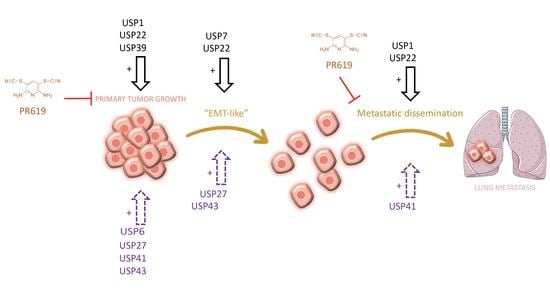
Overexpression of the Ubiquitin Specific Proteases USP43, USP41, USP27x and USP6 in Osteosarcoma Cell Lines: Inhibition of Osteosarcoma Tumor Growth and Lung Metastasis Development by the USP Antagonist PR619
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Real-Time Polymerase Chain Reaction
2.3. RNA Sequencing and Analysis
2.4. Proliferation Assay
2.5. Osteosarcoma Tissue Microarray
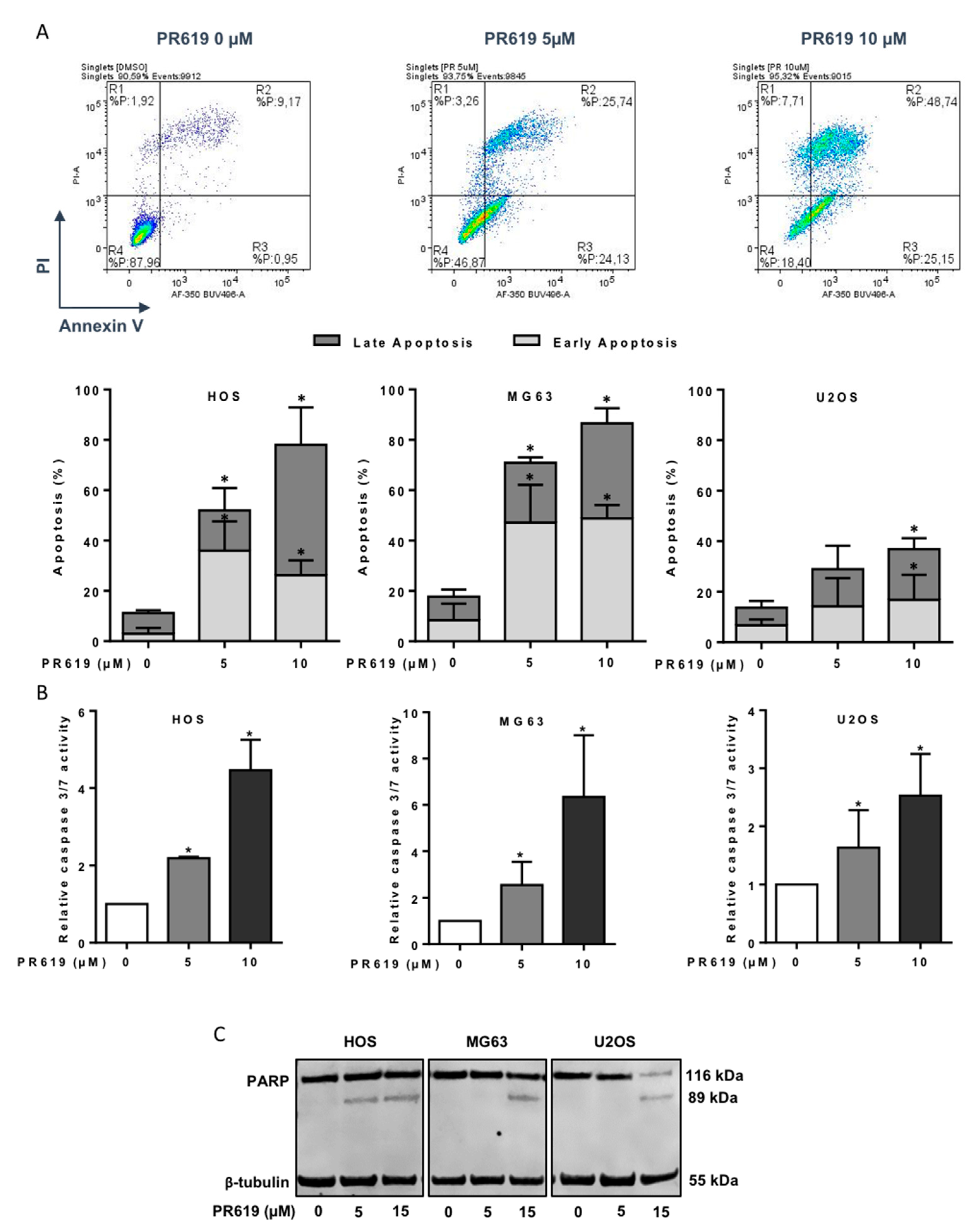
2.6. Annexin V Assay and Caspase Activity
2.7. Western Blot Analysis
2.8. Osteosarcoma (OS) Mouse Model
2.9. Cell Transfection with SiRNA against USPs
2.10. Migration Assay
2.11. Statistical Analysis
3. Results
3.1. Elevation of USPs Gene Expression in OS Cell Lines Compared to Mesenchymal Stem Cells
3.2. Expression of USP6, USP27x, USP41 and USP43 in OS Patient Biopsies
3.3. Correlation between USP6 and USP41 Gene Expression and Patient Survival
3.4. PR619 Inhibits Primary Tumor Growth and Lung Metastases Development in an Orthotopic Model of OS
3.5. PR619 Induces In Vitro Cell Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heare, T.; Hensley, M.A.; Dell’Orfano, S. Bone tumors: Osteosarcoma and Ewing’s sarcoma. Curr. Opin. Pediatr. 2009, 21, 365–372. [Google Scholar] [CrossRef]
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A.; Schwartz, C.L.; Krailo, M.D.; Healey, J.H.; Bernstein, M.L.; Betcher, D.; Ferguson, W.S.; Gebhardt, M.C.; Goorin, A.M.; Harris, M.; et al. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J. Clin. Oncol. 2008, 26, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, N.; Occean, B.V.; Pacquement, H.; Bompas, E.; Bouvier, C.; Brisse, H.J.; Castex, M.P.; Cheurfa, N.; Corradini, N.; Delaye, J.; et al. Results of methotrexate-etoposide-ifosfamide based regimen (M-EI) in osteosarcoma patients included in the French OS2006/sarcome-09 study. Eur. J. Cancer 2018, 88, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, T.G.; Alonso, M.; Avnet, S.; Banito, A.; Burdach, S.; Cidre-Aranaz, F.; Di Pompo, G.; Distel, M.; Dorado-Garcia, H.; Garcia-Castro, J.; et al. Sarcoma treatment in the era of molecular medicine. EMBO Mol. Med. 2010, 12, e11131. [Google Scholar] [CrossRef]
- Friebele, J.C.; Peck, J.; Pan, X.; Abdel-Rasoul, M.; Mayerson, J.L. Osteosarcoma: A Meta-Analysis and Review of the Literature. Am. J. Orthop 2015, 44, 547–553. [Google Scholar] [PubMed]
- Duchman, K.R.; Gao, Y.; Miller, B.J. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) Program database. Cancer Epidemiol. 2015, 39, 593–599. [Google Scholar] [CrossRef]
