Electroconvulsive Shock, but Not Transcranial Magnetic Stimulation, Transiently Elevates Cell Proliferation in the Adult Mouse Hippocampus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and General Experimental Design
2.2. Electroconvulsive Shock
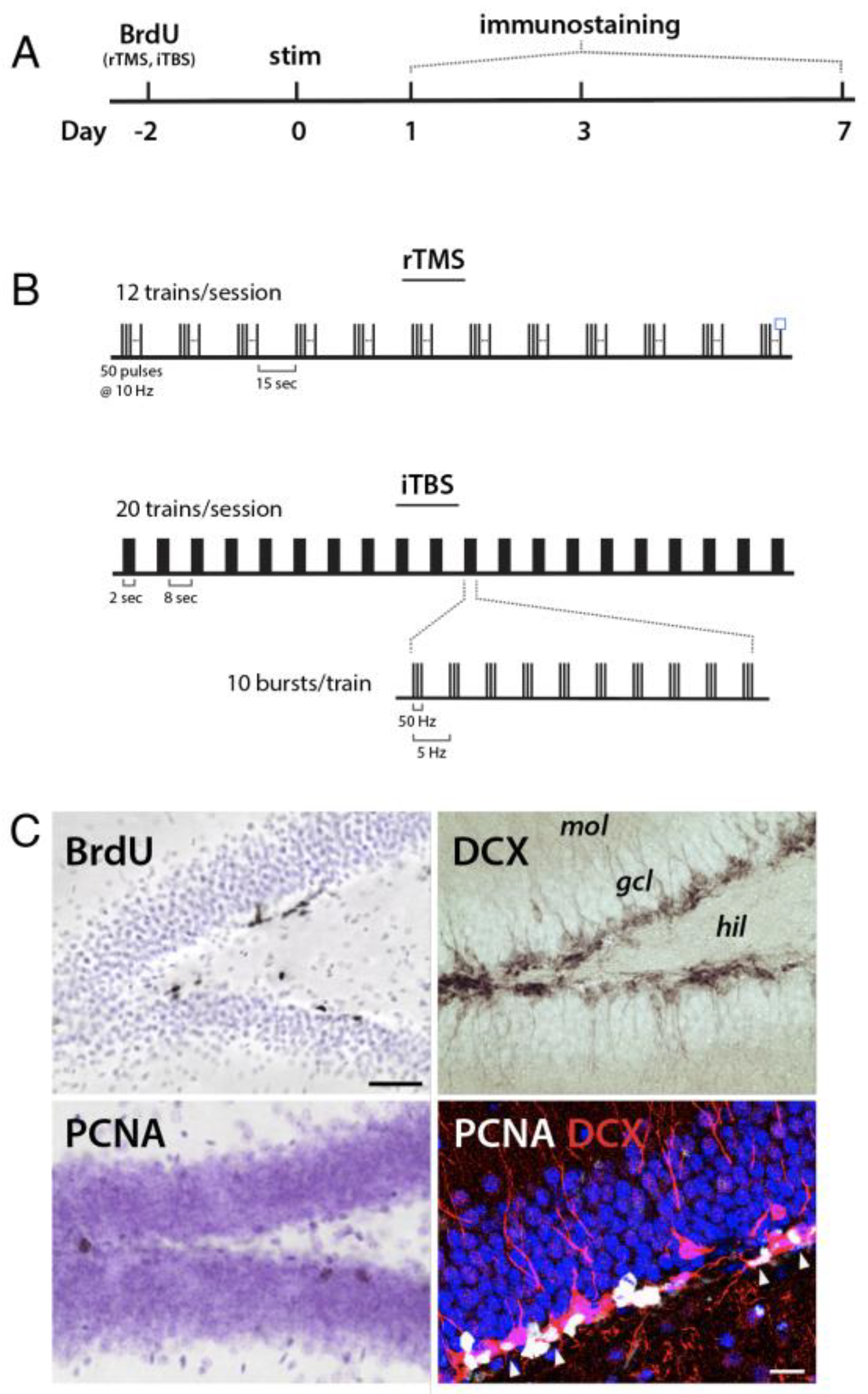
2.3. Transcranial Magnetic Stimulation
2.4. Tissue Preparation and Immunohistochemistry
2.5. Quantification and Statistical Analysis
3. Results
3.1. Effects of Acute ECS on Dentate Gyrus Neurogenesis
3.2. Effects of Acute rTMS and iTBS on Dentate Gyrus Neurogenesis
4. Discussion
4.1. ECS and Neurogenesis
4.2. Parallels with Clinical Findings
4.3. Limitations
4.4. Conclusion and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yun, S.; Reynolds, R.; Masiulis, I.; Eisch, A.J. Re-evaluating the link between neuropsychiatric disorders and dysregulated adult neurogenesis. Nat. Med. 2016, 22, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, M.C.; Yucel, K.; Nazarov, A.; MacQueen, G.M. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J. Psychiatry Neurosci. 2009, 34, 41–54. [Google Scholar]
- Van Bokhoven, P.; Oomen, C.A.; Hoogendijk, W.J.G.; Smit, A.B.; Lucassen, P.J.; Spijker, S. Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment. Eur. J. Neurosci. 2011, 33, 1833–1840. [Google Scholar] [CrossRef]
- Czéh, B.; Michaelis, T.; Watanabe, T.; Frahm, J.; de Biurrun, G.; van Kampen, M.; Bartolomucci, A.; Fuchs, E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. USA 2001, 98, 12796–12801. [Google Scholar] [CrossRef] [Green Version]
- Anacker, C.; Luna, V.M.; Stevens, G.S.; Millette, A.; Shores, R.; Jimenez, J.C.; Chen, B.; Hen, R. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nat. Cell Biol. 2018, 559, 98–102. [Google Scholar] [CrossRef]
- Schloesser, R.J.; Lehmann, M.; Martinowich, K.; Manji, H.K.; Herkenham, M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol. Psychiatry 2010, 15, 1152–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, J.S.; Radik, R.; Wojtowicz, J.M.; Cameron, H. Anatomical gradients of adult neurogenesis and activity: Young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus 2009, 19, 360–370. [Google Scholar] [CrossRef] [Green Version]
- Kempermann, G.; Kuhn, H.-G.; Gage, F.H. More hippocampal neurons in adult mice living in an enriched environment. Nat. Cell Biol. 1997, 386, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Malberg, J.E.; Eisch, A.; Nestler, E.J.; Duman, R.S. Chronic Antidepressant Treatment Increases Neurogenesis in Adult Rat Hippocampus. J. Neurosci. 2000, 20, 9104–9110. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults with Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Focus 2018, 16, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Micallef-Trigona, B. Comparing the Effects of Repetitive Transcranial Magnetic Stimulation and Electroconvulsive Therapy in the Treatment of Depression: A Systematic Review and Meta-Analysis. Depression Res. Treat. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pagnin, D.; De Queiroz, V.; Pini, S.; Cassano, G.B. Efficacy of ECT in Depression: A Meta-Analytic Review. J. ECT 2004, 20, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, J.; Wei, Q. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: A meta-analysis of stimulus parameter effects. Neurol. Res. 2013, 35, 1084–1091. [Google Scholar] [CrossRef]
- UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: A systematic review and meta-analysis. Lancet 2003, 361, 799–808. [Google Scholar] [CrossRef]
- Madsen, T.M.; Treschow, A.; Bengzon, J.; Bolwig, T.G.; Lindvall, O.; Tingström, A. Increased neurogenesis in a model of electroconvulsive therapy. Biol. Psychiatry 2000, 47, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Ueno, M.; Sugimoto, M.; Ohtsubo, K.; Sakai, N.; Endo, A.; Shikano, K.; Imoto, Y.; Segi-Nishida, E.; Endoh, A. The effect of electroconvulsive seizure on survival, neuronal differentiation, and expression of the maturation marker in the adult mouse hippocampus. J. Neurochem. 2019, 149, 488–498. [Google Scholar] [CrossRef]
- Schloesser, R.J.; Orvoen, S.; Jimenez, D.V.; Hardy, N.F.; Maynard, K.; Sukumar, M.; Manji, H.K.; Gardier, A.M.; David, D.; Martinowich, K. Antidepressant-like Effects of Electroconvulsive Seizures Require Adult Neurogenesis in a Neuroendocrine Model of Depression. Brain Stimul. 2015, 8, 862–867. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-J.; Zhao, L.-B.; Liu, Y.-Y.; Fan, S.-H.; Xie, P. Comparative efficacy and acceptability of electroconvulsive therapy versus repetitive transcranial magnetic stimulation for major depression: A systematic review and multiple-treatments meta-analysis. Behav. Brain Res. 2017, 320, 30–36. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J.; et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet 2018, 391, 1683–1692. [Google Scholar] [CrossRef]
- O’Reardon, J.P.; Solvason, H.B.; Janicak, P.G.; Sampson, S.; Isenberg, K.E.; Nahas, Z.; McDonald, W.M.; Avery, D.; Fitzgerald, P.; Loo, C.; et al. Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biol. Psychiatry 2007, 62, 1208–1216. [Google Scholar] [CrossRef]
- Guo, F.; Lou, J.; Han, X.; Deng, Y.; Huang, X. Repetitive Transcranial Magnetic Stimulation Ameliorates Cognitive Impairment by Enhancing Neurogenesis and Suppressing Apoptosis in the Hippocampus in Rats with Ischemic Stroke. Front. Physiol. 2017, 8, 559. [Google Scholar] [CrossRef] [PubMed]
- Ogiue-Ikeda, M.; Kawato, S.; Ueno, S. The effect of repetitive transcranial magnetic stimulation on long-term potentiation in rat hippocampus depends on stimulus intensity. Brain Res. 2003, 993, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Wang, X.; Shang, X.; Zhang, H.; Liu, Z.; Yin, T.; Zhang, T. Repetitive transcranial magnetic stimulation effectively facilitates spatial cognition and synaptic plasticity associated with increasing the levels of BDNF and synaptic proteins in Wistar rats. Neurobiol. Learn. Mem. 2016, 134, 369–378. [Google Scholar] [CrossRef]
- Sachdev, P.S.; McBride, R.; Loo, C.; Mitchell, P.M.; Malhi, G.S.; Croker, V. Effects of different frequencies of transcranial magnetic stimulation (TMS) on the forced swim test model of depression in rats. Biol. Psychiatry 2002, 51, 474–479. [Google Scholar] [CrossRef]
