Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction
Abstract
:1. Introduction
2. Clinical Complications with Implants
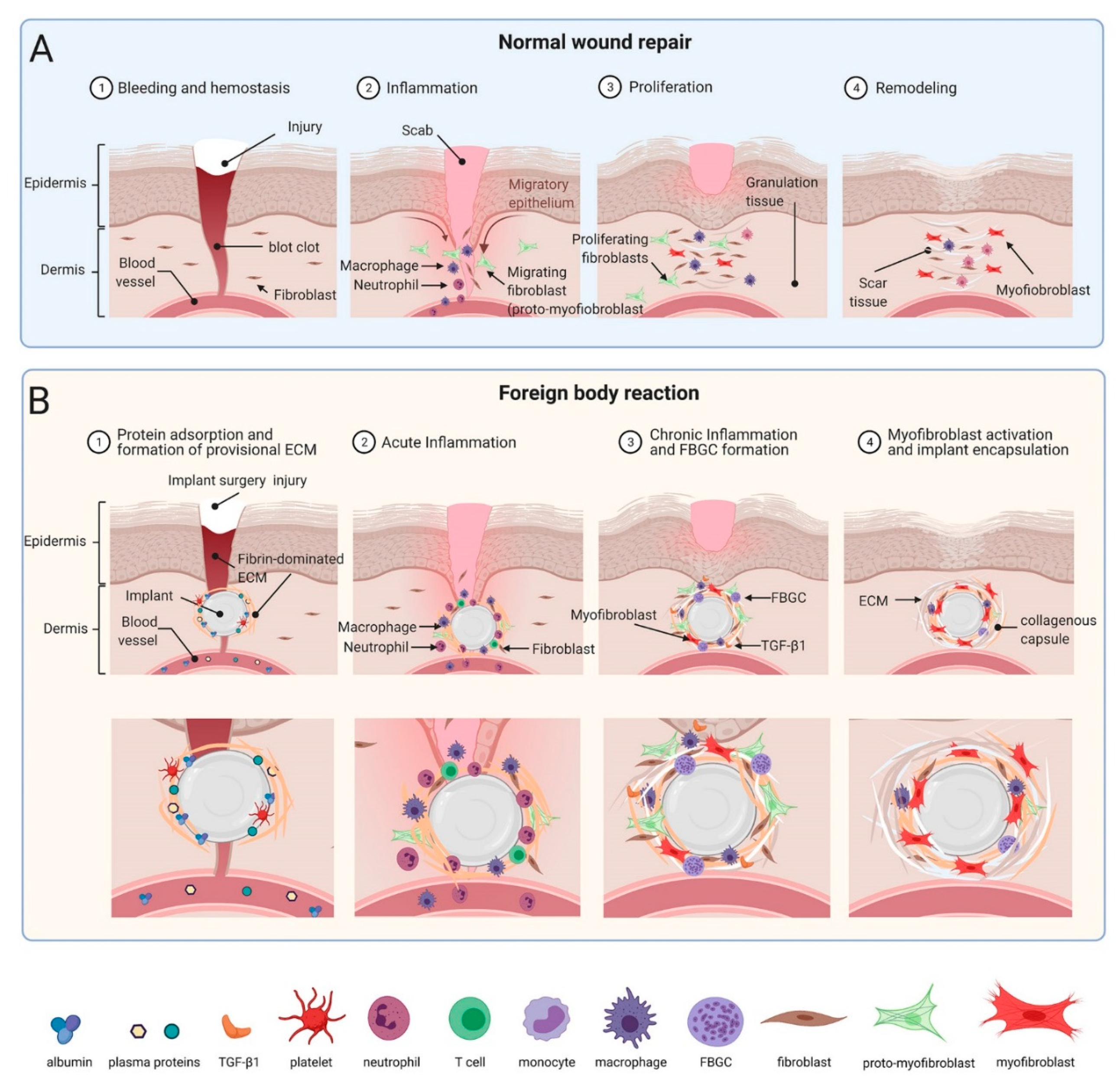
3. Commonalities between the FBR and Normal Wound Healing
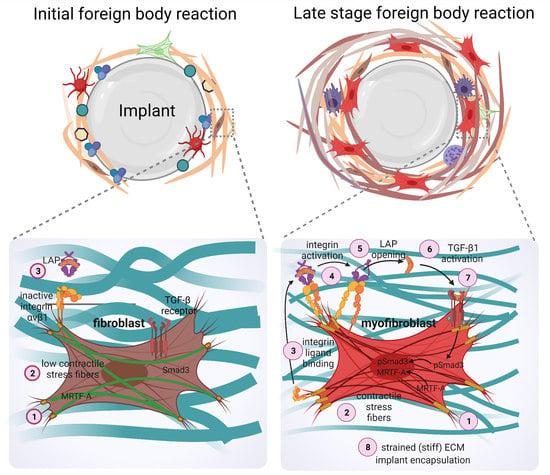
4. What Is Different between Normal Wound Healing and the FBR? The Implant
5. Implant Surfaces from the Perspective of a Macrophage
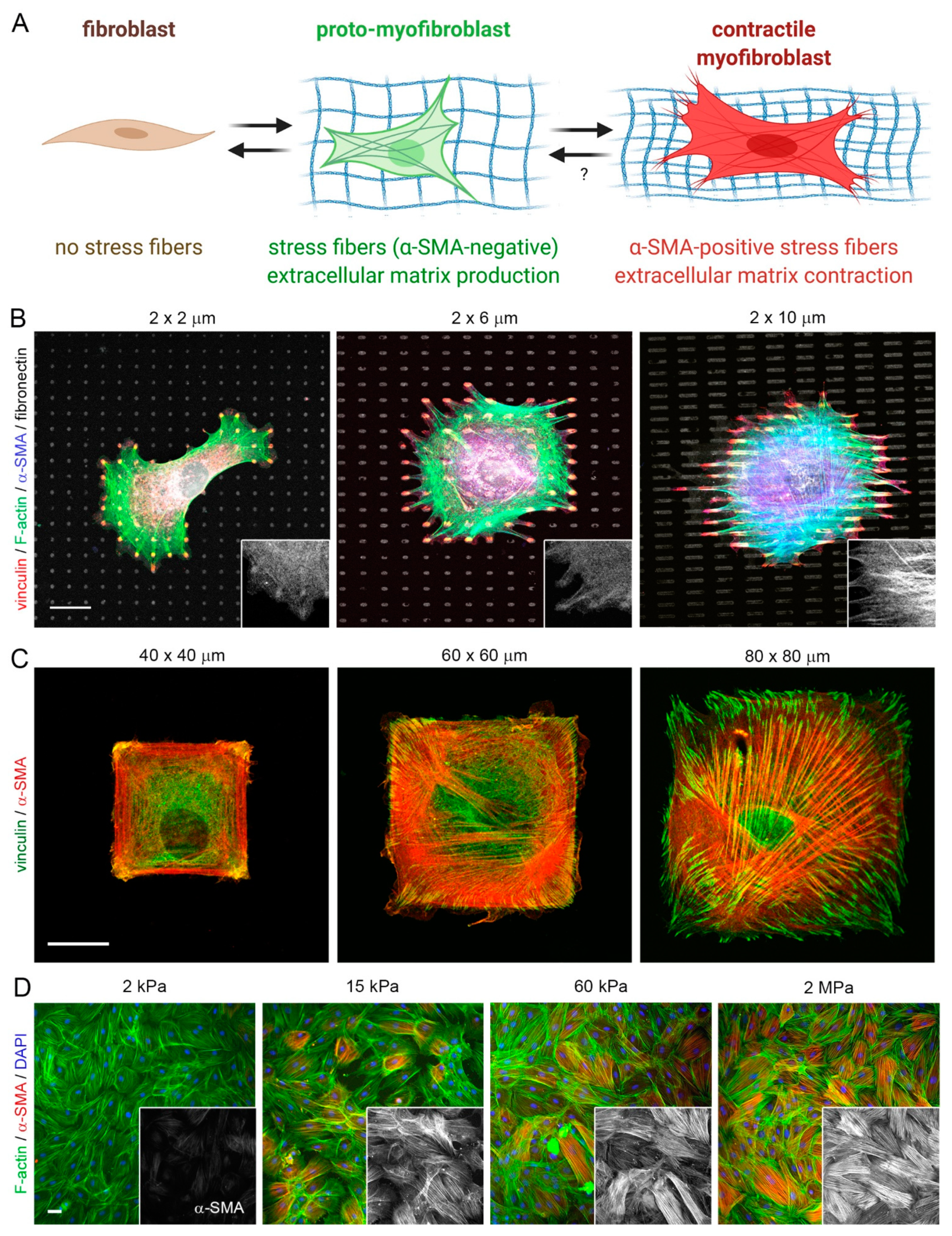
6. A Fibroblast View on the Implant: The Origins of Implant Fibrosis
7. Outlook: Lessons to Learn from Anti-Fibrosis Strategies against Cell Mechanosensing?
8. Conclusions and Open Questions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratner, B.D. Biomaterials: Been There, Done That, and Evolving into the Future. Annu. Rev. Biomed. Eng. 2019, 21, 171–191. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M. Exploiting the inflammatory response on biomaterials research and development. J. Mater. Sci. Mater. Electron. 2015, 26, 121. [Google Scholar] [CrossRef]
- Witherel, C.E.; Abebayehu, D.; Barker, T.H.; Spiller, K.L. Macrophage and Fibroblast Interactions in Biomaterial-Mediated Fibrosis. Adv. Health Mater. 2019, 8, e1801451. [Google Scholar] [CrossRef]
- Veiseh, O.; Vegas, A.J. Domesticating the foreign body response: Recent advances and applications. Adv. Drug Deliv. Rev. 2019, 144, 148–161. [Google Scholar] [CrossRef]
- Williams, D.F. Biocompatibility Pathways: Biomaterials-Induced Sterile Inflammation, Mechanotransduction, and Principles of Biocompatibility Control. ACS Biomater. Sci. Eng. 2017, 3, 2–35. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. Part A 2017, 105, 927–940. [Google Scholar] [CrossRef]
- Grainger, D.W. All charged up about implanted biomaterials. Nat. Biotechnol. 2013, 31, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Adusei, K.M.; Ngo, T.B.; Sadtler, K. T lymphocytes as critical mediators in tissue regeneration, fibrosis, and the foreign body response. Acta Biomater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.M.; Sales, A.; Chen, H.; Biela, S.A.; Kaufmann, D.; Kemkemer, R. Nano- and microstructured materials for in vitro studies of the physiology of vascular cells. Beilstein J. Nanotechnol. 2016, 7, 1620–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abaricia, J.O.; Farzad, N.; Heath, T.J.; Simmons, J.; Morandini, L.; Olivares-Navarrete, R. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 2021. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosner, G.A.; Fonacier, L.S. Hypersensitivity to biomedical implants: Prevention and diagnosis. Allergy Asthma Proc. 2017, 38, 177–183. [Google Scholar] [CrossRef]
- Schalock, P.C.; Thyssen, J.P. Patch Testers’ Opinions Regarding Diagnostic Criteria for Metal Hypersensitivity Reactions to Metallic Implants. Dermatitis 2013, 24, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.A.; Von Fraunhofer, J.A. Success or failure of dental implants? A literature review with treatment considerations. Gen. Dent. 2005, 53, 423. [Google Scholar] [PubMed]
- Liaw, K.; Delfini, R.H.; Abrahams, J.J. Dental Implant Complications. Semin. Ultrasound CT MR 2015, 36, 427–433. [Google Scholar] [CrossRef]
- Tolman, D.E.; Laney, W.R.; Tolman, D.E.; Laney, W.R. Tissue-integrated prosthesis complications. Int. J. Oral Maxillofac. Implant. 1992, 7, 477–484. [Google Scholar] [CrossRef]
- Quirynen, M.; De Soete, M.; Van Steenberghe, D. Infectious risks for oral implants: A review of the literature. Clin. Oral Implant. Res. 2002, 13, 1–19. [Google Scholar] [CrossRef]
- Gibon, E.; Amanatullah, D.F.; Loi, F.; Pajarinen, J.; Nabeshima, A.; Yao, Z.; Hamadouche, M.; Goodman, S.B. The biological response to orthopaedic implants for joint replacement: Part I: Metals. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2162–2173. [Google Scholar] [CrossRef]
- Urban, R.M.; Jacobs, J.; Tomlinson, M.J.; Gavrilovic, J.; Black, J.; Peoc’H, M. Dissemination of Wear Particles to the Liver, Spleen, and Abdominal Lymph Nodes of Patients with Hip or Knee Replacement. J. Bone Jt. Surg. Am. Vol. 2000, 82, 457–477. [Google Scholar] [CrossRef]
- Hauser, R.G.; Katsiyiannis, W.T.; Gornick, C.C.; Almquist, A.K.; Kallinen, L.M. Deaths and cardiovascular injuries due to device-assisted implantable cardioverter-defibrillator and pacemaker lead extraction. Europace 2009, 12, 395–401. [Google Scholar] [CrossRef]
- Nof, E.; Epstein, L. Complications of cardiac implants: Handling device infections. Eur. Hear. J. 2013, 34, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Tarakji, K.G.; Chan, E.J.; Cantillon, D.J.; Doonan, A.L.; Hu, T.; Schmitt, S.; Fraser, T.G.; Kim, A.; Gordon, S.M.; Wilkoff, B. Cardiac implantable electronic device infections: Presentation, management, and patient outcomes. Hear. Rhythm. 2010, 7, 1043–1047. [Google Scholar] [CrossRef]
- Baman, T.S.; Gupta, S.K.; Valle, J.A.; Yamada, E. Risk Factors for Mortality in Patients with Cardiac Device-Related Infection. Circ. Arrhythmia Electrophysiol. 2009, 2, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Keiler, J.; Schulze, M.; Sombetzki, M.; Heller, T.; Tischer, T.; Grabow, N.; Wree, A.; Bänsch, D. Neointimal fibrotic lead encapsulation—Clinical challenges and demands for implantable cardiac electronic devices. J. Cardiol. 2017, 70, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Epstein, L.M.; Love, C.J.; Wilkoff, B.; Chung, M.K.; Hackler, J.W.; Bongiorni, M.G.; Segreti, L.; Carrillo, R.G.; Baltodano, P.; Fischer, A.; et al. Superior Vena Cava Defibrillator Coils Make Transvenous Lead Extraction More Challenging and Riskier. J. Am. Coll. Cardiol. 2013, 61, 987–989. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.C.; Love, C.J. Extraction of Transvenous Pacing and ICD Leads. Pacing Clin. Electrophysiol. 2008, 31, 736–752. [Google Scholar] [CrossRef] [PubMed]
- Yakish, S.J.; Narula, A.; Foley, R.; Kohut, A.; Kutalek, S. Superior Vena Cava Echocardiography as a Screening Tool to Predict Cardiovascular Implantable Electronic Device Lead Fibrosis. J. Cardiovasc. Ultrasound 2015, 23, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, A.; Friedman, O. Combined Breast Implant Explantation and Multilevel Mastopexy Technique. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2429. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A.; Maxwell, G.P. The Evolution of Breast Implants. Clin. Plast. Surg. 2015, 42, 399–404. [Google Scholar] [CrossRef] [PubMed]
- American_Society_of_Plastic_Surgeons. Cosmetic Surgery Procedures. National Plastic Surgery Statistics. 2020. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-report-2020.pdf (accessed on 14 July 2021).
- Deva, A.K.; Adams, W.P., Jr.; Vickery, K. The Role of Bacterial Biofilms in Device-Associated Infection. Plast. Reconstr. Surg. 2013, 132, 1319–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coroneos, C.J.; Selber, J.C.; Offodile, A.C., 2nd; Butler, C.E.; Clemens, M.W. US FDA Breast Implant Postapproval Studies: Long-term Outcomes in 99,993 Patients. Ann Surg. 2019, 269, 30–36. [Google Scholar] [CrossRef]
- Headon, H.; Kasem, A.; Mokbel, K. Capsular Contracture after Breast Augmentation: An Update for Clinical Practice. Arch. Plast. Surg. 2015, 42, 532–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiert, A.; Sorg, H.; Boyce, M. Capsular contracture by silicone breast implants: Possible causes, biocompatibility, and prophylactic strategies. Med. Devices Évid. Res. 2013, 6, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collett, D.J.; Rakhorst, H.; Lennox, P.; Magnusson, M.; Cooter, R.; Deva, A. Current Risk Estimate of Breast Implant–Associated Anaplastic Large Cell Lymphoma in Textured Breast Implants. Plast. Reconstr. Surg. 2019, 143, 30S–40S. [Google Scholar] [CrossRef]