- Gibson, T.M.; Mostoufi-Moab, S.; Stratton, K.L.; Leisenring, W.M.; Barnea, D.; Chow, E.J.; Donaldson, S.S.; Howell, R.M.; Hudson, M.M.; Mahajan, A.; et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970-99: A report from the Childhood Cancer Survivor Study cohort. Lancet. Oncol. 2018, 19, 1590–1601. [Google Scholar] [CrossRef]
- Simpson, E.; Brown, H.L. Understanding osteosarcomas. JAAPA 2018, 31, 15–19. [Google Scholar] [CrossRef]
- Gianferante, D.M.; Mirabello, L.; Savage, S.A. Germline and somatic genetics of osteosarcoma–connecting aetiology, biology and therapy. Nat. Rev. Endocrinol. 2017, 13, 480–491. [Google Scholar] [CrossRef]
- Bousquet, M.; Noirot, C.; Accadbled, F.; Sales de Gauzy, J.; Castex, M.P.; Brousset, P.; Gomez-Brouchet, A. Whole-exome sequencing in osteosarcoma reveals important heterogeneity of genetic alterations. Ann. Oncol. 2016, 27, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.H.; Heazlewood, J.L.; Giglione, C.; Holdsworth, M.J.; Bachmair, A.; Schulze, W.X. The Scope, Functions, and Dynamics of Posttranslational Protein Modifications. Annu. Rev. Plant. Biol. 2019, 70, 119–151. [Google Scholar] [CrossRef] [PubMed]
- Mennerich, D.; Kubaichuk, K.; Kietzmann, T. DUBs, Hypoxia, and Cancer. Trends Cancer 2019, 5, 632–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M.-J.; Hsu, K.-C.; Lin, T.E.; Chang, W.-C.; Hung, J.-J. The role of ubiquitin-specific peptidases in cancer progression. J. Biomed. Sci. 2019, 26, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komander, D. The emerging complexity of protein ubiquitination. Biochem. Soc. Trans. 2009, 37, 937–953. [Google Scholar] [CrossRef] [Green Version]
- Nijman, S.M.B.; Luna-Vargas, M.P.A.; Velds, A.; Brummelkamp, T.R.; Dirac, A.M.G.; Sixma, T.K.; Bernards, R. A Genomic and Functional Inventory of Deubiquitinating Enzymes. Cell 2005, 123, 773–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfoh, R.; Lacdao, I.K.; Georges, A.A.; Capar, A.; Zheng, H.; Frappier, L.; Saridakis, V. Crystal Structure of USP7 Ubiquitin-like Domains with an ICP0 Peptide Reveals a Novel Mechanism Used by Viral and Cellular Proteins to Target USP7. PLoS Pathog. 2015, 11, e1004950. [Google Scholar] [CrossRef]
- Pal, A.; Young, M.A.; Donato, N.J. Emerging potential of therapeutic targeting of ubiquitin-specific proteases in the treatment of cancer. Cancer Res. 2014, 74, 4955–4966. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhu, H.; Zhong, N.; Jiang, Z.; Xu, L.; Deng, Y.; Jiang, Z.; Wang, H.; Wang, J. Gene silencing of USP1 by lentivirus effectively inhibits proliferation and invasion of human osteosarcoma cells. Int. J. Oncol. 2016, 49, 2549–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Q.; Li, Z.; Zhao, X.; Guo, L.; Yu, C.; Qin, J.; Zhang, S.; Zhang, Y.; Yang, X. Ubiquitin-specific protease 7 promotes osteosarcoma cell metastasis by inducing epithelial-mesenchymal transition. Oncol. Rep. 2019, 41, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Jiang, F.; Wang, X.; Li, G. Downregulation of Ubiquitin-Specific Protease 22 Inhibits Proliferation, Invasion, and Epithelial-Mesenchymal Transition in Osteosarcoma Cells. Oncol. Res. 2017, 25, 743–751. [Google Scholar] [CrossRef]
