Maidstone, R.; Baxter, M.; Bagnall, J.; Begley, N.; Saer, B.; Hussell, T.; et al. The clock gene Bmal1 inhibits macrophage motility, phagocytosis, and impairs defense against pneumonia. Proc. Natl. Acad. Sci. USA 2020, 117, 1543–1551. [Google Scholar] [CrossRef] [Green Version]
- Imamura, K.; Yoshitane, H.; Hattori, K.; Yamaguchi, M.; Yoshida, K.; Okubo, T.; Naguro, I.; Ichijo, H.; Fukada, Y. ASK family kinases mediate cellular stress and redox signaling to circadian clock. Proc. Natl. Acad. Sci. USA 2018, 115, 3646–3651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, L.X.; Li, X.N.; Yang, G.Y.; Zhang, X.; Li, W.X.; Zhang, Q.Q.; Pan, H.X.; Zhang, H.H.; Zhou, M.Y.; Wang, Y.D.; et al. Circadian misalignment alters insulin sensitivity during the light phase and shifts glucose tolerance rhythms in female mice. PLoS ONE 2019, 14, e0225813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolbe, I.; Leinweber, B.; Brandenburger, M.; Oster, H. Circadian clock network desynchrony promotes weight gain and alters glucose homeostasis in mice. Mol. Metab. 2019, 30, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Chalfant, J.M.; Howatt, D.A.; Tannock, L.R.; Daugherty, A.; Pendergast, J.S. Circadian disruption with constant light exposure exacerbates atherosclerosis in male ApolipoproteinE-deficient mice. Sci. Rep. 2020, 10, 9920. [Google Scholar] [CrossRef]
- Martino, T.A.; Oudit, G.Y.; Herzenberg, A.M.; Tata, N.; Koletar, M.M.; Kabir, G.M.; Belsham, D.D.; Backx, P.H.; Ralph, M.R.; Sole, M.J. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1675–R1683. [Google Scholar] [CrossRef] [Green Version]
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef]
- Fabbian, F.; Bhatia, S.; De Giorgi, A.; Maietti, E.; Bhatia, S.; Shanbhag, A.; Deshmukh, A. Circadian Periodicity of Ischemic Heart Disease: A Systematic Review of the Literature. Heart Fail. Clin. 2017, 13, 673–680. [Google Scholar] [CrossRef]
- Fournier, S.; Taffé, P.; Radovanovic, D.; Von Elm, E.; Morawiec, B.; Stauffer, J.C.; Erne, P.; Beggah, A.; Monney, P.; Pascale, P.; et al. Myocardial infarct size and mortality depend on the time of day-a large multicenter study. PLoS ONE 2015, 10, e0119157. [Google Scholar] [CrossRef] [Green Version]
- Ammirati, E.; Cristell, N.; Cianflone, D.; Vermi, A.C.; Marenzi, G.; De Metrio, M.; Uren, N.G.; Hu, D.; Ravasi, T.; Maseri, A.; et al. Questing for circadian dependence in ST-segment-elevation acute myocardial infarction: A multicentric and multiethnic study. Circ. Res. 2013, 112, e110–e114. [Google Scholar] [CrossRef] [Green Version]
- Cheng, B.; Anea, C.B.; Yao, L.; Chen, F.; Patel, V.; Merloiu, A.; Pati, P.; Caldwell, R.W.; Fulton, D.J.; Rudic, R.D. Tissue-intrinsic dysfunction of circadian clock confers transplant arteriosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108, 17147–17152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podobed, P.; Pyle, W.G.; Ackloo, S.; Alibhai, F.J.; Tsimakouridze, E.V.; Ratcliffe, W.F.; Mackay, A.; Simpson, J.; Wright, D.C.; Kirby, G.M.; et al. The day/night proteome in the murine heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R121–R137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Bradfield, C.A.; Hussain, M.M. Global and hepatocyte-specific ablation of Bmal1 induces hyperlipidaemia and enhances atherosclerosis. Nat. Commun. 2016, 7, 13011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Colson, J.C.; Jin, C.; Becker, B.K.; Rhoads, M.K.; Pati, P.; Neder, T.H.; King, M.A.; Valcin, J.A.; Tao, B.; et al. Timing of Food Intake Drives the Circadian Rhythm of Blood Pressure. Function 2021, 2, zqaa034. [Google Scholar] [CrossRef] [PubMed]