- Stack, A.; Ali, N.; Khan, N. Breast Implant-associated Anaplastic Large Cell Lymphoma: A Review with Emphasis on the Role of Brentuximab Vedotin. J. Cell Immunol. 2020, 2, 80–89. [Google Scholar] [PubMed]
- Clemens, M.W.; Medeiros, L.J.; Butler, C.E.; Hunt, K.K.; Fanale, M.A.; Horwitz, S.; Weisenburger, D.D.; Liu, J.; Morgan, E.; Kanagal-Shamanna, R.; et al. Complete Surgical Excision Is Essential for the Management of Patients with Breast Implant–Associated Anaplastic Large-Cell Lymphoma. J. Clin. Oncol. 2016, 34, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemens, M.W.; Brody, G.S.; Mahabir, R.C.; Miranda, R.N. How to Diagnose and Treat Breast Implant-Associated Anaplastic Large Cell Lymphoma. Plast Reconstr. Surg. 2018, 141, 586e–599e. [Google Scholar] [CrossRef]
- Sue, G.R.; Long, C.; Lee, G.K. Management of Mastectomy Skin Necrosis in Implant Based Breast Reconstruction. Ann. Plast. Surg. 2017, 78, S208–S211. [Google Scholar] [CrossRef]
- Hultman, C.S.; Daiza, S. Skin-Sparing Mastectomy Flap Complications After Breast Reconstruction: Review of Incidence, Management, and Outcome. Ann. Plast. Surg. 2003, 50, 249–255. [Google Scholar] [CrossRef]
- Rivera-Chacon, D.M.; Alvarado-Velez, M.; Acevedo-Morantes, C.; Singh, S.P.; Gultepe, E.; Nagesha, D.; Sridhar, S.; Ramirez-Vick, J. Fibronectin and Vitronectin Promote Human Fetal Osteoblast Cell Attachment and Proliferation on Nanoporous Titanium Surfaces. J. Biomed. Nanotechnol. 2013, 9, 1092–1097. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Z.; Bai, T.; Carr, L.R.; Ella-Menye, J.-R.; Irvin, C.; Ratner, B.D.; Jiang, S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013, 31, 553–556. [Google Scholar] [CrossRef]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef]
- Jenney, C.R.; Anderson, J.M. Adsorbed serum proteins responsible for surface dependent human macrophage behavior. J. Biomed. Mater. Res. 1999, 49, 435–447. [Google Scholar] [CrossRef]
- Wells, L.A.; Guo, H.; Emili, A.; Sefton, M.V. The profile of adsorbed plasma and serum proteins on methacrylic acid copolymer beads: Effect on complement activation. Biomaterials 2017, 118, 74–83. [Google Scholar] [CrossRef]
- Zdolsek, J.; Eaton, J.W.; Tang, L. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J. Transl. Med. 2007, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Wulff, B.C.; Wilgus, T.A. Mast cell activity in the healing wound: More than meets the eye? Exp. Dermatol. 2013, 22, 507–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komi, D.E.A.; Khomtchouk, K.; Maria, P.L.S. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.; Liu, L.; Rung, S.; Wang, Y.; Ma, Y.; Hu, C.; Zhao, X.; Man, Y.; Qu, Y. Modulation of foreign body reaction and macrophage phenotypes concerning microenvironment. J. Biomed. Mater. Res. Part A 2020, 108, 127–135. [Google Scholar] [CrossRef]
- Van Dyken, S.J.; Locksley, R.M. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: Roles in homeostasis and disease. Annu. Rev. Immunol. 2013, 31, 317–343. [Google Scholar] [CrossRef] [Green Version]
- Kenneth Ward, W. A review of the foreign-body response to subcutaneously-implanted devices: The role of macrophages and cytokines in biofouling and fibrosis. J. Diabetes Sci. Technol. 2008, 2, 768–777. [Google Scholar] [CrossRef] [Green Version]
- Hinz, B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Safferling, K.; Sütterlin, T.; Westphal, K.; Ernst, C.; Breuhahn, K.; James, M.; Jäger, D.; Halama, N.; Grabe, N. Wound healing revised: A novel reepithelialization mechanism revealed by in vitro and in silico models. J. Cell Biol. 2013, 203, 691–709. [Google Scholar] [CrossRef] [Green Version]
- Pakshir, P.; Noskovicova, N.; Lodyga, M.; Son, D.O.; Schuster, R.; Goodwin, A.; Karvonen, H.; Hinz, B. The myofibroblast at a glance. J. Cell Sci. 2020, 133, jcs227900. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.; Rockel, J.S.; Kapoor, M.; Hinz, B. The inflammatory speech of fibroblasts. Immunol. Rev. 2021. [Google Scholar] [CrossRef]
- Hinz, B.; Lagares, D. Evasion of apoptosis by myofibroblasts: A hallmark of fibrotic diseases. Nat. Rev. Rheumatol. 2020, 16, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Luttikhuizen, D.T.; Harmsen, M.C.; Van Luyn, M.J. Cellular and Molecular Dynamics in the Foreign Body Reaction. Tissue Eng. 2006, 12, 1955–1970. [Google Scholar] [CrossRef] [PubMed]
- Dorin, R.P.; Pohl, H.G.; De Filippo, R.E.; Yoo, J.J.; Atala, A. Tubularized urethral replacement with unseeded matrices: What is the maximum distance for normal tissue regeneration? World J. Urol. 2008, 26, 323–326. [Google Scholar] [CrossRef]
- Atala, A. Engineering organs. Curr. Opin. Biotechnol. 2009, 20, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Doloff, J.; Ma, M.; Vegas, A.F.; Tam, H.H.; Bader, A.R.; Li, J.; Langan, E.; Wyckoff, J.; Loo, W.; et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015, 14, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Vroman, L.; Adams, A.L.; Fischer, G.C.; Munoz, P.C. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood 1980, 55, 156–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swartzlander, M.D.; Barnes, C.A.; Blakney, A.K.; Kaar, J.L.; Kyriakides, T.R.; Bryant, S.J. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials 2015, 41, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thang, L.; Thevenot, P.; Hu, W. Surface Chemistry Influences Implant Biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Loppnow, H.; Groth, T. A macrophage/fibroblast co-culture system using a cell migration chamber to study inflammatory effects of biomaterials. Acta Biomater. 2015, 26, 54–63. [Google Scholar] [CrossRef]
- Sussman, E.; Halpin, M.C.; Muster, J.; Moon, R.; Ratner, B.D. Porous Implants Modulate Healing and Induce Shifts in Local Macrophage Polarization in the Foreign Body Reaction. Ann. Biomed. Eng. 2014, 42, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Pullen, N.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef] [Green Version]
- Noskovicova, N.; Schuster, R.; van Putten, S.; Ezzo, M.; Koehler, A.; Boo, S.; Coelho, N.M.; Griggs, D.; Ruminski, P.; McCulloch, C.A.; et al. Suppression of the fibrotic encapsulation of silicone implants by inhibiting the mechanical activation of pro-fibrotic TGF-beta. Nat. Biomed. Eng. 2021. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; Swartzlander, M.D.; Bryant, S.J. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. A 2012, 100, 1375–1386. [Google Scholar] [CrossRef] [Green Version]
- Prasad, B.R.; Brook, M.A.; Smith, T.; Zhao, S.; Chen, Y.; Sheardown, H.; D’Souza, R.; Rochev, Y. Controlling cellular activity by manipulating silicone surface roughness. Colloids Surf. B Biointerfaces 2010, 78, 237–242. [Google Scholar] [CrossRef]
- Ananth, H.; Kundapur, V.; Mohammed, H.S.; Anand, M.; Amarnath, G.S.; Mankar, S. A Review on Biomaterials in Dental Implantology. Int. J. Biomed. Sci. IJBS 2015, 11, 113–120. [Google Scholar] [PubMed]
- Ermis, M.; Antmen, E.; Hasirci, V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: A review from the tissue engineering perspective. Bioact. Mater. 2018, 3, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Doloff, J.C.; Veiseh, O.; de Mezerville, R.; Sforza, M.; Perry, T.A.; Haupt, J.; Jamiel, M.; Chambers, C.; Nash, A.; Aghlara-Fotovat, S.; et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nat. Biomed. Eng. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Clegg, R.; Leavesley, D.; Pearcy, M. Mediation of Biomaterial–Cell Interactions by Adsorbed Proteins: A Review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Liamas, E.; Kubiak-Ossowska, K.; Black, R.A.; Thomas, O.R.; Zhang, Z.J.; Mulheran, P.A. Adsorption of Fibronectin Fragment on Surfaces Using Fully Atomistic Molecular Dynamics Simulations. Int. J. Mol. Sci. 2018, 19, 3321. [Google Scholar] [CrossRef] [Green Version]
