Comparison of a New 68Ga-Radiolabelled PET Imaging Agent sCD146 and RGD Peptide for In Vivo Evaluation of Angiogenesis in Mouse Model of Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Mouse Model of Myocardial Infarction
2.3. Ultrasound Imaging
2.4. Radiochemistry
2.5. MicroPET/CT Imaging
2.6. Histological Sirius Red Staining
2.7. Statistical Analysis
3. Results
3.1. Evaluation of the Myocardial Infarction Defect
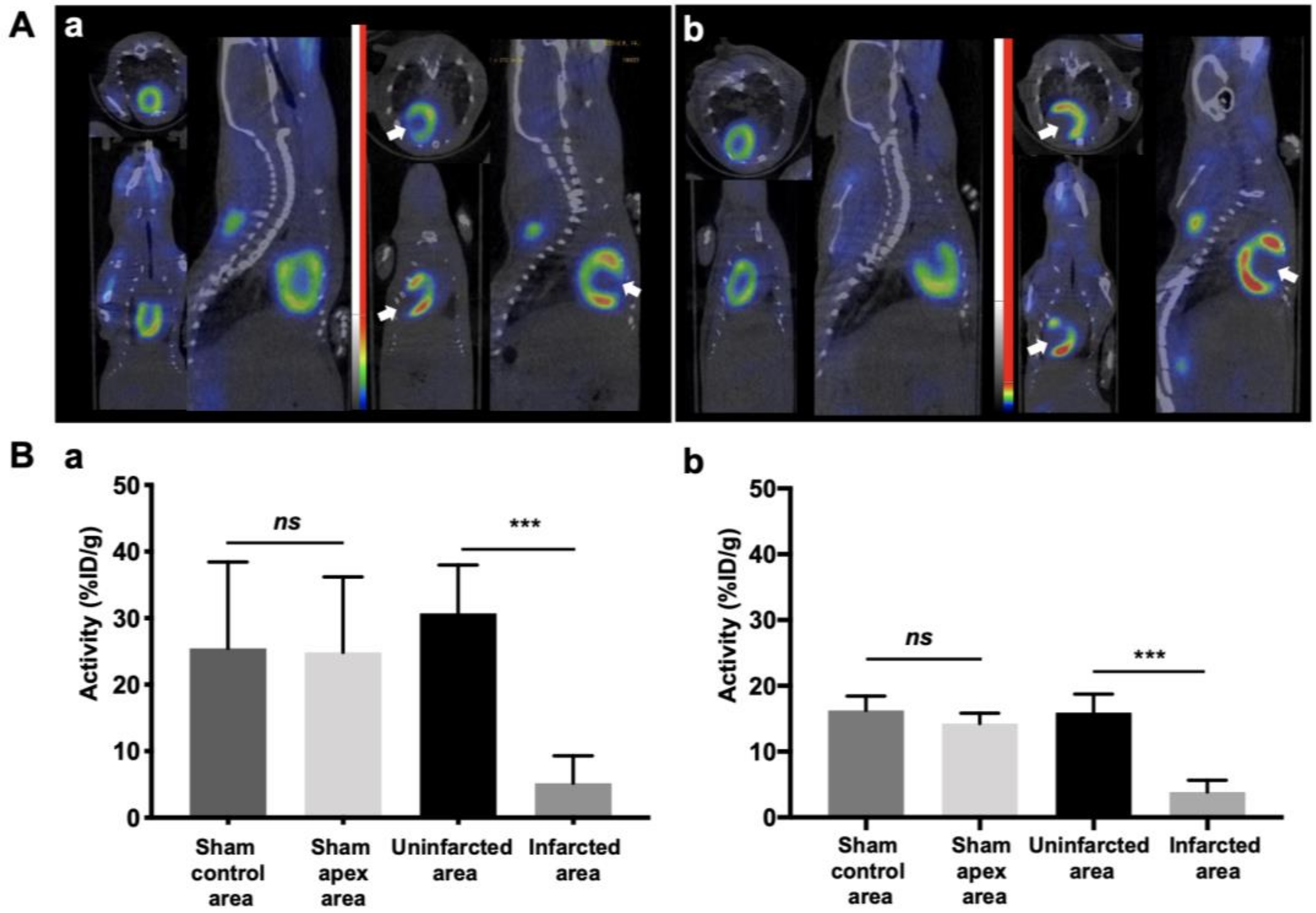
3.2. 68Ga-sCD146 Showed an Early and Sustained Increase of AMOT Expression whereas 68Ga-RGD2 Showed No Significant Increase in Integrin Expression at Day 15 following Myocardial Infarction
3.3. Early 68Ga-sCD146 PET Signal Intensity on Day 15 Correlated with Delayed Residual Myocardial Perfusion
3.4. Early 68Ga-sCD146 PET Signal Intensity Was Inversely Correlated with Delayed Myocardial Fibrosis
3.5. Early 68Ga-sCD146 PET Signal Correlated with Myocardial Functional Recovery
3.6. Early 18F-FDG PET Signal Intensity Was Inversely Correlated with Delayed Myocardial Fibrosis and Wasn’t Correlated with Myocardial Functional Recovery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keeley, E.C.; Boura, J.A.; Grines, C.L. Comparison of Primary and Facilitated Percutaneous Coronary Interventions for ST-Elevation Myocardial Infarction: Quantitative Review of Randomised Trials. Lancet 2006, 367, 579–588. [Google Scholar] [CrossRef]
- Wu, X.; Reboll, M.R.; Korf-Klingebiel, M.; Wollert, K.C. Angiogenesis after Acute Myocardial Infarction. Cardiovasc. Res. 2021, 117, 1257–1273. [Google Scholar] [CrossRef]
- Vagnozzi, R.J.; Maillet, M.; Sargent, M.A.; Khalil, H.; Johansen, A.K.Z.; Schwanekamp, J.A.; York, A.J.; Huang, V.; Nahrendorf, M.; Sadayappan, S. An Acute Immune Response Underlies the Benefit of Cardiac Stem Cell Therapy. Nature 2020, 577, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.S.; Afshari, J.T.; Esmaeili, S.-A.; Saburi, E.; Joneidi, Z.; Momtazi-Borojeni, A.A. Cardioprotective MicroRNAs: Lessons from Stem Cell-Derived Exosomale MicroRNAs to Treat Cardiovascular Disease. Atherosclerosis 2019, 285, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beer, L.; Mildner, M.; Gyöngyösi, M.; Ankersmit, H.J. Peripheral Blood Mononuclear Cell Secretome for Tissue Repair. Apoptosis 2016, 21, 1336–1353. [Google Scholar] [CrossRef] [Green Version]
- Florea, A.; Mottaghy, F.M.; Bauwens, M. Molecular Imaging of Angiogenesis in Oncology: Current Preclinical and Clinical Status. IJMS 2021, 22, 5544. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, G.; Vöö, S.; Bauwens, M.; Post, M.J.; Mottaghy, F.M. SPECT and PET Imaging of Angiogenesis and Arteriogenesis in Pre-Clinical Models of Myocardial Ischemia and Peripheral Vascular Disease. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2433–2447. [Google Scholar] [CrossRef] [Green Version]