- Pati, P.; Valcin, J.A.; Zhang, D.; Neder, T.H.; Millender-Swain, T.; Allan, J.M.; Sedaka, R.S.; Jin, C.; Becker, B.K.; Pollock, D.M.; et al. Liver circadian clock disruption alters perivascular adipose tissue gene expression and aortic function in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021. [Google Scholar] [CrossRef]
- Knutsson, A.; Akerstedt, T.; Jonsson, B.G.; Orth-Gomer, K. Increased risk of ischaemic heart disease in shift workers. Lancet 1986, 2, 89–92. [Google Scholar] [CrossRef]
- Kawachi, I.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Manson, J.E.; Speizer, F.E.; Hennekens, C.H. Prospective study of shift work and risk of coronary heart disease in women. Circulation 1995, 92, 3178–3182. [Google Scholar] [CrossRef]
- Niedhammer, I.; Lert, F.; Marne, M.J. Prevalence of overweight and weight gain in relation to night work in a nurses’cohort. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 625–633. [Google Scholar] [PubMed]
- Suwazono, Y.; Sakata, K.; Okubo, Y.; Harada, H.; Oishi, M.; Kobayashi, E.; Uetani, M.; Kido, T.; Nogawa, K. Long-term longitudinal study on the relationship between alternating shift work and the onset of diabetes mellitus in male Japanese workers. J. Occup. Environ. Med. 2006, 48, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Suwazono, Y.; Dochi, M.; Sakata, K.; Okubo, Y.; Oishi, M.; Tanaka, K.; Kobayashi, E.; Kido, T.; Nogawa, K. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity 2008, 16, 1887–1893. [Google Scholar] [CrossRef]
- Yamasaki, F.; Schwartz, J.E.; Gerber, L.M.; Warren, K.; Pickering, T.G. Impact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholamines. Hypertension 1998, 32, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Nam, J.; Lee, J.K.; Oh, S.S.; Kang, H.T.; Koh, S.B. Association between night work and cardiovascular diseases: Analysis of the 3rd Korean working conditions survey. Ann. Occup. Environ. Med. 2015, 27, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, B.C.; van der Putten, K.; Van Someren, E.J.; Wielders, J.P.; Ter Wee, P.M.; Nagtegaal, J.E.; Gaillard, C.A. Impairment of endogenous melatonin rhythm is related to the degree of chronic kidney disease (CREAM study). Nephrol. Dial. Transpl. 2010, 25, 513–519. [Google Scholar] [CrossRef]
- Russcher, M.; Chaves, I.; Lech, K.; Koch, B.C.; Nagtegaal, J.E.; Dorsman, K.F.; Jong, A.; Kayser, M.; van Faassen, H.M.; Kema, I.P.; et al. An observational study on disturbed peripheral circadian rhythms in hemodialysis patients. Chronobiol. Int. 2015, 32, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Crespo, J.J.; Domínguez-Sardiña, M.; Otero, A.; Moyá, A.; Ríos, M.T.; Sineiro, E.; Castiñeira, M.C.; Callejas, P.A.; Pousa, L.; et al. Bedtime hypertension treatment improves cardiovascular risk reduction: The Hygia Chronotherapy Trial. Eur. Heart J. 2020, 41, 4565–4576. [Google Scholar] [CrossRef]
- Rahman, A.; Hasan, A.U.; Nishiyama, A.; Kobori, H. Altered Circadian Timing System-Mediated Non-Dipping Pattern of Blood Pressure and Associated Cardiovascular Disorders in Metabolic and Kidney Diseases. Int. J. Mol. Sci. 2018, 19, 400. [Google Scholar] [CrossRef] [Green Version]
- Makimoto, H.; Shimizu, K.; Fujiu, K.; Lin, T.; Oshima, T.; Amiya, E.; Yamagata, K.; Kojima, T.; Daimon, M.; Nagatomo, R.; et al. Effect of Sympatholytic Therapy on Circadian Cardiac Autonomic Activity in Non-Diabetic Chronic Kidney Disease. Int. Heart J. 2018, 59, 1352–1358. [Google Scholar] [CrossRef] [Green Version]