- Felgueiras, H.P.; Evans, M.D.M.; Migonney, V. Contribution of fibronectin and vitronectin to the adhesion and morphology of MC3T3-E1 osteoblastic cells to poly(NaSS) grafted Ti6Al4V. Acta Biomater. 2015, 28, 225–233. [Google Scholar] [CrossRef]
- Xu, L.C.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef]
- Tan, F.; Al-Rubeai, M. Customizable Implant-specific and Tissue-Specific Extracellular Matrix Protein Coatings Fabricated Using Atmospheric Plasma. Front. Bioeng. Biotechnol. 2019, 7, 247. [Google Scholar] [CrossRef] [PubMed]
- Khandwekar, A.; Rho, C.K. Modulation of cellular responses on engineered polyurethane implants. J. Biomed. Mater. Res. Part A 2012, 100, 2211–2222. [Google Scholar] [CrossRef]
- He, Y.; Hower, J.; Chen, S.; Bernards, M.T.; Chang, Y.; Jiang, S. Molecular Simulation Studies of Protein Interactions with Zwitterionic Phosphorylcholine Self-Assembled Monolayers in the Presence of Water. Langmuir 2008, 24, 10358–10364. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandor, M.; Singh, D.; Silverman, R.P.; Xu, H.; De Deyne, P.G. Comparative Host Response of 2 Human Acellular Dermal Matrices in a Primate Implant Model. Eplasty 2014, 14, e7. [Google Scholar] [PubMed]
- Mathieu, V.; Vayron, R.; Richard, G.; Lambert, G.; Naili, S.; Meningaud, J.-P.; Haiat, G. Biomechanical determinants of the stability of dental implants: Influence of the bone–implant interface properties. J. Biomech. 2014, 47, 3–13. [Google Scholar] [CrossRef]
- Vagaská, B.; Bacáková, L.; Filová, E.; Balík, K. Osteogenic cells on bio-inspired materials for bone tissue engineering. Physiol. Res. 2009, 59, 309–322. [Google Scholar]
- Tang, D.; Yang, L.-Y.; Ou, K.-L.; Oreffo, R. Repositioning Titanium: An In Vitro Evaluation of Laser-Generated Microporous, Microrough Titanium Templates as a Potential Bridging Interface for Enhanced Osseointegration and Durability of Implants. Front. Bioeng. Biotechnol. 2017, 5, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.Y.; Hansen, J.C.; Siedlecki, C.A.; Runt, J.; Donahue, H.J. Human foetal osteoblastic cell response to polymer-demixed nanotopographic interfaces. J. R. Soc. Interface 2005, 2, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, S.E.; Shao, J.; Beucken, J.J.J.P.V.D. Combinatorial Surface Roughness Effects on Osteoclastogenesis and Osteogenesis. ACS Appl. Mater. Interfaces 2018, 10, 36652–36663. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.G.; Hill, E.; Bayat, A. Designing implant surface topography for improved biocompatibility. Expert Rev. Med. Devices 2013, 10, 257–267. [Google Scholar] [CrossRef]
- Wixtrom, R.N.; Garadi, V.; Leopold, J.; Canady, J.W. Device-Specific Findings of Imprinted-Texture Breast Implants: Characteristics, Risks, and Benefits. Aesthetic Surg. J. 2019, 40, 167–173. [Google Scholar] [CrossRef]
- Wong, C.-H.; Samuel, M.; Tan, B.-K.; Song, C. Capsular Contracture in Subglandular Breast Augmentation with Textured versus Smooth Breast Implants: A Systematic Review. Plast. Reconstr. Surg. 2006, 118, 1224–1236. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, L.; Pan, F.; Gao, Y.; Yuan, X.; Fan, D. Comparison of the Postoperative Incidence Rate of Capsular Contracture among Different Breast Implants: A Cumulative Meta-Analysis. PLoS ONE 2015, 10, e0116071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, B.H.; Kim, B.H.; Kim, S.; Lee, K.; Bin Choy, Y.; Heo, C.Y. Silicone breast implant modification review: Overcoming capsular contracture. Biomater. Res. 2018, 22, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnsley, G.P.; Sigurdson, L.J.; Barnsley, S.E. Textured Surface Breast Implants in the Prevention of Capsular Contracture among Breast Augmentation Patients: A Meta-Analysis of Randomized Controlled Trials. Plast. Reconstr. Surg. 2006, 117, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.G.; Nahabedian, M.Y.; Calobrace, M.B.; Harrington, J.L.; Capizzi, P.J.; Cohen, R.; d’Incelli, R.C.; Beckstrand, M. Risk factor analysis for capsular contracture: A 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2013, 132, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Derby, B.M.; Codner, M.A. Textured silicone breast implant use in primary augmentation: Core data update and review. Plast. Reconstr. Surg. 2015, 135, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.; Hill, E.; Bayat, A. Development, fabrication and evaluation of a novel biomimetic human breast tissue derived breast implant surface. Acta Biomater. 2017, 49, 260–271. [Google Scholar] [CrossRef] [Green Version]
- Loch-Wilkinson, A.; Beath, K.J.; Knight, R.J.W.; Wessels, W.L.F.; Magnusson, M.; Papadopoulos, T.; Connell, T.; Lofts, J.; Locke, M.; Hopper, I.; et al. Breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk. Plast. Reconstr. Surg. 2017, 140, 645–654. [Google Scholar] [CrossRef]
- Tevis, S.E.; Hunt, K.K.; Miranda, R.N.; Lange, C.; Pinnix, C.C.; Iyer, S.; Butler, C.E.; Clemens, M.W. Breast Implant-Associated Anaplastic Large Cell Lymphoma: A Prospective Series of 52 Patients. Ann. Surg. 2020. [Google Scholar] [CrossRef]
- Sforza, M.; Zaccheddu, R.; Alleruzzo, A.; Seno, A.; Mileto, D.; Paganelli, A.; Sulaiman, H.; Payne, M.; Maurovich-Horvat, L. Preliminary 3-Year Evaluation of Experience with SilkSurface and VelvetSurface Motiva Silicone Breast Implants: A Single-Center Experience With 5813 Consecutive Breast Augmentation Cases. Aesthetic Surg. J. 2018, 38, S62–S73. [Google Scholar] [CrossRef]
- Wolf, M.T.; Dearth, C.L.; Ranallo, C.A.; LoPresti, S.T.; Carey, L.E.; Daly, K.A.; Brown, B.N.; Badylak, S.F. Macrophage polarization in response to ECM coated polypropylene mesh. Biomaterials 2014, 35, 6838–6849. [Google Scholar] [CrossRef] [Green Version]
- Bracaglia, L.G.; Fisher, J.P. Extracellular Matrix-Based Biohybrid Materials for Engineering Compliant, Matrix-Dense Tissues. Adv. Heal. Mater. 2015, 4, 2475–2487. [Google Scholar] [CrossRef] [Green Version]
- Oakes, R.; Polei, M.D.; Skousen, J.L.; Tresco, P.A. An astrocyte derived extracellular matrix coating reduces astrogliosis surrounding chronically implanted microelectrode arrays in rat cortex. Biomaterials 2018, 154, 1–11. [Google Scholar] [CrossRef]
- Robotti, F.; Bottan, S.; Fraschetti, F.; Mallone, A.; Pellegrini, G.; Lindenblatt, N.; Starck, C.; Falk, V.; Poulikakos, D.; Ferrari, A. A micron-scale surface topography design reducing cell adhesion to implanted materials. Sci. Rep. 2018, 8, 10887. [Google Scholar] [CrossRef] [PubMed]
- Klos, A.; Sedao, X.; Itina, T.E.; Helfenstein-Didier, C.; Donnet, C.; Peyroche, S.; Vico, L.; Guignandon, A.; Dumas, V. Ultrafast Laser Processing of Nanostructured Patterns for the Control of Cell Adhesion and Migration on Titanium Alloy. Nanomaterials 2020, 10, 864. [Google Scholar] [CrossRef]
- Padmanabhan, J.; Kinser, E.R.; Stalter, M.A.; Duncan-Lewis, C.; Balestrini, J.L.; Sawyer, A.J.; Schroers, J.; Kyriakides, T.R. Engineering Cellular Response Using Nanopatterned Bulk Metallic Glass. ACS Nano 2014, 8, 4366–4375. [Google Scholar] [CrossRef]
- Tan, J.L.; Tien, J.; Pirone, D.M.; Gray, D.S.; Bhadriraju, K.; Chen, C.S. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA 2003, 100, 1484–1489. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, D.; Peng, F.; Qiu, J.; Ouyang, L.; Qiao, Y.; Liu, X. Nanostructural Surfaces with Different Elastic Moduli Regulate the Immune Response by Stretching Macrophages. Nano Lett. 2019, 19, 3480–3489. [Google Scholar] [CrossRef] [PubMed]