- Gan, Z.; Han, K.; Lin, S.; Hu, H.; Shen, Z.; Min, D. Knockdown of ubiquitin-specific peptidase 39 inhibited the growth of osteosarcoma cells and induced apoptosis in vitro. Biol. Res. 2017, 50, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuijjer, M.L.; Peterse, E.F.P.; Van Den Akker, B.E.W.M.; Briaire de Bruijn, I.H.; Serra, M.; Meza-Zepeda, L.A.; Myklebost, O.; Hassan, A.B.; Hogendoorn, P.C.W.; Cleton-Jansen, A.M.; et al. IR/IGF1R signaling as potential target for treatment of high-grade osteosarcoma. BMC Cancer 2013, 13, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuijjer, M.L.; Van Den Akker, B.E.; Hilhorst, R.; Mommersteeg, M.; Buddingh, E.P.; Serra, M.; Bürger, H.; Hogendoorn, P.C.W.; Cleton-Jansen, A.M. Kinome and mRNA expression profiling of high-grade osteosarcoma cell lines implies Akt signaling as possible target for therapy. BMC Med. Genom. 2014, 7, 4. [Google Scholar] [CrossRef]
- Yuan, T.; Yan, F.; Ying, M.; Cao, J.; He, Q.; Zhu, H.; Yang, B. Inhibition of Ubiquitin-Specific Proteases as a Novel Anticancer Therapeutic Strategy. Front. Pharmacol. 2018, 9, 1080. [Google Scholar] [CrossRef] [Green Version]
- Poondla, N.; Chandrasekaran, A.P.; Kim, K.-S.; Ramakrishna, S. Deubiquitinating enzymes as cancer biomarkers: New therapeutic opportunities? BMB Rep. 2019, 52, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Arcy, P.; Wang, X.; Linder, S. Deubiquitinase inhibition as a cancer therapeutic strategy. Pharmacol. Ther. 2015, 147, 32–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.M.; Hsi, B.L.; Weremowicz, S.; Rosenberg, A.E.; Dal Cin, P.; Joseph, N.; Bridge, J.A.; Perez-Atayde, A.R.; Fletcher, J.A. USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res. 2004, 64, 1920–1923. [Google Scholar] [CrossRef] [Green Version]
- Pringle, L.M.; Young, R.; Quick, L.; Riquelme, D.N.; Oliveira, A.M.; May, M.J.; Chou, M.M. Atypical mechanism of NF-κB activation by TRE17/ubiquitin-specific protease 6 (USP6) oncogene and its requirement in tumorigenesis. Oncogene 2012, 31, 3525–3535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Pringle, L.M.; Lau, A.W.; Riquelme, D.N.; Wang, H.; Jiang, T.; Lev, D.; Welman, A.; Blobel, G.A.; Oliveira, A.M.; et al. TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-kappaB. Oncogene 2010, 29, 3619–3629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Chen, B.; Jiang, K.; Lao, L.; Shen, H.; Chen, Z. Activation of TNF-α/NF-κB axis enhances CRL4BDCAF 11 E3 ligase activity and regulates cell cycle progression in human osteosarcoma cells. Mol. Oncol. 