- Motohashi, H.; Tahara, Y.; Whittaker, D.S.; Wang, H.B.; Yamaji, T.; Wakui, H.; Haraguchi, A.; Yamazaki, M.; Miyakawa, H.; Hama, K.; et al. The circadian clock is disrupted in mice with adenine-induced tubulointerstitial nephropathy. Kidney Int. 2020, 97, 728–740. [Google Scholar] [CrossRef] [Green Version]
- Moe, S.M.; Chen, N.X. Mechanisms of vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2008, 19, 213–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; London, G.M. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001, 38, 938–942. [Google Scholar] [CrossRef] [Green Version]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M.; Jono, S.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H. Vascular calcification and inorganic phosphate. Am. J. Kidney Dis. 2001, 38, S34–S37. [Google Scholar] [CrossRef] [PubMed]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 87, E10–E17. [Google Scholar] [CrossRef]
- Rukov, J.L.; Gravesen, E.; Mace, M.L.; Hofman-Bang, J.; Vinther, J.; Andersen, C.B.; Lewin, E.; Olgaard, K. Effect of chronic uremia on the transcriptional profile of the calcified aorta analyzed by RNA sequencing. Am. J. Physiol. Ren. Physiol. 2016, 310, F477–F491. [Google Scholar] [CrossRef] [Green Version]
- Egstrand, S.; Nordholm, A.; Morevati, M.; Mace, M.L.; Hassan, A.; Naveh-Many, T.; Rukov, J.L.; Gravesen, E.; Olgaard, K.; Lewin, E. A molecular circadian clock operates in the parathyroid gland and is disturbed in chronic kidney disease associated bone and mineral disorder. Kidney Int. 2020, 98, 1461–1475. [Google Scholar] [CrossRef]
- Egstrand, S.; Olgaard, K.; Lewin, E. Circadian rhythms of mineral metabolism in chronic kidney disease-mineral bone disorder. Curr. Opin. Nephrol. Hypertens 2020, 29, 367–377. [Google Scholar] [CrossRef]
- Bonny, O.; Firsov, D. Circadian regulation of renal function and potential role in hypertension. Curr. Opin. Nephrol. Hypertens 2013, 22, 439–444. [Google Scholar] [CrossRef]
- Ansermet, C.; Centeno, G.; Nikolaeva, S.; Maillard, M.P.; Pradervand, S.; Firsov, D. The intrinsic circadian clock in podocytes controls glomerular filtration rate. Sci. Rep. 2019, 9, 16089. [Google Scholar] [CrossRef]
- Zuber, A.M.; Centeno, G.; Pradervand, S.; Nikolaeva, S.; Maquelin, L.; Cardinaux, L.; Bonny, O.; Firsov, D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc. Natl. Acad. Sci. USA 2009, 106, 16523–16528. [Google Scholar] [CrossRef] [Green Version]
- Solocinski, K.; Gumz, M.L. The Circadian Clock in the Regulation of Renal Rhythms. J. Biol. Rhythm. 2015, 30, 470–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, J.; Cheng, K.Y.; All, S.; Skopis, G.; Jeffers, L.; Lynch, I.J.; Wingo, C.S.; Gumz, M.L. A role for the circadian clock protein Per1 in the regulation of aldosterone levels and renal Na+ retention. Am. J. Physiol. Ren. Physiol. 2013, 305, F1697–F1704. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [Green Version]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.K.; Panda, S.; Miraglia, L.J.; Reyes, T.M.; Rudic, R.D.; McNamara, P.; Naik, K.A.; FitzGerald, G.A.; Kay, S.A.; Hogenesch, J.B. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 2004, 43, 527–537. [Google Scholar] [CrossRef] [Green Version]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Rey, G.; Cesbron, F.; Rougemont, J.; Reinke, H.; Brunner, M.; Naef, F. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 2011, 9, e1000595. [Google Scholar] [CrossRef] [Green Version]