- Zuo, C.; Cao, H.; Ding, F.; Zhao, J.; Huang, Y.; Li, G.; Huang, S.; Jiang, H.; Jiang, Y.; Wang, F. Neuroprotective efficacy of different levels of high-frequency repetitive transcranial magnetic stimulation in mice with CUMS-induced depression: Involvement of the p11/BDNF/Homer1a signaling pathway. J. Psychiatr. Res. 2020, 125, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, E.; Ukai, S.; Ogawa, A.; Yamamoto, M.; Kawaguchi, S.; Ishii, R.; Shinosaki, K. Chronic repetitive transcranial magnetic stimulation increases hippocampal neurogenesis in rats. Psychiatry Clin. Neurosci. 2011, 65, 77–81. [Google Scholar] [CrossRef]
- Czeh, B.; Welt, T.; Fischer, A.K.; Erhardt, A.; Schmitt, W.; Müller, M.B.; Toschi, N.; Fuchs, E.; Keck, M.E. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: Effects on stress hormone levels and adult hippocampal neurogenesis. Biol. Psychiatry 2002, 52, 1057–1065. [Google Scholar] [CrossRef]
- Chung, S.W.; Hoy, K.; Fitzgerald, P.B. Theta-Burst Stimulation: A New Form of TMS Treatment for Depression? Depress. Anxiety 2015, 32, 182–192. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta Burst Stimulation of the Human Motor Cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, J.; Lynch, G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science 1986, 232, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Larson, J.; Wong, D.; Lynch, G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986, 368, 347–350. [Google Scholar] [CrossRef]
- Berry, S.D.; Seager, M.A. Hippocampal Theta Oscillations and Classical Conditioning. Neurobiol. Learn. Mem. 2001, 76, 298–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizumori, S.; Perez, G.; Alvarado, M.C.; Barnes, C.; McNaughton, B. Reversible inactivation of the medial septum differentially affects two forms of learning in rats. Brain Res. 1990, 528, 12–20. [Google Scholar] [CrossRef]
- Seidenbecher, T.; Laxmi, T.R.; Stork, O.; Pape, H.-C. Amygdalar and Hippocampal Theta Rhythm Synchronization During Fear Memory Retrieval. Science 2003, 301, 846–850. [Google Scholar] [CrossRef] [Green Version]
- Winson, J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science 1978, 201, 160–163. [Google Scholar] [CrossRef]
- Nokia, M.S.; Sisti, H.M.; Choksi, M.R.; Shors, T.J. Learning to Learn: Theta Oscillations Predict New Learning, which Enhances Related Learning and Neurogenesis. PLoS ONE 2012, 7, e31375. [Google Scholar] [CrossRef]
- Kanzari, A.; Bourcier-Lucas, C.; Freyssin, A.; Abrous, N.; Haddjeri, N.; Lucas, G. Inducing a long-term potentiation in the dentate gyrus is sufficient to produce rapid antidepressant-like effects. Mol. Psychiatry 2017, 23, 587–596. [Google Scholar] [CrossRef]
- Jonckheere, J.; Deloulme, J.C.; Dall’Igna, G.; Chauliac, N.; Pelluet, A.; Nguon, A.-S.; Lentini, C.; Brocard, J.; Denarier, E.; Brugière, S.; et al. Short- and long-term efficacy of electroconvulsive stimulation in animal models of depression: The essential role of neuronal survival. Brain Stimul. 2018, 11, 1336–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, M.; Seki, T.; Liu, J.; Nakamura, K.; Namba, T.; Matsubara, Y.; Suzuki, T.; Arai, H. Effects of repeated electroconvulsive seizure on cell proliferation in the rat hippocampus. Synapse 2010, 64, 814–821. [Google Scholar] [CrossRef]