2018, 12, 476–494. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Yu, L.; Bai, C.; Liu, L.; Long, H.; Shi, L.; Lin, Z. USP27-mediated Cyclin E stabilization drives cell cycle progression and hepatocellular tumorigenesis. Oncogene 2018, 37, 2702–2713. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Yang, S.; Zu, L.; Li, Y.; Li, Y. Deubiquitinating enzyme USP41 promotes lung cancer cell proliferation and migration. Thorac. Cancer 2021, 12, 1041–1047. [Google Scholar] [CrossRef]
- Ye, D.-X.; Wang, S.-S.; Huang, Y.; Wang, X.-J.; Chi, P. USP43 directly regulates ZEB1 protein, mediating proliferation and metastasis of colorectal cancer. J. Cancer 2021, 12, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Xie, Z.; Chang, L.; Li, W.; Wang, L.; Hou, Y.; Li, L.; Zhu, J.; Xia, Y.; He, W.; et al. USP43 promotes tumorigenesis through regulating cell cycle and EMT in breast cancer. Int. J. Clin. Exp. Pathol. 2017, 10, 11014–11021. [Google Scholar] [PubMed]
- Yang, G.; Yuan, J.; Li, K. EMT transcription factors: Implication in osteosarcoma. Med. Oncol. 2013, 30, 697. [Google Scholar] [CrossRef]
- Verrecchia, F.; Rédini, F. Transforming Growth Factor-β Signaling Plays a Pivotal Role in the Interplay between Osteosarcoma Cells and Their Microenvironment. Front. Oncol. 2018, 8, 133. [Google Scholar] [CrossRef]
- Shen, A.; Zhang, Y.; Yang, H.; Xu, R.; Huang, G. Overexpression of ZEB1 relates to metastasis and invasion in osteosarcoma. J. Surg. Oncol. 2012, 105, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Díaz, V.M. USP27X, a new player on EMT and fibroblast activation. Oncotarget 2018, 9, 36724–36725. [Google Scholar] [CrossRef]
- Sharili, A.-S.; Allen, S.; Smith, K.; Hargreaves, J.; Price, J.; McGonnell, I. Expression of Snail2 in long bone osteosarcomas correlates with tumour malignancy. Tumour Biol. 2011, 32, 515–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrigan, J.A.; Jacq, X.; Martin, N.M.; Jackson, S.P. Deubiquitylating enzymes and drug discovery: Emerging opportunities. Nat. Rev. Drug Discov. 2018, 17, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-T.; Huang, K.-Y.; Lu, M.-C.; Huang, H.-L.; Chen, C.-Y.; Cheng, Y.-L.; Yu, H.-C.; Liu, S.-Q.; Lai, N.-S.; Huang, H.-B. TGF-β upregulates the translation of USP15 via the PI3K/AKT pathway to promote p53 stability. Oncogene 2017, 36, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, P.J.A.; Rodón, L.; Gonzàlez-Juncà, A.; Dirac, A.; Gili, M.; Martínez-Sáez, E.; Aura, C.; Barba, I.; Peg, V.; Prat, A.; et al. USP15 stabilizes TGF-β receptor I and promotes oncogenesis through the activation of TGF-β signaling in glioblastoma. Nat. Med. 2012, 18, 429–435. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, F.; Drabsch, Y.; Gao, R.; Snaar-Jagalska, B.E.; Mickanin, C.; Huang, H.; Sheppard, K.A.; Porter, J.A.; Lu, C.X.; et al. USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-β type I receptor. Nat. Cell Biol. 2012, 14, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Lamora, A.; Mullard, M.; Amiaud, J.; Brion, R.; Heymann, D.; Rédini, F.; Verrecchia, F. Anticancer activity of halofuginone in a preclinical model of osteosarcoma: Inhibition of tumor growth and lung metastases. Oncotarget 2015, 6, 14413–14427. [Google Scholar] [CrossRef] [Green Version]
- Lamora, A.; Talbot, J.; Bougras, G.; Amiaud, J.; Leduc, M.; Chesneau, J.; Taurelle, J.; Stresing, V.; Le Deley, M.C.; Heymann, M.F.; et al. Overexpression of smad7 blocks primary tumor growth and lung metastasis development in osteosarcoma. Clin. Cancer Res. 2014, 20, 5097–5112. [Google Scholar] [CrossRef] [Green Version]
- Lamora, A.; Talbot, J.; Mullard, M.; Royer, B.L.; Redini, F.; Verrecchia, F. TGF-β Signaling in Bone Remodeling and Osteosarcoma Progression. J. Clin. Med. 2016, 5, 96. [Google Scholar] [CrossRef]





| Forward Primers | Reverse Primers | |
|---|---|---|
| B2M | TTCTGGCCTGGAGGCTATC | TCAGGAAATTTGACTTTCCATTC |
| HPRT | TGACCTTGATTTATTTTGCATACC | CGAGCAAGACGTTCAGTCCT |
| USP6 | CATGCCATCTCTTCCTGACAGC | CAATGGCATTCCAAAGAGGCTGG |
| USP27X | ACCAAGGAACCTTGGAGAGTGG | CCTTCACTGTCCAGTACGTCCT |
| USP41 | TGAATGTGGACTTCGCCAGG | ATGTTGGACAAACAGGGGCA |
| USP43 | TGGGCATTACACAGCCTACTG | AGACAGGGAGGAGCTGGTAG |
| Patient | Core Position | Age | Gender | Localisation | Stage | Fibroblastic Osteosarcoma | Osteo Chondroblastic Osteosarcoma | Mixte Osteosarcoma |
|---|---|---|---|---|---|---|---|---|
| 1 | A1/A2 | 13 | F | femur | IB | +/+ | ||
| 2 | A3/A4 | 12 | F | femur | IIB | +/+ | ||
| 3 | A5/A6 | 23 | F | femur | IIB | |||
| 4 | A7/A8 | 21 | F | femur | IB | +/+ | ||
| 5 | A9/A10 | 42 | F | femur | IIB | +/+ | ||
| 6 | B1/B2 | 16 | F | femur | IIB | +/+ | ||
| 7 | B3/B4 | 15 | F | femur | IB | +/+ | ||
| 8 | B5/B6 | 35 | F | femur | IB | +/+ | ||
| 9 | B7/B8 | 64 | F | femur | IB | +/+ | ||
| 10 | B9/B10 | 29 | F | femur | IA | +/+ | ||
| 11 | C1/C2 | 30 | F | femur | IB | +/+ | ||
| 12 | C3/C4 | 51 | F | femur | IB | +/+ | ||
| 13 | C5/C6 | 11 | F | femur | IA | +/+ | ||
| 14 | C7/C8 | 47 | F | femur | IB | +/+ | ||
| 15 | C9/C10 | 47 | F | femur | IIB | +/+ | ||
| 16 | D1/D2 | 16 | F | femur | IB | +/+ | ||
| 17 | D3/D4 | 12 | F | femur | IIB | +/+ | ||
| 18 | D5/D6 | 16 | F | femur | IB | |||
| 19 | D7/D8 | 16 | F | femur | IIB | +/+ | ||
| 20 | D9/D10 | 37 | F | femur | IA | +/+ | ||
| 21 | E1/E2 | 14 | F | femur | IIB | +/+ | ||