- Achterberg, V.F.; Buscemi, L.; Diekmann, H.; Smith-Clerc, J.; Schwengler, H.; Meister, J.-J.; Wenck, H.; Gallinat, S.; Hinz, B. The Nano-Scale Mechanical Properties of the Extracellular Matrix Regulate Dermal Fibroblast Function. J. Investig. Dermatol. 2014, 134, 1862–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ, A.F.; Franze, K.; Gautier, H.O.B.; Moshayedi, P.; Fawcett, J.; Franklin, R.; Karadottir, R.T.; Guck, J. Mechanical difference between white and gray matter in the rat cerebellum measured by scanning force microscopy. J. Biomech. 2010, 43, 2986–2992. [Google Scholar] [CrossRef] [PubMed]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Georges, P.; Hui, J.-J.; Gombos, Z.; McCormick, M.E.; Wang, A.Y.; Uemura, M.; Mick, R.; Janmey, P.A.; Furth, E.E.; Wells, R.G. Increased stiffness of the rat liver precedes matrix deposition: Implications for fibrosis. Am. J. Physiol. Liver Physiol. 2007, 293, G1147–G1154. [Google Scholar] [CrossRef] [PubMed]
- Majkut, S.F.; Discher, D.E. Cardiomyocytes from late embryos and neonates do optimal work and striate best on substrates with tissue-level elasticity: Metrics and mathematics. Biomech. Model. Mechanobiol. 2012, 11, 1219–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, S.P.; Marshall, S.J.; Ryder, M.I.; Marshall, G.W. The tooth attachment mechanism defined by structure, chemical composition and mechanical properties of collagen fibers in the periodontium. Biomaterials 2007, 28, 5238–5245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janmey, P.A.; Fletcher, D.A.; Reinhart-King, C.A. Stiffness Sensing by Cells. Physiol. Rev. 2020, 100, 695–724. [Google Scholar] [CrossRef]
- Discher, D.E.; Smith, L.; Cho, S.; Colasurdo, M.; Garcia, A.J.; Safran, S. Matrix Mechanosensing: From Scaling Concepts in Omics Data to Mechanisms in the Nucleus, Regeneration, and Cancer. Annu. Rev. Biophys. 2017, 46, 295–315. [Google Scholar] [CrossRef]
- Franze, K.; Janmey, P.A.; Guck, J. Mechanics in Neuronal Development and Repair. Annu. Rev. Biomed. Eng. 2013, 15, 227–251. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Minev, I.R.; Musienko, P.; Hirsch, A.; Barraud, Q.; Wenger, N.; Moraud, E.M.; Gandar, J.; Capogrosso, M.; Milekovic, T.; Asboth, L.; et al. Electronic dura mater for long-term multimodal neural interfaces. Science 2015, 347, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Moshayedi, P.; Ng, G.; Kwok, J.; Yeo, G.S.H.; Bryant, C.E.; Fawcett, J.; Franze, K.; Guck, J. The relationship between glial cell mechanosensitivity and foreign body reactions in the central nervous system. Biomaterials 2014, 35, 3919–3925. [Google Scholar] [CrossRef] [Green Version]
- Sindhu, K.; Bansode, N.; Rémy, M.; Morel, C.; Bareille, R.; Hagedorn, M.; Hinz, B.; Barthélémy, P.; Chassande, O.; Boiziau, C. New injectable self-assembled hydrogels that promote angiogenesis through a bioactive degradation product. Acta Biomater. 2020, 115, 197–209. [Google Scholar] [CrossRef]
- LeComte, A.; Descamps, E.; Bergaud, C. A review on mechanical considerations for chronically-implanted neural probes. J. Neural. Eng. 2018, 15, 031001. [Google Scholar] [CrossRef]
- Du, Z.J.; Kolarcik, C.L.; Kozai, T.D.; Luebben, S.D.; Sapp, S.A.; Zheng, X.S.; Nabity, J.A.; Cui, X.T. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 2017, 53, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Doloff, J.; Veiseh, O.; Vegas, A.J.; Tam, H.H.; Farah, S.; Ma, M.; Li, J.; Bader, A.; Chiu, A.; Sadraei, A.; et al. Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates. Nat. Mater. 2017, 16, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Bosshardt, D.D. Multinucleated Giant Cells: Good Guys or Bad Guys? Tissue Eng. Part B Rev. 2018, 24, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Dondossola, E.; Holzapfel, B.M.; Alexander, S.; Filippini, S.; Hutmacher, D.W.; Friedl, P. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat. Biomed. Eng. 2017, 1, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M.; McNally, A.K. Biocompatibility of implants: Lymphocyte/macrophage interactions. Semin. Immunopathol. 2011, 33, 221–233. [Google Scholar] [CrossRef]
- Chung, L.; Maestas, D.R., Jr.; Housseau, F.; Elisseeff, J.H. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2017, 114, 184–192. [Google Scholar] [CrossRef]
- Pakshir, P.; Hinz, B. The big five in fibrosis: Macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018, 68, 81–93. [Google Scholar] [CrossRef]
- Mooney, J.E.; Rolfe, B.; Osborne, G.; Sester, D.P.; van Rooijen, N.; Campbell, G.R.; Hume, D.; Campbell, J.H. Cellular Plasticity of Inflammatory Myeloid Cells in the Peritoneal Foreign Body Response. Am. J. Pathol. 2010, 176, 369–380. [Google Scholar] [CrossRef]
- Smith, T.D.; Nagalla, R.R.; Chen, E.Y.; Liu, W.F. Harnessing macrophage plasticity for tissue regeneration. Adv. Drug Deliv. Rev. 2017, 114, 193–205. [Google Scholar] [CrossRef]
- Martin, K.E.; García, A.J. Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies. Acta Biomater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Graney, P.; Lurier, E.B.; Spiller, K.L. Biomaterials and Bioactive Factor Delivery Systems for the Control of Macrophage Activation in Regenerative Medicine. ACS Biomater. Sci. Eng. 2017, 4, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.C. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004, 15, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Sadtler, K.; Wolf, M.T.; Ganguly, S.; Moad, C.A.; Chung, L.; Majumdar, S.; Housseau, F.; Pardoll, D.M.; Elisseeff, J.H. Divergent immune responses to synthetic and biological scaffolds. Biomaterials 2019, 192, 405–415. [Google Scholar] [CrossRef]
- McNally, A.K.; Anderson, J.M. Phenotypic expression in human monocyte-derived interleukin-4-induced foreign body giant cells and macrophages in vitro: Dependence on material surface properties. J. Biomed. Mater. Res. A 2015, 103, 1380–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [Green Version]
- Milde, R.; Ritter, J.; Tennent, G.A.; Loesch, A.; Martinez, F.O.; Gordon, S.; Pepys, M.B.; Verschoor, A.; Helming, L. Multinucleated Giant Cells Are Specialized for Complement-Mediated Phagocytosis and Large Target Destruction. Cell Rep. 2015, 13, 1937–1948. [Google Scholar] [CrossRef] [Green Version]
- Aiyelabegan, H.T.; Sadroddiny, E. Fundamentals of protein and cell interactions in biomaterials. Biomed. Pharmacother. 2017, 88, 956–970. [Google Scholar] [CrossRef]
- Altieri, D.C.; Mannucci, P.M.; Capitanio, A.M. Binding of fibrinogen to human monocytes. J. Clin. Investig. 1986, 78, 968–976. [Google Scholar] [CrossRef] [Green Version]
- Love, R.J.; Jones, K.S. The recognition of biomaterials: Pattern recognition of medical polymers and their adsorbed biomolecules. J. Biomed. Mater. Res. Part A 2013, 101, 2740–2752. [Google Scholar] [CrossRef]
- Lee, T.T.; Garcia, J.R.; Paez, J.I.; Singh, A.; Phelps, E.A.; Weis, S.; Shafiq, Z.; Shekaran, A.; Del Campo, A.; Garcia, A.; et al. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat. Mater. 2015, 14, 352–360. [Google Scholar] [CrossRef]
- Helming, L.; Gordon, S. Molecular mediators of macrophage fusion. Trends Cell Biol. 2009, 19, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Cavanagh, B.; Cameron, A.R.; Kelly, D.J.; O’Brien, F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019, 89, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.; Goswami, R.; Rahaman, S.O. TRPV4 Plays a Role in Matrix Stiffness-Induced Macrophage Polarization. Front. Immunol. 2020, 11, 3150. [Google Scholar] [CrossRef]