- Storch, K.F.; Lipan, O.; Leykin, I.; Viswanathan, N.; Davis, F.C.; Wong, W.H.; Weitz, C.J. Extensive and divergent circadian gene expression in liver and heart. Nature 2002, 417, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [Green Version]
- Myung, J.; Wu, M.Y.; Lee, C.Y.; Rahim, A.R.; Truong, V.H.; Wu, D.; Piggins, H.D.; Wu, M.S. The Kidney Clock Contributes to Timekeeping by the Master Circadian Clock. Int. J. Mol. Sci. 2019, 20, 2765. [Google Scholar] [CrossRef] [Green Version]
- McNamara, P.; Seo, S.B.; Rudic, R.D.; Sehgal, A.; Chakravarti, D.; FitzGerald, G.A. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: A humoral mechanism to reset a peripheral clock. Cell 2001, 105, 877–889. [Google Scholar] [CrossRef] [Green Version]
- Nonaka, H.; Emoto, N.; Ikeda, K.; Fukuya, H.; Rohman, M.S.; Raharjo, S.B.; Yagita, K.; Okamura, H.; Yokoyama, M. Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation 2001, 104, 1746–1748. [Google Scholar] [CrossRef] [Green Version]
- Takeda, N.; Maemura, K.; Horie, S.; Oishi, K.; Imai, Y.; Harada, T.; Saito, T.; Shiga, T.; Amiya, E.; Manabe, I.; et al. Thrombomodulin is a clock-controlled gene in vascular endothelial cells. J. Biol. Chem. 2007, 282, 32561–32567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welsh, D.K.; Yoo, S.H.; Liu, A.C.; Takahashi, J.S.; Kay, S.A. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr. Biol. 2004, 14, 2289–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, A.W.C.; Li, H.; Xia, N. Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis. Int. J. Mol. Sci. 2021, 22, 676. [Google Scholar] [CrossRef]
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M.; Clendenin, C.; Radcliffe, L.A.; Hogenesch, J.B.; Simon, M.C.; Takahashi, J.S.; Bradfield, C.A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000, 103, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anea, C.B.; Zhang, M.; Stepp, D.W.; Simkins, G.B.; Reed, G.; Fulton, D.J.; Rudic, R.D. Vascular disease in mice with a dysfunctional circadian clock. Circulation 2009, 119, 1510–1517. [Google Scholar] [CrossRef] [Green Version]
- Debruyne, J.P.; Noton, E.; Lambert, C.M.; Maywood, E.S.; Weaver, D.R.; Reppert, S.M. A clock shock: Mouse CLOCK is not required for circadian oscillator function. Neuron 2006, 50, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Dubrovsky, Y.V.; Samsa, W.E.; Kondratov, R.V. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging 2010, 2, 936–944. [Google Scholar] [CrossRef] [Green Version]
- Viswambharan, H.; Carvas, J.M.; Antic, V.; Marecic, A.; Jud, C.; Zaugg, C.E.; Ming, X.F.; Montani, J.P.; Albrecht, U.; Yang, Z. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation 2007, 115, 2188–2195. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Zhao, X.; Hatori, M.; Yu, R.T.; Barish, G.D.; Lam, M.T.; Chong, L.W.; DiTacchio, L.; Atkins, A.R.; Glass, C.K.; et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 2012, 485, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Hurd, M.W.; Ralph, M.R. The significance of circadian organization for longevity in the golden hamster. J. Biol. Rhythm. 1998, 13, 430–436. [Google Scholar] [CrossRef]