- Yanpallewar, S.U.; Barrick, C.A.; Palko, M.E.; Fulgenzi, G.; Tessarollo, L. Tamalin is a critical mediator of electroconvulsive shock-induced adult neuroplasticity. J. Neurosci. 2012, 32, 2252–2262. [Google Scholar] [CrossRef] [Green Version]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Snyder, J.S.; Glover, L.R.; Sanzone, K.M.; Kamhi, J.F.; Cameron, H. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus 2009, 19, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Cahill, S.P.; Yu, R.Q.; Green, D.; Todorova, E.V.; Snyder, J.S. Early survival and delayed death of developmentally-born dentate gyrus neurons. Hippocampus 2017, 27, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, J.; Wennström, M.; Mohapel, P.; Ekdahl, C.T.; Bengzon, J.; Tingström, A. Electroconvulsive seizures increase hippocampal neurogenesis after chronic corticosterone treatment. Eur. J. Neurosci. 2002, 16, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Segi-Nishida, E.; Warner-Schmidt, J.L.; Duman, R.S. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc. Natl. Acad. Sci. USA 2008, 105, 11352–11357. [Google Scholar] [CrossRef] [Green Version]
- Ekstrand, J.; Hellsten, J.; Wennström, M.; Tingström, A. Differential inhibition of neurogenesis and angiogenesis by corticosterone in rats stimulated with electroconvulsive seizures. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1466–1472. [Google Scholar] [CrossRef]
- Inta, D.; Lima-Ojeda, J.M.; Lau, T.; Tang, W.; Dormann, C.; Sprengel, R.; Schloss, P.; Sartorius, A.; Meyer-Lindenberg, A.; Gass, P. Electroconvulsive Therapy Induces Neurogenesis in Frontal Rat Brain Areas. PLoS ONE 2013, 8, e69869. [Google Scholar] [CrossRef] [Green Version]
- Olesen, M.V.; Wörtwein, G.; Pakkenberg, B. Electroconvulsive stimulation, but not chronic restraint stress, causes structural alterations in adult rat hippocampus-A stereological study. Hippocampus 2014, 25, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.; Wojtowicz, J.; Burnham, W. Neurogenesis in the Dentate Gyrus of the Rat Following Electroconvulsive Shock Seizures. Exp. Neurol. 2000, 165, 231–236. [Google Scholar] [CrossRef]
- Jun, H.; Hussaini, S.M.Q.; Cho, C.H.; Welby, J.; Jang, M.-H. Gadd45b Mediates Electroconvulsive Shock Induced Proliferation of Hippocampal Neural Stem Cells. Brain Stimul. 2015, 8, 1021–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanti, A.; Rainer, Q.; Minier, F.; Surget, A.; Belzung, C. Differential environmental regulation of neurogenesis along the septo-temporal axis of the hippocampus. Neuropharmacol. 2012, 63, 374–384. [Google Scholar] [CrossRef]
- Sierra, A.; Martín-Suárez, S.; Valcárcel-Martín, R.; Pascual-Brazo, J.; Aelvoet, S.-A.; Abiega, O.; Deudero, J.J.; Brewster, A.L.; Bernales, I.; Anderson, A.E.; et al. Neuronal Hyperactivity Accelerates Depletion of Neural Stem Cells and Impairs Hippocampal Neurogenesis. Cell Stem Cell 2015, 16, 488–503. [Google Scholar] [CrossRef] [Green Version]