- Arya, R.K.; Goswami, R.; Rahaman, S.O. Mechanotransduction via a TRPV4-Rac1 signaling axis plays a role in multinucleated giant cell formation. J. Biol. Chem. 2021, 296, 100129. [Google Scholar] [CrossRef] [PubMed]
- Boersema, G.S.; Grotenhuis, N.; Bayon, Y.; Lange, J.F.; Bastiaansen-Jenniskens, Y.M. The Effect of Biomaterials Used for Tissue Regeneration Purposes on Polarization of Macrophages. Biores. Open Access 2016, 5, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Abaricia, J.O.; Shah, A.H.; Chaubal, M.; Hotchkiss, K.M.; Olivares-Navarrete, R. Wnt signaling modulates macrophage polarization and is regulated by biomaterial surface properties. Biomaterials 2020, 243, 119920. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Jansen, J.A.; Yang, F.; Beucken, J.J.V.D. Titanium surfaces characteristics modulate macrophage polarization. Mater. Sci. Eng. C 2019, 95, 143–151. [Google Scholar] [CrossRef]
- Wang, T.; Luu, T.U.; Chen, A.; Khine, M.; Liu, W.F. Topographical modulation of macrophage phenotype by shrink-film multi-scale wrinkles. Biomater. Sci. 2016, 4, 948–952. [Google Scholar] [CrossRef]
- Luu, T.; Gott, S.C.; Woo, B.W.K.; Rao, M.; Liu, W.F. Micro- and Nanopatterned Topographical Cues for Regulating Macrophage Cell Shape and Phenotype. ACS Appl. Mater. Interfaces 2015, 7, 28665–28672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McWhorter, F.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Jones, J.A.; Xu, Y.; Low, H.-Y.; Anderson, J.M.; Leong, K.W. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials 2010, 31, 3479–3491. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Cremmel, C.V.M.; Kulpa, A.; Jaeger, N.A.F.; Kappelhoff, R.; Overall, C.M.; Waterfield, J.D.; Brunette, D.M. Novel grooved substrata stimulate macrophage fusion, CCL2 and MMP-9 secretion. J. Biomed. Mater. Res. Part A 2016, 104, 2243–2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shayan, M.; Padmanabhan, J.; Morris, A.H.; Cheung, B.; Smith, R.; Schroers, J.; Kyriakides, T.R. Nanopatterned bulk metallic glass-based biomaterials modulate macrophage polarization. Acta Biomater. 2018, 75, 427–438. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Pan, H.-A.; Hung, Y.-C.; Huang, G.S. Control of growth and inflammatory response of macrophages and foam cells with nanotopography. Nanoscale Res. Lett. 2012, 7, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padmanabhan, J.; Augelli, M.J.; Cheung, B.; Kinser, E.R.; Cleary, B.; Kumar, P.; Wang, R.; Sawyer, A.J.; Li, R.; Schwarz, U.; et al. Regulation of cell-cell fusion by nanotopography. Sci. Rep. 2016, 6, 33277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Previtera, M.L.; Sengupta, A. Substrate Stiffness Regulates Proinflammatory Mediator Production through TLR4 Activity in Macrophages. PLoS ONE 2015, 10, e0145813. [Google Scholar] [CrossRef] [PubMed]
- Irwin, E.F.; Saha, K.; Rosenbluth, M.; Gamble, L.J.; Castner, D.G.; Healy, K.E. Modulus-dependent macrophage adhesion and behavior. J. Biomater. Sci. Polym. Ed. 2008, 19, 1363–1382. [Google Scholar] [CrossRef] [Green Version]
- Adlerz, K.M.; Aranda-Espinoza, H.; Hayenga, H.N. Substrate elasticity regulates the behavior of human monocyte-derived macrophages. Eur. Biophys. J. 2016, 45, 301–309. [Google Scholar] [CrossRef]
- Féréol, S.; Fodil, R.; Labat, B.; Galiacy, S.; Laurent, V.M.; Louis, B.; Isabey, D.; Planus, E. Sensitivity of alveolar macrophages to substrate mechanical and adhesive properties. Cell Motil. Cytoskelet. 2006, 63, 321–340. [Google Scholar] [CrossRef]
- Scott, R.A.; Kiick, K.L.; Akins, R.E. Substrate stiffness directs the phenotype and polarization state of cord blood derived macrophages. Acta Biomater. 2021, 122, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.R.; Bole, M.; Chen, C.; Hardin, C.C.; Kho, A.T.; Mih, J.; Deng, L.; Butler, J.; Tschumperlin, D.; Fredberg, J.J.; et al. Cell elasticity determines macrophage function. PLoS ONE 2012, 7, e41024. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.Y.; Yang, Z.; Han, B. Switch of macrophage fusion competency by 3D matrices. Sci. Rep. 2020, 10, 10348. [Google Scholar] [CrossRef]
- Akilbekova, D.; Bratlie, K.M. Quantitative Characterization of Collagen in the Fibrotic Capsule Surrounding Implanted Polymeric Microparticles through Second Harmonic Generation Imaging. PLoS ONE 2015, 10, e0130386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.H.; Efendy, J.L.; Campbell, G.R. Novel Vascular Graft Grown Within Recipient’s Own Peritoneal Cavity. Circ. Res. 1999, 85, 1173–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffield, J.S.; Lupher, M.L.; Thannickal, V.J.; Wynn, T.A. Host Responses in Tissue Repair and Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2013, 8, 241–276. [Google Scholar] [CrossRef] [Green Version]
- Schuppan, D.; Ashfaq-Khan, M.; Yang, A.T.; Kim, Y.O. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018, 68-69, 435–451. [Google Scholar] [CrossRef]
- Fell, S.; Wang, Z.; Blanchard, A.; Nanthakumar, C.; Griffin, M. Transglutaminase 2: A novel therapeutic target for idiopathic pulmonary fibrosis using selective small molecule inhibitors. Amino Acids 2021, 53, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.; Wodtke, R.; Tondera, C.; Wodtke, J.; Neffe, A.T.; Hampe, J.; Lendlein, A.; Löser, R.; Pietzsch, J. Characterization of Tissue Transglutaminase as a Potential Biomarker for Tissue Response toward Biomaterials. ACS Biomater. Sci. Eng. 2018, 5, 5979–5989. [Google Scholar] [CrossRef] [PubMed]
- Piersma, B.; Bank, R.A. Collagen cross-linking mediated by lysyl hydroxylase 2: An enzymatic battlefield to combat fibrosis. Essays Biochem. 2019, 63, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Vallet, S.D.; Ricard-Blum, S. Lysyl oxidases: From enzyme activity to extracellular matrix cross-links. Essays Biochem. 2019, 63, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.; Li, W. LOX/LOXL in pulmonary fibrosis: Potential therapeutic targets. J. Drug Target. 2018, 27, 790–796. [Google Scholar] [CrossRef]
- Tschumperlin, D.J.; Lagares, D. Mechano-therapeutics: Targeting Mechanical Signaling in Fibrosis and Tumor Stroma. Pharmacol. Ther. 2020, 212, 107575. [Google Scholar] [CrossRef]
- Hinz, B.; McCulloch, C.A.; Coelho, N.M. Mechanical regulation of myofibroblast phenoconversion and collagen contraction. Exp. Cell Res. 2019, 379, 119–128. [Google Scholar] [CrossRef]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Neff, L.S.; Bradshaw, A.D. Cross your heart? Collagen cross-links in cardiac health and disease. Cell. Signal. 2021, 79, 109889. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-Smooth Muscle Actin Expression Upregulates Fibroblast Contractile Activity. Mol. Biol. Cell 2001, 12, 2730–2741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, B.; Dugina, V.; Ballestrem, C.; Wehrle-Haller, B.; Chaponnier, C. Alpha-smooth muscle actin Is crucial for focal adhesion maturation in myofibroblasts. Mol. Biol. Cell. 2003, 14, 2508–2519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goffin, J.M.; Pittet, P.; Csucs, G.; Lussi, J.W.; Meister, J.-J.; Hinz, B. Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J. Cell Biol. 2006, 172, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Godbout, C.; Castella, L.F.; Smith, E.A.; Talele, N.; Chow, M.L.; Garonna, A.; Hinz, B. The Mechanical Environment Modulates Intracellular Calcium Oscillation Activities of Myofibroblasts. PLoS ONE 2013, 8, e64560. [Google Scholar] [CrossRef] [PubMed]