| 22 | E3/E4 | 14 | F | femur | IIB | + | /+ | |
| 23 | E5/E6 | 32 | F | femur | IIB | +/+ | ||
| 24 | E7/E8 | 46 | F | femur | IB | +/+ | ||
| 25 | E9/E10 | 16 | F | femur | IIB | +/+ | ||
| 26 | F1/F2 | 17 | F | tibia | IB | +/+ | ||
| 27 | F3/F4 | 17 | F | tibia | IB | +/+ | ||
| 28 | F5/F6 | 14 | F | tibia | IIB | +/+ | ||
| 29 | F7/F8 | 38 | F | tibia | IIB | +/+ | ||
| 30 | F9/F10 | 20 | F | tibia | IIB | +/+ | ||
| 31 | G1/G2 | 60 | F | tibia | IIB | +/+ | ||
| 32 | G3/G4 | 31 | F | tibia | IIB | +/+ | ||
| 33 | G5/G6 | 18 | F | tibia | IB | +/+ | ||
| 34 | G7/G8 | 32 | F | tibia | IB | +/+ | ||
| 35 | G9/G10 | 41 | F | tibia | IA | +/+ | ||
| 36 | H1/H2 | 30 | M | rib | IA | + | /+ | |
| 37 | H3/H4 | 32 | M | rib rib | IIA | +/+ | ||
| 38 | H5/H6 | 33 | M | upper jaw | IA | +/+ | ||
| 39 | H7/H8 | 15 | M | upper jaw | IVB | + | /+ | |
| 40 | H9/H10 | 51 | M | fibula | IIB | +/+ | ||
| H11 | 42 | M | Pheochromocytoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavaud, M.; Mullard, M.; Tesfaye, R.; Amiaud, J.; Legrand, M.; Danieau, G.; Brion, R.; Morice, S.; Regnier, L.; Dupuy, M.; et al. Overexpression of the Ubiquitin Specific Proteases USP43, USP41, USP27x and USP6 in Osteosarcoma Cell Lines: Inhibition of Osteosarcoma Tumor Growth and Lung Metastasis Development by the USP Antagonist PR619. Cells 2021, 10, 2268. https://doi.org/10.3390/cells10092268
Lavaud M, Mullard M, Tesfaye R, Amiaud J, Legrand M, Danieau G, Brion R, Morice S, Regnier L, Dupuy M, et al. Overexpression of the Ubiquitin Specific Proteases USP43, USP41, USP27x and USP6 in Osteosarcoma Cell Lines: Inhibition of Osteosarcoma Tumor Growth and Lung Metastasis Development by the USP Antagonist PR619. Cells. 2021; 10(9):2268. https://doi.org/10.3390/cells10092268
Chicago/Turabian StyleLavaud, Mélanie, Mathilde Mullard, Robel Tesfaye, Jérôme Amiaud, Mélanie Legrand, Geoffroy Danieau, Régis Brion, Sarah Morice, Laura Regnier, Maryne Dupuy, and et al. 2021. "Overexpression of the Ubiquitin Specific Proteases USP43, USP41, USP27x and USP6 in Osteosarcoma Cell Lines: Inhibition of Osteosarcoma Tumor Growth and Lung Metastasis Development by the USP Antagonist PR619" Cells 10, no. 9: 2268. https://doi.org/10.3390/cells10092268
APA StyleLavaud, M., Mullard, M., Tesfaye, R., Amiaud, J., Legrand, M., Danieau, G., Brion, R., Morice, S., Regnier, L., Dupuy, M., Brounais-Le Royer, B., Lamoureux, F., Ory, B., Rédini, F., & Verrecchia, F. (2021). Overexpression of the Ubiquitin Specific Proteases USP43, USP41, USP27x and USP6 in Osteosarcoma Cell Lines: Inhibition of Osteosarcoma Tumor Growth and Lung Metastasis Development by the USP Antagonist PR619. Cells, 10(9), 2268. https://doi.org/10.3390/cells10092268