- Shang, X.; Pati, P.; Anea, C.B.; Fulton, D.J.R.; Rudic, R.D. Differential Regulation of BMAL1, CLOCK, and Endothelial Signaling in the Aortic Arch and Ligated Common Carotid Artery. J. Vasc. Res. 2016, 53, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anea, C.B.; Ali, M.I.; Osmond, J.M.; Sullivan, J.C.; Stepp, D.W.; Merloiu, A.M.; Rudic, R.D. Matrix metalloproteinase 2 and 9 dysfunction underlie vascular stiffness in circadian clock mutant mice. Arterioscler. Thromb Vasc. Biol. 2010, 30, 2535–2543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatwadekar, A.D.; Beli, E.; Diao, Y.; Chen, J.; Luo, Q.; Alex, A.; Caballero, S.; Dominguez, J.M., 2nd; Salazar, T.E.; Busik, J.V.; et al. Conditional Deletion of Bmal1 Accentuates Microvascular and Macrovascular Injury. Am. J. Pathol. 2017, 187, 1426–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westgate, E.J.; Cheng, Y.; Reilly, D.F.; Price, T.S.; Walisser, J.A.; Bradfield, C.A.; FitzGerald, G.A. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation 2008, 117, 2087–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Su, W.; Liu, S.; Zhao, G.; Esser, K.; Schroder, E.A.; Lefta, M.; Stauss, H.M.; Guo, Z.; Gong, M.C. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J. Clin. Investig. 2015, 125, 324–336. [Google Scholar] [CrossRef] [Green Version]
- Lutshumba, J.; Liu, S.; Zhong, Y.; Hou, T.; Daugherty, A.; Lu, H.; Guo, Z.; Gong, M.C. Deletion of BMAL1 in Smooth Muscle Cells Protects Mice From Abdominal Aortic Aneurysms. Arterioscler. Thromb Vasc. Biol. 2018, 38, 1063–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hruska, K.A.; Seifert, M.; Sugatani, T. Pathophysiology of the chronic kidney disease-mineral bone disorder. Curr. Opin. Nephrol. Hypertens 2015, 24, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Mace, M.L.; Gravesen, E.; Nordholm, A.; Egstrand, S.; Morevati, M.; Nielsen, C.; Kjaer, A.; Behets, G.; D’Haese, P.; Olgaard, K.; et al. Chronic Kidney Disease-Induced Vascular Calcification Impairs Bone Metabolism. J. Bone Miner. Res. 2021, 36, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Hara, R.; Wan, K.; Wakamatsu, H.; Aida, R.; Moriya, T.; Akiyama, M.; Shibata, S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells 2001, 6, 269–278. [Google Scholar] [CrossRef]
- Yoshitane, H.; Asano, Y.; Sagami, A.; Sakai, S.; Suzuki, Y.; Okamura, H.; Iwasaki, W.; Ozaki, H.; Fukada, Y. Functional D-box sequences reset the circadian clock and drive mRNA rhythms. Commun. Biol. 2019, 2, 300. [Google Scholar] [CrossRef] [Green Version]
- Migita, H.; Morser, J.; Kawai, K. Rev-erbalpha upregulates NF-kappaB-responsive genes in vascular smooth muscle cells. FEBS Lett. 2004, 561, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Raspé, E.; Duez, H.; Mansén, A.; Fontaine, C.; Fiévet, C.; Fruchart, J.C.; Vennström, B.; Staels, B. Identification of Rev-erbalpha as a physiological repressor of apoC-III gene transcription. J. Lipid Res. 2002, 43, 2172–2179. [Google Scholar] [CrossRef] [Green Version]
- Sitaula, S.; Billon, C.; Kamenecka, T.M.; Solt, L.A.; Burris, T.P. Suppression of atherosclerosis by synthetic REV-ERB agonist. Biochem. Biophys. Res. Commun. 2015, 460, 566–571. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Sakurai, T.; Ogasawara, J.; Takahashi, M.; Izawa, T.; Imaizumi, K.; Taniguchi, N.; Ohno, H.; Kizaki, T. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J. Immunol. 2014, 192, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Noels, H.; Weber, C.; Koenen, R.R. Chemokines as Therapeutic Targets in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 583–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesch, G.H. MCP-1/CCL2: A new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2008, 294, F697–F701. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.; Osman, M.; Ferguson, C.M.; Nath, M.C.; Roy, B.; Lien, K.R.; Nath, K.A.; Garovic, V.D.; Lerman, L.O.; Grande, J.P. Ccl2 deficiency protects against chronic renal injury in murine renovascular hypertension. Sci. Rep. 2018, 8, 8598. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, S.; Warner, G.M.; Hartono, S.P.; Boyilla, R.; Knudsen, B.E.; Zubair, A.S.; Lien, K.; Nath, K.A.; Textor, S.C.; Lerman, L.O.; et al. Blockade of CCR2 reduces macrophage influx and development of chronic renal damage in murine renovascular hypertension. Am. J. Physiol. Ren. Physiol. 2016, 310, F372–F384. [Google Scholar] [CrossRef] [Green Version]
- Koh, K.K.; Quon, M.J.; Han, S.H.; Chung, W.J.; Ahn, J.Y.; Seo, Y.H.; Kang, M.H.; Ahn, T.H.; Choi, I.S.; Shin, E.K. Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation 2004, 110, 3687–3692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, K.K.; Ahn, J.Y.; Han, S.H.; Kim, D.S.; Jin, D.K.; Kim, H.S.; Shin, M.S.; Ahn, T.H.; Choi, I.S.; Shin, E.K. Pleiotropic effects of angiotensin II receptor blocker in hypertensive patients. J. Am. Coll. Cardiol. 2003, 42, 905–910. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.D.; Fentress, S.J.; Qiu, Y.; Yun, K.; Cox, J.S.; Chawla, A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 2013, 341, 1483–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papayianni, A.; Alexopoulos, E.; Giamalis, P.; Gionanlis, L.; Belechri, A.M.; Koukoudis, P.; Memmos, D. Circulating levels of ICAM-1, VCAM-1, and MCP-1 are increased in haemodialysis patients: Association with inflammation, dyslipidaemia, and vascular events. Nephrol. Dial. Transplant. 2002, 17, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Stenvinkel, P.; Lindholm, B.; Heimbürger, M.; Heimbürger, O. Elevated serum levels of soluble adhesion molecules predict death in pre-dialysis patients: Association with malnutrition, inflammation, and cardiovascular disease. Nephrol. Dial. Transplant. 2000, 15, 1624–1630. [Google Scholar] [CrossRef]
- Buck, C.A. Immunoglobulin superfamily: Structure, function and relationship to other receptor molecules. Semin. Cell Biol. 1992, 3, 179–188. [Google Scholar] [CrossRef]
- Filippi, M.D. Mechanism of Diapedesis: Importance of the Transcellular Route. Adv. Immunol. 2016, 129, 25–53. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Holtkamp, S.; Hergenhan, S.M.; Kraus, K.; de Juan, A.; Weber, J.; Bradfield, P.; Grenier, J.M.P.; Pelletier, J.; Druzd, D.; et al. Circadian Expression of Migratory Factors Establishes Lineage-Specific Signatures that Guide the Homing of Leukocyte Subsets to Tissues. Immunity 2018, 49, 1175–1190.e1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, J.E.; Blaikley, J.; Beesley, S.; Matthews, L.; Simpson, K.D.; Boyce, S.H.; Farrow, S.N.; Else, K.J.; Singh, D.; Ray, D.W.; et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Barrientos, A.; López-Romero, P.; Vivas, D.; Castro-Ferreira, F.; Núñez-Gil, I.; Franco, E.; Ruiz-Mateos, B.; García-Rubira, J.C.; Fernández-Ortiz, A.; Macaya, C.; et al. Circadian variations of infarct size in acute myocardial infarction. Heart 2011, 97, 970–976. [Google Scholar] [CrossRef] [Green Version]
- Schloss, M.J.; Horckmans, M.; Nitz, K.; Duchene, J.; Drechsler, M.; Bidzhekov, K.; Scheiermann, C.; Weber, C.; Soehnlein, O.; Steffens, S. The time-of-day of myocardial infarction onset affects healing through oscillations in cardiac neutrophil recruitment. EMBO Mol. Med. 2016, 8, 937–948. [Google Scholar] [CrossRef]