- Barker, A.; Jalinous, R.; Freeston, I. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 325, 1106–1107. [Google Scholar] [CrossRef]
- Tang, A.; Thickbroom, G.; Rodger, J. Repetitive Transcranial Magnetic Stimulation of the Brain: Mechanisms from Animal and Experimental Models. Neuroscientist 2017, 23, 82–94. [Google Scholar] [CrossRef]
- Buzsáki, G.; Moser, E.I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013, 16, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Yang, C.-H.; Hsu, K.-S.; Ming, G.-L.; Song, H. A Critical Period for Enhanced Synaptic Plasticity in Newly Generated Neurons of the Adult Brain. Neuron 2007, 54, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Hieber, C.; Jonas, P.; Bischofberger, J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. e-Neuroforum 2004, 10, 244–246. [Google Scholar] [CrossRef]
- Harrison, P.J. The hippocampus in schizophrenia: A review of the neuropathological evidence and its pathophysiological implications. Psychopharmacol. 2004, 174, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.; Petersen, R.C.; Xu, Y.; O’Brien, P.C.; Smith, G.; Ivnik, R.J.; Boeve, B.F.; Tangalos, E.G.; Kokmen, E. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology 2000, 55, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.S.; Soumier, A.; Brewer, M.; Pickel, J.; Cameron, H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011, 476, 458–461. [Google Scholar] [CrossRef]
- Surget, A.; Saxe, M.; Leman, S.; Ibarguen-Vargas, Y.; Chalon, S.; Griebel, G.; Hen, R.; Belzung, C. Drug-Dependent Requirement of Hippocampal Neurogenesis in a Model of Depression and of Antidepressant Reversal. Biol. Psychiatry 2008, 64, 293–301. [Google Scholar] [CrossRef]
- Lehmann, M.L.; Brachman, R.; Martinowich, K.; Schloesser, R.J.; Herkenham, M. Glucocorticoids Orchestrate Divergent Effects on Mood through Adult Neurogenesis. J. Neurosci. 2013, 33, 2961–2972. [Google Scholar] [CrossRef] [Green Version]
- Tunc-Ozcan, E.; Peng, C.-Y.; Zhu, Y.; Dunlop, S.R.; Contractor, A.; Kessler, J.A. Activating newborn neurons suppresses depression and anxiety-like behaviors. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Hill, A.S.; Sahay, A.; Hen, R. Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology 2015, 40, 2368–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenfeld, T.J.; McCausland, H.C.; Morris, H.D.; Padmanaban, V.; Cameron, H.A. Stress and Loss of Adult Neurogenesis Differentially Reduce Hippocampal Volume. Biol. Psychiatry 2017, 82, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Gbyl, K.; Videbech, P. Electroconvulsive therapy increases brain volume in major depression: A systematic review and meta-analysis. Acta Psychiatr. Scand. 2018, 138, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, S.; Nakamura, M.; Noda, Y.; Izuno, T.; Saeki, T.; Iwanari, H.; Hirayasu, Y. Lateralized hippocampal volume increase following high-frequency left prefrontal repetitive transcranial magnetic stimulation in patients with major depression. Psychiatry Clin. Neurosci. 2017, 71, 747–758. [Google Scholar] [CrossRef] [Green Version]