- Saraste, A.; Knuuti, J. PET Imaging in Heart Failure: The Role of New Tracers. Heart Fail. Rev. 2017, 22, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Makowski, M.R.; Rischpler, C.; Ebersberger, U.; Keithahn, A.; Kasel, M.; Hoffmann, E.; Rassaf, T.; Kessler, H.; Wester, H.-J.; Nekolla, S.G. Multiparametric PET and MRI of Myocardial Damage after Myocardial Infarction: Correlation of Integrin Avβ3 Expression and Myocardial Blood Flow. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1070–1080. [Google Scholar] [CrossRef]
- Sun, Y.; Zeng, Y.; Zhu, Y.; Feng, F.; Xu, W.; Wu, C.; Xing, B.; Zhang, W.; Wu, P.; Cui, L.; et al. Application of 68Ga-PRGD2 PET/CT for Avβ3-Integrin Imaging of Myocardial Infarction and Stroke. Theranostics 2014, 4, 778–786. [Google Scholar] [CrossRef] [Green Version]
- Moyon, A.; Garrigue, P.; Balasse, L.; Fernandez, S.; Brige, P.; Nollet, M.; Hache, G.; Blot-Chabaud, M.; Dignat-George, F.; Guillet, B. Early Prediction of Revascularisation by Angiomotin-Targeting Positron Emission Tomography. Theranostics 2018, 8, 4985–4994. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.-H.; Wang, G.-D.; Yang, C.-T.; Otecko, N.O.; Liu, F.; Wu, S.-F.; Wang, L.; Yu, L.; Zhang, Y.-P. Identifying Molecular Signatures of Hypoxia Adaptation from Sex Chromosomes: A Case for Tibetan Mastiff Based on Analyses of X Chromosome. Sci. Rep. 2016, 6, 35004. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-C.; Chen, K.-D.; Su, M.-C.; Chin, C.-H.; Chen, C.-J.; Liou, C.-W.; Chen, T.-W.; Chang, Y.-C.; Huang, K.-T.; Wang, C.-C.; et al. Genome-Wide Gene Expression Array Identifies Novel Genes Related to Disease Severity and Excessive Daytime Sleepiness in Patients with Obstructive Sleep Apnea. PLoS ONE 2017, 12, e0176575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arigoni, M.; Barutello, G.; Lanzardo, S.; Longo, D.; Aime, S.; Curcio, C.; Iezzi, M.; Zheng, Y.; Barkefors, I.; Holmgren, L.; et al. A Vaccine Targeting Angiomotin Induces an Antibody Response Which Alters Tumor Vessel Permeability and Hampers the Growth of Established Tumors. Angiogenesis 2012, 15, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Levchenko, T.; Veitonmäki, N.; Lundkvist, A.; Gerhardt, H.; Ming, Y.; Berggren, K.; Kvanta, A.; Carlsson, R.; Holmgren, L. Therapeutic Antibodies Targeting Angiomotin Inhibit Angiogenesis in Vivo. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Kikuno, R.; Nagase, T.; Ishikawa, K.; Hirosawa, M.; Miyajima, N.; Tanaka, A.; Kotani, H.; Nomura, N.; Ohara, O. Prediction of the Coding Sequences of Unidentified Human Genes. XIV. The Complete Sequences of 100 New CDNA Clones from Brain Which Code for Large Proteins in Vitro. DNA Res. 1999, 6, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levchenko, T.; Aase, K.; Troyanovsky, B.; Bratt, A.; Holmgren, L. Loss of Responsiveness to Chemotactic Factors by Deletion of the C-Terminal Protein Interaction Site of Angiomotin. J. Cell Sci. 2003, 116, 3803–3810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troyanovsky, B.; Levchenko, T.; Månsson, G.; Matvijenko, O.; Holmgren, L. Angiomotin: An Angiostatin Binding Protein That Regulates Endothelial Cell Migration and Tube Formation. J. Cell Biol. 2001, 152, 8. [Google Scholar] [CrossRef]