- Schloss, M.J.; Hilby, M.; Nitz, K.; Guillamat Prats, R.; Ferraro, B.; Leoni, G.; Soehnlein, O.; Kessler, T.; He, W.; Luckow, B.; et al. Ly6C(high) Monocytes Oscillate in the Heart During Homeostasis and After Myocardial Infarction-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Durgan, D.J.; Pulinilkunnil, T.; Villegas-Montoya, C.; Garvey, M.E.; Frangogiannis, N.G.; Michael, L.H.; Chow, C.W.; Dyck, J.R.; Young, M.E. Short communication: Ischemia/reperfusion tolerance is time-of-day-dependent: Mediation by the cardiomyocyte circadian clock. Circ. Res. 2010, 106, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Carmona, P.; Mendez, N.; Ili, C.G.; Brebi, P. The Role of Clock Genes in Fibrinolysis Regulation: Circadian Disturbance and Its Effect on Fibrinolytic Activity. Front. Physiol. 2020, 11, 129. [Google Scholar] [CrossRef]
- Somanath, P.R.; Podrez, E.A.; Chen, J.; Ma, Y.; Marchant, K.; Antoch, M.; Byzova, T.V. Deficiency in core circadian protein Bmal1 is associated with a prothrombotic and vascular phenotype. J. Cell Physiol. 2011, 226, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravesen, E.; Nordholm, A.; Mace, M.; Morevati, M.; Hogdall, E.; Nielsen, C.; Kjaer, A.; Olgaard, K.; Lewin, E. Effect of inhibition of CBP-coactivated beta-catenin-mediated Wnt signalling in uremic rats with vascular calcifications. PLoS ONE 2018, 13, e0201936. [Google Scholar] [CrossRef]

| Gene | Name | Control Aorta (rpkm) | Uremic Calcified Aorta (rpkm) | p-Value |
|---|---|---|---|---|
| Bmal1/Arntl | Aryl hydrocarbon receptor nuclear translocator-like 1 | 7 | 8 | 0.52 |
| Clock | Circadian locomotor output cycles kaput | 2 | 5 | <0.001 |
| Per1 | Period circadian clock 1 | 87 | 51 | <0.001 |
| Per2 | Period circadian clock 2 | 51 | 32 | 0.003 |
| Per3 | Period circadian clock 3 | 16 | 7 | <0.001 |
| Cry1 | Cryptochrome 1 | 3 | 3 | 1 |
| Cry2 | Cryptochrome 2 | 10 | 11 | 0.91 |
| Rev-Erbα/ Nr1d1 | Nuclear Receptor Subfamily 1, Group D, Member 1 | 145 | 42 | <0.05 |
| Rev-Erbβ/ Nr1d2 | Nuclear Receptor Subfamily 1, Group D, Member 2 | 10 | 7 | <0.05 |
| Dbp | D site of albumin promoter (albumin D-box) binding protein | 206 | 28 | <0.001 |
| Nfil3 | Nuclear factor, interleukin 3 regulated | 73 | 32 | <0.001 |
| Wee1 | WEE1 G2 checkpoint kinase | 26 | 10 | <0.001 |
| Ccdn1 | Cyclin D1 | 22 | 86 | <0.001 |
| Icam1 | Intercellular adhesion molecule 1 | 12 | 28 | <0.001 |
| Vcam1 | Vascular cell adhesion molecule 1 | 80 | 293 | <0.001 |
| Ccl2 | Chemokine ligand 2 | 2 | 26 | <0.001 |
| Thbd | Thrombomodulin | 40 | 23 | <0.001 |
| Vwf | von Willebrand factor | 7 | 16 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egstrand, S.; Mace, M.L.; Olgaard, K.; Lewin, E. The Vascular Circadian Clock in Chronic Kidney Disease. Cells 2021, 10, 1769. https://doi.org/10.3390/cells10071769
Egstrand S, Mace ML, Olgaard K, Lewin E. The Vascular Circadian Clock in Chronic Kidney Disease. Cells. 2021; 10(7):1769. https://doi.org/10.3390/cells10071769
Chicago/Turabian StyleEgstrand, Søren, Maria L. Mace, Klaus Olgaard, and Ewa Lewin. 2021. "The Vascular Circadian Clock in Chronic Kidney Disease" Cells 10, no. 7: 1769. https://doi.org/10.3390/cells10071769
APA StyleEgstrand, S., Mace, M. L., Olgaard, K., & Lewin, E. (2021). The Vascular Circadian Clock in Chronic Kidney Disease. Cells, 10(7), 1769. https://doi.org/10.3390/cells10071769