- Smithmyer, M.E.; Sawicki, L.A.; Kloxin, A.M. Hydrogel scaffolds as in vitro models to study fibroblast activation in wound healing and disease. Biomater. Sci. 2014, 2, 634–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majd, H.; Scherer, S.S.; Boo, S.; Ramondetti, S.; Cambridge, E.; Raffoul, W.; Friedrich, M.; Pittet, B.; Pioletti, D.; Hinz, B.; et al. Novel micropatterns mechanically control fibrotic reactions at the surface of silicone implants. Biomaterials 2015, 54, 136–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, D.J.; Oikonomou, A.; Hill, E.; Bayat, A. Development and functional evaluation of biomimetic silicone surfaces with hierarchical micro/nano-topographical features demonstrates favourable in vitro foreign body response of breast-derived fibroblasts. Biomaterials 2015, 52, 88–102. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A Common Pathway to Organ Injury and Failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef]
- Fernández-Colino, A.; Iop, L.; Ferreira, M.S.V.; Mela, P. Fibrosis in tissue engineering and regenerative medicine: Treat or trigger? Adv. Drug Deliv. Rev. 2019, 146, 17–36. [Google Scholar] [CrossRef]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nat. Cell Biol. 2020, 587, 555–566. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Love, R.J.; Jones, K.S. Biomaterials, fibrosis, and the use of drug delivery systems in future antifibrotic strategies. Crit. Rev. Biomed. Eng. 2009, 37, 259–281. [Google Scholar] [CrossRef]
- Gancedo, M.; Ruiz-Corro, L.; Salazar-Montes, A.; Rincón, A.R.; Armendáriz-Borunda, J. Pirfenidone Prevents Capsular Contracture After Mammary Implantation. Aesthetic Plast. Surg. 2008, 32, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Arnold, T.D.; Katamura, Y.; Giacomini, M.M.; Rodriguez, J.D.; McCarty, J.H. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 2013, 19, 1617–1624. [Google Scholar] [CrossRef] [Green Version]
- Rujitanaroj, P.-O.; Jao, B.; Yang, R.; Wang, F.; Anderson, J.M.; Wang, J.; Chew, S.Y. Controlling fibrous capsule formation through long-term down-regulation of collagen type I (COL1A1) expression by nanofiber-mediated siRNA gene silencing. Acta Biomater. 2013, 9, 4513–4524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodyga, M.; Hinz, B. TGF-beta1—A truly transforming growth factor in fibrosis and immunity. Semin. Cell Dev. Biol. 2020, 101, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. Targeting the myofibroblast to improve wound healing. Volume I: “Therapies and regeneration“. In Wound Healing Biomaterials; Ågren, M.S., Ed.; Woodhead Publishing Limited: Cambridge, UK; Springer Science and Business Media Publishing: Cambridge, UK, 2016; pp. 69–100. [Google Scholar]
- Distler, J.H.W.; Gyorfi, A.H.; Ramanujam, M.; Whitfield, M.L.; Konigshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef]
- Takahashi, H.; Wang, Y.; Grainger, D.W. Device-based local delivery of siRNA against mammalian target of rapamycin (mTOR) in a murine subcutaneous implant model to inhibit fibrous encapsulation. J. Control Release 2010, 147, 400–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saika, S.; Kawashima, Y.; Miyamoto, T.; Okada, Y.; Ichitanakaa, S.-; Ohmi, S.; Minamide, A.; Yamanaka, O.; Ohnishi, Y.; Ooshima, A.; et al. Immunolocalization of Prolyl 4-Hydroxylase Subunits, α-Smooth Muscle Actin, and Extracellular Matrix Components in Human Lens Capsules with Lens Implants. Exp. Eye Res. 1998, 66, 283–294. [Google Scholar] [CrossRef]
- Zeplin, P.H.; Larena-Avellaneda, A.; Schmidt, K. Surface Modification of Silicone Breast Implants by Binding the Antifibrotic Drug Halofuginone Reduces Capsular Fibrosis. Plast. Reconstr. Surg. 2010, 126, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Li, A.G.; Quinn, M.J.; Siddiqui, Y.; Wood, M.D.; Federiuk, I.F.; Duman, H.M. Elevation of transforming growth factor beta (TGFbeta) and its downstream mediators in subcutaneous foreign body capsule tissue. J. Biomed. Mater. Res. A 2007, 82, 498–508. [Google Scholar] [CrossRef]
- Avula, M.N.; Rao, A.N.; McGill, L.D.; Grainger, D.W.; Solzbacher, F. Modulation of the foreign body response to implanted sensor models through device-based delivery of the tyrosine kinase inhibitor, masitinib. Biomaterials 2013, 34, 9737–9746. [Google Scholar] [CrossRef]
- Ward, W.K.; Li, A.G.; Siddiqui, Y.; Federiuk, I.F.; Wang, X.-J. Increased expression of Interleukin-13 and connective tissue growth factor, and their potential roles during foreign body encapsulation of subcutaneous implants. J. Biomater. Sci. Polym. Ed. 2008, 19, 1065–1072. [Google Scholar] [CrossRef]
- Castro, P.; Marques, S.M.; Viana, C.T.; Campos, P.P.; Ferreira, M.A.; Barcelos, L.S.; Andrade, S.P. Deletion of the chemokine receptor CCR2 attenuates foreign body reaction to implants in mice. Microvasc. Res. 2014, 95, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; McNally, A.K.; Chang, D.T.; Qin, L.A.; Meyerson, H.; Colton, E.; Kwon, I.K.; Matsuda, T.; Anderson, J.M. Matrix metalloproteinases and their inhibitors in the foreign body reaction on biomaterials. J. Biomed. Mater. Res. Part A 2008, 84, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Ouyang, L.; Ci, L.; Chen, B.; Lv, D.; Li, Q.; Sun, Y.; Fei, J.; Bao, S.; Liu, X.; et al. Pravastatin regulates host foreign-body reaction to polyetheretherketone implants via miR-29ab1-mediated SLIT3 upregulation. Biomaterials 2019, 203, 12–22. [Google Scholar] [CrossRef]
- Klueh, U.; Dorsky, D.I.; Kreutzer, D.L. Enhancement of implantable glucose sensor function in vivo using gene transfer-induced neovascularization. Biomaterials 2005, 26, 1155–1163. [Google Scholar] [CrossRef]
- Tschumperlin, D.J.; Ligresti, G.; Hilscher, M.B.; Shah, V.H. Mechanosensing and fibrosis. J. Clin. Investig. 2018, 128, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.; Lagares, D. Matrix Stiffness: The Conductor of Organ Fibrosis. Curr. Rheumatol. Rep. 2018, 20, 2. [Google Scholar] [CrossRef]
- Kuehlmann, B.; Bonham, C.A.; Zucal, I.; Prantl, L.; Gurtner, G.C. Mechanotransduction in Wound Healing and Fibrosis. J. Clin. Med. 2020, 9, 1423. [Google Scholar] [CrossRef] [PubMed]
- Martinac, B. The ion channels to cytoskeleton connection as potential mechanism of mechanosensitivity. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 682–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, J.; Nansa, S.A.; Kim, D.-H. Molecular Regulators of Cellular Mechanoadaptation at Cell–Material Interfaces. Front. Bioeng. Biotechnol. 2020, 8, 608569. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Kukkurainen, S.; Hytonen, V.P.; Wehrle-Haller, B. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef]
- Jansen, K.A.; Atherton, P.; Ballestrem, C. Mechanotransduction at the cell-matrix interface. Semin. Cell Dev. Biol. 2017, 71, 75–83. [Google Scholar] [CrossRef]
- Revach, O.-Y.; Grosheva, I.; Geiger, B. Biomechanical regulation of focal adhesion and invadopodia formation. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Oria, R.; Chen, Y.; Kosmalska, A.; Pérez-González, C.; Castro, N.; Zhu, C.; Trepat, X.; Roca-Cusachs, P. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol. 2016, 18, 540–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hintermann, E.; Christen, U. The Many Roles of Cell Adhesion Molecules in Hepatic Fibrosis. Cells 2019, 8, 1503. [Google Scholar] [CrossRef] [Green Version]
- Schnittert, J.; Bansal, R.; Storm, G.; Prakash, J. Integrins in wound healing, fibrosis and tumor stroma: High potential targets for therapeutics and drug delivery. Adv. Drug Deliv. Rev. 2018, 129, 37–53. [Google Scholar] [CrossRef]