- Stalin, J.; Harhouri, K.; Hubert, L.; Subrini, C.; Lafitte, D.; Lissitzky, J.-C.; Elganfoud, N.; Robert, S.; Foucault-Bertaud, A.; Kaspi, E.; et al. Soluble Melanoma Cell Adhesion Molecule (SMCAM/SCD146) Promotes Angiogenic Effects on Endothelial Progenitor Cells through Angiomotin. J. Biol. Chem. 2013, 288, 8991–9000. [Google Scholar] [CrossRef] [Green Version]
- Harhouri, K.; Kebir, A.; Guillet, B.; Foucault-Bertaud, A.; Voytenko, S.; Piercecchi-Marti, M.-D.; Berenguer, C.; Lamy, E.; Vely, F.; Pisano, P.; et al. Soluble CD146 Displays Angiogenic Properties and Promotes Neovascularization in Experimental Hind-Limb Ischemia. Blood 2010, 115, 3843–3851. [Google Scholar] [CrossRef]
- Stalin, J.; Nollet, M.; Garigue, P.; Fernandez, S.; Vivancos, L.; Essaadi, A.; Muller, A.; Bachelier, R.; Foucault-Bertaud, A.; Fugazza, L.; et al. Targeting Soluble CD146 with a Neutralizing Antibody Inhibits Vascularization, Growth and Survival of CD146-Positive Tumors. Oncogene 2016, 35, 5489–5500. [Google Scholar] [CrossRef] [PubMed]
- Kubena, P.; Arrigo, M.; Parenica, J.; Gayat, E.; Sadoune, M.; Ganovska, E.; Pavlusova, M.; Littnerova, S.; Spinar, J.; Mebazaa, A.; et al. Plasma Levels of Soluble CD146 Reflect the Severity of Pulmonary Congestion Better Than Brain Natriuretic Peptide in Acute Coronary Syndrome. Ann. Lab. Med. 2016, 36, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Gayat, E.; Caillard, A.; Laribi, S.; Mueller, C.; Sadoune, M.; Seronde, M.-F.; Maisel, A.; Bartunek, J.; Vanderheyden, M.; Desutter, J. Soluble CD146, a New Endothelial Biomarker of Acutely Decompensated Heart Failure. Int. J. Cardiol. 2015, 199, 241–247. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Kameishi, S.; Barutello, G.; Zheng, Y.; Tobin, N.P.; Nicosia, J.; Hennig, K.; Chiu, D.K.-C.; Balland, M.; et al. The Amot/Integrin Protein Complex Transmits Mechanical Forces Required for Vascular Expansion. Cell Biol. 2021, 26, 109616. [Google Scholar]
- Anderson, J.L.; Morrow, D.A. Acute Myocardial Infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef] [Green Version]
- Mamas, M.A.; Anderson, S.G.; O’Kane, P.D.; Keavney, B.; Nolan, J.; Oldroyd, K.G.; Perera, D.; Redwood, S.; Zaman, A.; Ludman, P.F. Impact of Left Ventricular Function in Relation to Procedural Outcomes Following Percutaneous Coronary Intervention: Insights from the British Cardiovascular Intervention Society. Eur. Heart J. 2014, 35, 3004–3012. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Palomares, J.F.; Gavara, J.; Ferreira-González, I.; Valente, F.; Rios, C.; Rodríguez-García, J.; Bonanad, C.; García del Blanco, B.; Miñana, G.; Mutuberria, M. Prognostic Value of Initial Left Ventricular Remodeling in Patients with Reperfused STEMI. JACC Cardiovasc. Imaging 2019, 12, 2445–2456. [Google Scholar] [CrossRef]