- Nordanskog, P.; Dahlstrand, U.; Larsson, M.R.; Larsson, E.M.; Knutsson, L.; Johanson, A. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: A volumetric magnetic resonance imaging study. J. ECT 2010, 26, 62–67. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Sanacora, G.; Bloch, M.H. Hippocampal Volume Changes Following Electroconvulsive Therapy: A Systematic Review and Meta-analysis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, B.; Luo, Q.; Fu, Y.; Du, L.; Qiu, T.; Yang, X.; Chen, X.; Chen, Q.; Soares, J.C.; Cho, R.Y.; et al. Predicting individual responses to the electroconvulsive therapy with hippocampal subfield volumes in major depression disorder. Sci. Rep. 2018, 8, 5434. [Google Scholar] [CrossRef] [Green Version]
- Nuninga, J.O.; Mandl, R.C.W.; Boks, M.P.; Bakker, S.; Somers, M.; Heringa, S.M.; Nieuwdorp, W.; Hoogduin, H.; Kahn, R.S.; Luijten, P.; et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol. Psychiatry 2020, 25, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, A.; Nuninga, J.O.; Mandl, R.C.W.; Sommer, I.E.C.; Mimura, M.; Kishimoto, T. Volume Increase of the Dentate Gyrus Induced by Electroconvulsive Therapy: Shedding Light on the Clinical Relevance of Plasticity in the Hippocampus. J. ECT 2019, 35, e57–e58. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, A.; Plitman, E.; Chung, J.K.; Chakravarty, M.; Graff-Guerrero, A.; Mimura, M.; Kishimoto, T. Acute and long-term effects of electroconvulsive therapy on human dentate gyrus. Neuropsychopharmacology 2019, 44, 1805–1811. [Google Scholar] [CrossRef]
- Boldrini, M.; Butt, T.H.; Santiago, A.N.; Tamir, H.; Dwork, A.J.; Rosoklija, G.B.; Arango, V.; Hen, R.; Mann, J.J. Benzodiazepines and the potential trophic effect of antidepressants on dentate gyrus cells in mood disorders. Int. J. Neuropsychopharmacol. 2014, 17, 1923–1933. [Google Scholar] [CrossRef] [Green Version]
- Mahar, I.; Bambico, F.R.; Mechawar, N.; Nobrega, J.N. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 2014, 38, 173–192. [Google Scholar] [CrossRef]
- Boldrini, M.; Santiago, A.N.; Hen, R.; Dwork, A.J.; Rosoklija, G.B.; Tamir, H.; Arango, V.; Mann, J.J. Hippocampal Granule Neuron Number and Dentate Gyrus Volume in Antidepressant-Treated and Untreated Major Depression. Neuropsychopharmacology 2013, 38, 1068–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flor-García, M.; Terreros-Roncal, J.; Moreno-Jiménez, E.P.; Ávila, J.; Rábano, A.; Llorens-Martín, M. Unraveling human adult hippocampal neurogenesis. Nat. Protoc. 2020, 15, 668–693. [Google Scholar] [CrossRef]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.-G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes, M.F.; Sorrells, S.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Does Adult Neurogenesis Persist in the Human Hippocampus? Cell Stem Cell 2018, 23, 780–781. [Google Scholar] [CrossRef] [Green Version]