- Schulz, J.N.; Plomann, M.; Sengle, G.; Gullberg, D.; Krieg, T.; Eckes, B. New developments on skin fibrosis—Essential signals emanating from the extracellular matrix for the control of myofibroblasts. Matrix Biol. 2018, 68, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, H.; Hagood, J.S. Cooperative signaling between integrins and growth factor receptors in fibrosis. J. Mol. Med. 2021, 99, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.; Briquez, P.S.; Güç, E.; Tortelli, F.; Kilarski, W.W.; Metzger, S.; Rice, J.J.; Kuhn, G.A.; Müller, R.; Swartz, M.A.; et al. Growth Factors Engineered for Super-Affinity to the Extracellular Matrix Enhance Tissue Healing. Science 2014, 343, 885–888. [Google Scholar] [CrossRef]
- Wipff, P.J.; Hinz, B. Integrins and the activation of latent transforming growth factor beta1—An intimate relationship. Eur. J. Cell Biol. 2008, 87, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. It has to be the alphav: Myofibroblast integrins activate latent TGF-beta1. Nat. Med. 2013, 19, 1567–1568. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.E. TGF beta -1, -2 and -3 in the modulation of fibrosis in the cornea and other organs. Exp. Eye Res. 2021, 207, 108594. [Google Scholar] [CrossRef]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. TGF-beta1 Signaling and Tissue Fibrosis. Cold Spring Harb. Perspect. Biol. 2018, 10, a022293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rifkin, D.B.; Rifkin, W.; Zilberberg, L. LTBPs in biology and medicine: LTBP diseases. Matrix Biol. 2018, 71, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Annes, J.P.; Chen, Y.; Munger, J.S.; Rifkin, D.B. Integrin αVβ6-mediated activation of latent TGF-β requires the latent TGF-β binding protein-1. J. Cell Biol. 2004, 165, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Munger, J.S.; Huang, X.; Kawakatsu, H.; Griffiths, M.J.; Dalton, S.L.; Wu, J.; Pittet, J.F.; Kaminski, N.; Garat, C.; Matthay, M.A.; et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999, 96, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Mu, D.; Cambier, S.; Fjellbirkeland, L.; Baron, J.L.; Munger, J.S.; Kawakatsu, H. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J. Cell Biol. 2002, 157, 493–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, G. The role of proteases in transforming growth factor-beta activation. Int. J. Biochem. Cell Biol. 2008, 40, 1068–1078. [Google Scholar] [CrossRef]
- Shi, M.; Zhu, J.; Wang, R.; Chen, X.; Mi, L.; Walz, T. Latent TGF-beta structure and activation. Nature 2011, 474, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Buscemi, L.; Ramonet, D.; Klingberg, F.; Formey, A.; Smith-Clerc, J.; Meister, J.J. The single-molecule mechanics of the latent TGF-beta1 complex. Curr. Biol. 2011, 21, 2046–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingberg, F.; Chow, M.L.; Koehler, A.; Boo, S.; Buscemi, L.; Quinn, T.M. Prestress in the extracellular matrix sensitizes latent TGF-beta1 for activation. J. Cell Biol. 2014, 207, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Sheppard, D. Integrin-mediated regulation of TGFbeta in fibrosis. Biochim. Biophys. Acta 2013, 1832, 891–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, N.I.; Jo, H.; Chen, C.; Tsujino, K.; Arnold, T.D.; DeGrado, W.F. The alphavbeta1 integrin plays a critical in vivo role in tissue fibrosis. Sci. Transl. Med. 2015, 7, 288ra79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derynck, R.; Budi, E.H. Specificity, versatility, and control of TGF-beta family signaling. Sci. Signal. 2019, 12, eaav5183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heldin, C.H.; Moustakas, A. Signaling Receptors for TGF-beta Family Members. Cold Spring Harb. Perspect. Biol. 2016, 8, a022053. [Google Scholar] [CrossRef] [Green Version]
- Follonier Castella, L.; Buscemi, L.; Godbout, C.; Meister, J.J.; Hinz, B. A new lock-step mechanism of matrix remodelling based on subcellular contractile events. J. Cell Sci. 2010, 123, 1751–1760. [Google Scholar] [CrossRef] [Green Version]
- Follonier Castella, L.; Gabbiani, G.; McCulloch, C.A.; Hinz, B. Regulation of myofibroblast activities: Calcium pulls some strings behind the scene. Exp. Cell Res. 2010, 316, 2390–2401. [Google Scholar] [CrossRef]
- Sakai, N.; Chun, J.; Duffield, J.S.; Lagares, D.; Wada, T.; Luster, A.D.; Tager, A.M. Lysophosphatidic acid signaling through its receptor initiates profibrotic epithelial cell fibroblast communication mediated by epithelial cell derived connective tissue growth factor. Kidney Int. 2017, 91, 628–641. [Google Scholar] [CrossRef] [Green Version]
- Ungefroren, H.; Gieseler, F.; Kaufmann, R.; Settmacher, U.; Lehnert, H.; Rauch, B.H. Signaling Crosstalk of TGF-beta/ALK5 and PAR2/PAR1: A Complex Regulatory Network Controlling Fibrosis and Cancer. Int. J. Mol. Sci. 2018, 19, 1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johan, M.Z.; Samuel, M.S. Rho–ROCK signaling regulates tumor-microenvironment interactions. Biochem. Soc. Trans. 2018, 47, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Dooling, L.J.; Discher, D.E. Inhibiting Tumor Fibrosis and Actomyosin through GPCR activation. Trends Cancer 2019, 5, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Haak, A.J.; Ducharme, M.T.; Espinosa, A.M.D.; Tschumperlin, D.J. Targeting GPCR Signaling for Idiopathic Pulmonary Fibrosis Therapies. Trends Pharmacol. Sci. 2020, 41, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nat. Cell Biol. 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Yu, F.-X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the Hippo-YAP Pathway by G-Protein-Coupled Receptor Signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, I.; McCollum, D. Control of cellular responses to mechanical cues through YAP/TAZ regulation. J. Biol. Chem. 2019, 294, 17693–17706. [Google Scholar] [CrossRef] [Green Version]
- Rausch, V.; Hansen, C.G. The Hippo Pathway, YAP/TAZ, and the Plasma Membrane. Trends Cell Biol. 2020, 30, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Totaro, A.; Panciera, T.; Piccolo, S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 2018, 20, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.-L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410.e14. [Google Scholar] [CrossRef]
- Kofler, M.; Speight, P.; Little, D.; Di Ciano-Oliveira, C.; Szászi, K.; Kapus, A. Mediated nuclear import and export of TAZ and the underlying molecular requirements. Nat. Commun. 2018, 9, 4966. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, S.; Saito, A.; Nagase, T. YAP/TAZ Signaling as a Molecular Link between Fibrosis and Cancer. Int. J. Mol. Sci. 2018, 19, 3674. [Google Scholar] [CrossRef] [Green Version]
- Piersma, B.; Bank, R.A.; Boersema, M. Signaling in Fibrosis: TGF-beta, WNT, and YAP/TAZ Converge. Front. Med. 2015, 2, 59. [Google Scholar] [CrossRef]
- Pan, D. The Hippo Signaling Pathway in Development and Cancer. Dev. Cell 2010, 19, 491–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szeto, S.G.; Narimatsu, M.; Lu, M.; He, X.; Sidiqi, A.M.; Tolosa, M.F. YAP/TAZ Are Mechanoregulators of TGF-beta-Smad Signaling and Renal Fibrogenesis. J. Am. Soc. Nephrol. 2016, 27, 3117–3128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, K.; Pritchett, J.; Llewellyn, J.; Mullan, A.F.; Athwal, V.S.; Dobie, R.; Harvey, E.; Zeef, L.; Farrow, S.; Streuli, C.; et al. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nat. Commun. 2016, 7, 12502. [Google Scholar] [CrossRef]
- Piersma, B.; de Rond, S.; Werker, P.M.; Boo, S.; Hinz, B.; van Beuge, M.M.; Bank, R.A. YAP1 Is a Driver of Myofibroblast Differentiation in Normal and Diseased Fibroblasts. Am. J. Pathol. 2015, 185, 3326–3337. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Lagares, D.; Choi, K.M.; Stopfer, L.; Marinković, A.; Vrbanac, V.; Probst, C.K.; Hiemer, S.E.; Sisson, T.H.; Horowitz, J.; et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Cell. Mol. Physiol. 2015, 308, L344–L357. [Google Scholar] [CrossRef] [Green Version]