- Stone, G.W.; Sabik, J.F.; Serruys, P.W.; Simonton, C.A.; Généreux, P.; Puskas, J.; Kandzari, D.E.; Morice, M.-C.; Lembo, N.; Brown III, W.M. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N. Engl. J. Med. 2016, 375, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Solomonidis, E.G.; Meloni, M.; Taylor, R.S.; Duffin, R.; Dobie, R.; Magalhaes, M.S.; Henderson, B.E.; Louwe, P.A.; D’Amico, G. Single-Cell Transcriptome Analyses Reveal Novel Targets Modulating Cardiac Neovascularization by Resident Endothelial Cells Following Myocardial Infarction. Eur. Heart J. 2019, 40, 2507–2520. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Huang, K.; Ye, Y.; Su, T.; Qiao, L.; Hensley, M.T.; Caranasos, T.G.; Zhang, J.; Gu, Z. Cardiac Cell–Integrated Microneedle Patch for Treating Myocardial Infarction. Sci. Adv. 2018, 4, eaat9365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bratt, A.; Birot, O.; Sinha, I.; Veitonmäki, N.; Aase, K.; Ernkvist, M.; Holmgren, L. Angiomotin Regulates Endothelial Cell-Cell Junctions and Cell Motility. J. Biol. Chem. 2005, 280, 34859–34869. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Fei, Y.; Zheng, L.; Wang, J.; Xiao, W.; Wen, J.; Xu, Y.; Wang, Y.; He, L.; Guan, J. Expression of Endothelial Cell Injury Marker Cd146 Correlates with Disease Severity and Predicts the Renal Outcomes in Patients with Diabetic Nephropathy. Cell. Physiol. Biochem. 2018, 48, 63–74. [Google Scholar] [CrossRef]
- Bardin, N.; Anfosso, F.; Massé, J.-M.; Cramer, E.; Sabatier, F.; Bivic, A.L.; Sampol, J.; Dignat-George, F. Identification of CD146 as a Component of the Endothelial Junction Involved in the Control of Cell-Cell Cohesion. Blood 2001, 98, 3677–3684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grönman, M.; Tarkia, M.; Kiviniemi, T.; Halonen, P.; Kuivanen, A.; Savunen, T.; Tolvanen, T.; Teuho, J.; Käkelä, M.; Metsälä, O.; et al. Imaging of Avβ3 Integrin Expression in Experimental Myocardial Ischemia with [68Ga]NODAGA-RGD Positron Emission Tomography. J. Transl. Med. 2017, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Kiugel, M.; Dijkgraaf, I.; Kytö, V.; Helin, S.; Liljenbäck, H.; Saanijoki, T.; Yim, C.-B.; Oikonen, V.; Saukko, P.; Knuuti, J.; et al. Dimeric [68Ga]DOTA-RGD Peptide Targeting Avβ3 Integrin Reveals Extracellular Matrix Alterations after Myocardial Infarction. Mol. Imaging Biol. 2014, 16, 793–801. [Google Scholar] [CrossRef]