- Tartt, A.N.; Fulmore, C.A.; Liu, Y.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; Hen, R.; Mann, J.J.; Boldrini, M. Considerations for Assessing the Extent of Hippocampal Neurogenesis in the Adult and Aging Human Brain. Cell Stem Cell 2018, 23, 782–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, J.S. Recalibrating the Relevance of Adult Neurogenesis. Trends Neurosci. 2019, 42, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.D.; Espinueva, D.F.; Seib, D.R.; Ash, A.M.; Cooke, M.B.; Cahill, S.P.; O’Leary, T.P.; Kwan, S.S.; Snyder, J.S. Adult-Born Hippocampal Neurons Undergo Extended Development and Are Morphologically Distinct from Neonatally-Born Neurons. J. Neurosci. 2020, 40, 5740–5756. [Google Scholar] [CrossRef]
- Alvarez, D.D.; Giacomini, D.; Yang, S.M.; Trinchero, M.F.; Temprana, S.G.; Büttner, K.A.; Beltramone, N.; Schinder, A.F. A disynaptic feedback network activated by experience promotes the integration of new granule cells. Science 2016, 354, 459–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergami, M.; Masserdotti, G.; Temprana, S.G.; Motori, E.; Eriksson, T.M.; Göbel, J.; Yang, S.M.; Conzelmann, K.-K.; Schinder, A.; Götz, M.; et al. A Critical Period for Experience-Dependent Remodeling of Adult-Born Neuron Connectivity. Neuron 2015, 85, 710–717. [Google Scholar] [CrossRef] [Green Version]
- Tronel, S.; Fabre, A.; Charrier, V.; Oliet, S.H.R.; Gage, F.H.; Abrous, N. Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proc. Natl. Acad. Sci. USA 2010, 107, 7963–7968. [Google Scholar] [CrossRef] [Green Version]
- Koponen, L.M.; Stenroos, M.; Nieminen, J.O.; Jokivarsi, K.; Gröhn, O.; Ilmoniemi, R.J. Individual head models for estimating the TMS-induced electric field in rat brain. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Hermiller, M.; Chen, Y.F.; Parrish, T.B.; Voss, J.L. Evidence for Immediate Enhancement of Hippocampal Memory Encoding by Network-Targeted Theta-Burst Stimulation during Concurrent fMRI. J. Neurosci. 2020, 40, 7155–7168. [Google Scholar] [CrossRef]
- Dayer, A.G.; Ford, A.A.; Cleaver, K.M.; Yassaee, M.; Cameron, H. Short-term and long-term survival of new neurons in the rat dentate gyrus. J. Comp. Neurol. 2003, 460, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.R.; Guilherme, E.; Kesici, A.; Ash, A.M.; Vila-Rodriguez, F.; Snyder, J.S. Electroconvulsive Shock, but Not Transcranial Magnetic Stimulation, Transiently Elevates Cell Proliferation in the Adult Mouse Hippocampus. Cells 2021, 10, 2090. https://doi.org/10.3390/cells10082090
Zhang TR, Guilherme E, Kesici A, Ash AM, Vila-Rodriguez F, Snyder JS. Electroconvulsive Shock, but Not Transcranial Magnetic Stimulation, Transiently Elevates Cell Proliferation in the Adult Mouse Hippocampus. Cells. 2021; 10(8):2090. https://doi.org/10.3390/cells10082090
Chicago/Turabian StyleZhang, Tian Rui, Evelyn Guilherme, Aydan Kesici, Alyssa M. Ash, Fidel Vila-Rodriguez, and Jason S. Snyder. 2021. "Electroconvulsive Shock, but Not Transcranial Magnetic Stimulation, Transiently Elevates Cell Proliferation in the Adult Mouse Hippocampus" Cells 10, no. 8: 2090. https://doi.org/10.3390/cells10082090
APA StyleZhang, T. R., Guilherme, E., Kesici, A., Ash, A. M., Vila-Rodriguez, F., & Snyder, J. S. (2021). Electroconvulsive Shock, but Not Transcranial Magnetic Stimulation, Transiently Elevates Cell Proliferation in the Adult Mouse Hippocampus. Cells, 10(8), 2090. https://doi.org/10.3390/cells10082090