- Talele, N.; Fradette, J.; Davies, J.E.; Kapus, A.; Hinz, B. Expression of α-Smooth Muscle Actin Determines the Fate of Mesenchymal Stromal Cells. Stem Cell Rep. 2015, 4, 1016–1030. [Google Scholar] [CrossRef] [Green Version]
- Haak, A.J.; Kostallari, E.; Sicard, D.; Ligresti, G.; Choi, K.M.; Caporarello, N.; Jones, D.L.; Tan, Q.; Meridew, J.; Espinosa, A.M.D.; et al. Selective YAP/TAZ inhibition in fibroblasts via dopamine receptor D1 agonism reverses fibrosis. Sci. Transl. Med. 2019, 11, eaau6296. [Google Scholar] [CrossRef] [PubMed]
- Gau, D.; Roy, P. SRF’ing and SAP’ing—The role of MRTF proteins in cell migration. J. Cell Sci. 2018, 131, jcs218222. [Google Scholar] [CrossRef] [Green Version]
- Esnault, C.; Stewart, A.; Gualdrini, F.; East, P.; Horswell, S.; Matthews, N.; Treisman, R. Rho-actin signaling to the MRTF coactivators dominates the immediate transcriptional response to serum in fibroblasts. Genes Dev. 2014, 28, 943–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, E.M.; Thatcher, J.E.; Sutherland, L.B.; Kinoshita, H.; Gerard, R.D.; Richardson, J.A. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ. Res. 2010, 107, 294–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bialik, J.F.; Ding, M.; Speight, P.; Dan, Q.; Miranda, M.Z.; Di Ciano-Oliveira, C.; Kofler, M.M.; Rotstein, O.D.; Pedersen, S.F.; Szászi, K.; et al. Profibrotic epithelial phenotype: A central role for MRTF and TAZ. Sci. Rep. 2019, 9, 4323. [Google Scholar] [CrossRef]
- Song, E.; Ouyang, N.; Horbelt, M.; Antus, B.; Wang, M.; Exton, M.S. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol. 2000, 204, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ding, J.; Ma, Z.; Iwashina, T.; Tredget, E.E. Alternatively activated macrophages derived from THP-1 cells promote the fibrogenic activities of human dermal fibroblasts. Wound Repair Regen. 2017, 25, 377–388. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Lodyga, M.; Cambridge, E.; Karvonen, H.M.; Pakshir, P.; Wu, B.; Boo, S. Cadherin-11-mediated adhesion of macrophages to myofibroblasts establishes a profibrotic niche of active TGF-beta. Sci. Signal. 2019, 12, eaao3469. [Google Scholar] [CrossRef]
- Reinhart-King, C.A.; Dembo, M.; Hammer, D.A. Cell-Cell Mechanical Communication through Compliant Substrates. Biophys. J. 2008, 95, 6044–6051. [Google Scholar] [CrossRef] [Green Version]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.-M.; Wang, H.-B.; Dembo, M.; Wang, Y.-L. Cell Movement Is Guided by the Rigidity of the Substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Pakshir, P.; Alizadehgiashi, M.; Wong, B.; Coelho, N.M.; Chen, X.; Gong, Z. Dynamic fibroblast contractions attract remote macrophages in fibrillar collagen matrix. Nat. Commun. 2019, 10, 1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bank, R.A.; Zandstra, J.; Room, H.; Petersen, A.H.; van Putten, S.M. Biomaterial Encapsulation Is Enhanced in the Early Stages of the Foreign Body Reaction During Conditional Macrophage Depletion in Transgenic Macrophage Fas-Induced Apoptosis Mice. . Tissue Eng. Part A 2017, 23, 1078–1087. [Google Scholar] [CrossRef]
- Rodriguez, A.; Macewanm, S.R.; Meyerson, H.; Kirk, J.T.; Anderson, J.M. The foreign body reaction in T-cell-deficient mice. J. Biomed. Mater. Res. A 2009, 90, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Jao, B.; McNally, A.K.; Anderson, J.M. In vivo quantitative and qualitative assessment of foreign body giant cell formation on biomaterials in mice deficient in natural killer lymphocyte subsets, mast cells, or the interleukin-4 receptorα and in severe combined immunodeficient mice. J. Biomed. Mater. Res. A 2014, 102, 2017–2023. [Google Scholar] [CrossRef]
- Avula, M.N.; Rao, A.N.; McGill, L.D.; Grainger, D.W.; Solzbacher, F. Foreign body response to subcutaneous biomaterial implants in a mast cell-deficient Kit(w-Sh) murine model. Acta Biomater. 2014, 10, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Janmey, P.A.; McCulloch, C.A. Lateral boundary mechanosensing by adherent cells in a collagen gel system. Biomaterials 2014, 35, 1138–1149. [Google Scholar] [CrossRef]
- Hall, M.S.; Alisafaei, F.; Ban, E.; Feng, X.; Hui, C.Y.; Shenoy, V.B.; Wu, M. Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proc. Natl. Acad. Sci. USA 2016, 113, 14043–14048. [Google Scholar] [CrossRef] [Green Version]
- Baker, B.; Trappmann, B.; Wang, W.Y.; Sakar, M.S.; Kim, I.L.; Shenoy, V.B.; Burdick, J.A.; Chen, C. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 2015, 14, 1262–1268. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Abhilash, A.; Chen, C.; Wells, R.G.; Shenoy, V.B. Long-Range Force Transmission in Fibrous Matrices Enabled by Tension-Driven Alignment of Fibers. Biophys. J. 2014, 107, 2592–2603. [Google Scholar] [CrossRef] [Green Version]
- Bloise, N.; Rountree, I.; Polucha, C.; Montagna, G.; Visai, L.; Coulombe, K.L.K.; Munarin, F. Engineering Immunomodulatory Biomaterials for Regenerating the Infarcted Myocardium. Front. Bioeng. Biotechnol. 2020, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Hannan, R.T.; Peirce, S.; Barker, T.H. Fibroblasts: Diverse Cells Critical to Biomaterials Integration. ACS Biomater. Sci. Eng. 2018, 4, 1223–1232. [Google Scholar] [CrossRef] [PubMed]


| Inhibiting Compound | FBR Pathway Target | Implant Material | Implant Model | Species | Study Length (In Vivo) | References |
|---|---|---|---|---|---|---|
| Prolyl-4-hydroxylase inhibitors | Collagen synthesis | N/A | Intraocular implants | Human | 19 months | [192,193,195,199,200] |
| Col1 siRNA (Nanofiber scaffold-mediated RNA interference) | Collagen synthesis | siRNA–poly(caprolactone-co-ethylethylene phosphate) nanofibers | Posterior dorsal areas | Rat | 4 weeks | [195] |
| Rapamycin (mTOR) siRNA | Type I collagen synthesis | Poly(ethylene glycol) (PEG)-based hydrogel coatings | Subcutaneous siRNA-releasing device implantation | Mice | 2 weeks | [199] |
| Halofuginone | Type I collagen synthesis | Silicone discs | Subcutaneous implantation | Rat | 3 months | [201] |
| Relaxin, BMP-7, hepatocyte growth factor, SMAD7 | TGF-β | Mock biosensors | Subcutaneous implantation | Rat | 55 days | [192,193,202] |
| Pirfenidone | TGF-β and Collagen synthesis | Smooth and textured silicone implants | Submammary implantation | Rat | 8 weeks | [193] |
| Masitinib | Tyrosine-kinase | Polyester fiber model | Subcutaneous implantation | Mice | 4 weeks | [203] |
| Antisense oligonucleotides, cAMP, TNF | CCN2 | polyether-polyurethane sponges | Subcutaneous implantation | Mice | 14 days | [204,205] |
| Monoclonal antibodies specific for MMPs and TIMP-1 | Pharmacological inhibition of MMP-1,-8,-13, and -18 | In vitro human monocyte assay | In vitro | Human | In vitro | [206] |
| Pravastatin | Neovascularization and AMPK/mTOR pathway | Medical-grade Polyetheretherketone (SP) | Subcutaneous implantation | Mice | 4 weeks | [207] |
| VEGF | Neovascularization | Commercial glucose sensors | Subcutaneous implantation | Mice | 10 days | [208] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noskovicova, N.; Hinz, B.; Pakshir, P. Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells 2021, 10, 1794. https://doi.org/10.3390/cells10071794
Noskovicova N, Hinz B, Pakshir P. Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells. 2021; 10(7):1794. https://doi.org/10.3390/cells10071794
Chicago/Turabian StyleNoskovicova, Nina, Boris Hinz, and Pardis Pakshir. 2021. "Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction" Cells 10, no. 7: 1794. https://doi.org/10.3390/cells10071794