- Menichetti, L.; Kusmic, C.; Panetta, D.; Arosio, D.; Petroni, D.; Matteucci, M.; Salvadori, P.A.; Casagrande, C.; L’Abbate, A.; Manzoni, L. MicroPET/CT Imaging of Avβ3 Integrin via a Novel 68Ga-NOTA-RGD Peptidomimetic Conjugate in Rat Myocardial Infarction. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, W.S.; Vesey, A.T.; Stirrat, C.; Connell, M.; Lucatelli, C.; Neale, A.; Moles, C.; Vickers, A.; Fletcher, A.; Pawade, T. Cardiac AVβ3 Integrin Expression Following Acute Myocardial Infarction in Humans. Heart 2017, 103, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Fallahi, B.; Beiki, D.; Mousavi, S.A.; Gholamrezanezhad, A.; Eftekhari, M.; Fard-Esfahani, A.; Alimoghaddam, K.; Mirpour, S.; Eskandarian, A.; Saghari, M. 99mTc-MIBI Whole Body Scintigraphy and P-Glycoprotein for the Prediction of Multiple Drug Resistance in Multiple Myeloma Patients. Hell. J. Nucl. Med. 2009, 12, 255–259. [Google Scholar] [PubMed]
- Al-Shammari, A.; Elgazzar, A.; Ashkanani, R.A. 99mTc-MIBI Whole Body Scan: A Potentially Useful Technique for Evaluating Metabolic Bone Disease. World J. Nucl. Med. 2013, 12, 8. [Google Scholar]
- Chen, X.; Park, R.; Hou, Y.; Khankaldyyan, V.; Gonzales-Gomez, I.; Tohme, M.; Bading, J.R.; Laug, W.E.; Conti, P.S. MicroPET Imaging of Brain Tumor Angiogenesis with 18 F-Labeled PEGylated RGD Peptide. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1081–1089. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Z.-B.; Cai, W.; He, L.; Chin, F.T.; Li, F.; Chen, X. 18 F-Labeled Mini-PEG Spacered RGD Dimer (18 F-FPRGD2): Synthesis and MicroPET Imaging of α v β 3 Integrin Expression. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1823–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyon, A.; Garrigue, P.; Fernandez, S.; Hubert, F.; Balasse, L.; Brige, P.; Hache, G.; Nail, V.; Blot-Chabaud, M.; Dignat-George, F.; et al. Comparison of a New 68Ga-Radiolabelled PET Imaging Agent sCD146 and RGD Peptide for In Vivo Evaluation of Angiogenesis in Mouse Model of Myocardial Infarction. Cells 2021, 10, 2305. https://doi.org/10.3390/cells10092305
Moyon A, Garrigue P, Fernandez S, Hubert F, Balasse L, Brige P, Hache G, Nail V, Blot-Chabaud M, Dignat-George F, et al. Comparison of a New 68Ga-Radiolabelled PET Imaging Agent sCD146 and RGD Peptide for In Vivo Evaluation of Angiogenesis in Mouse Model of Myocardial Infarction. Cells. 2021; 10(9):2305. https://doi.org/10.3390/cells10092305
Chicago/Turabian StyleMoyon, Anaïs, Philippe Garrigue, Samantha Fernandez, Fabien Hubert, Laure Balasse, Pauline Brige, Guillaume Hache, Vincent Nail, Marcel Blot-Chabaud, Françoise Dignat-George, and et al. 2021. "Comparison of a New 68Ga-Radiolabelled PET Imaging Agent sCD146 and RGD Peptide for In Vivo Evaluation of Angiogenesis in Mouse Model of Myocardial Infarction" Cells 10, no. 9: 2305. https://doi.org/10.3390/cells10092305
APA StyleMoyon, A., Garrigue, P., Fernandez, S., Hubert, F., Balasse, L., Brige, P., Hache, G., Nail, V., Blot-Chabaud, M., Dignat-George, F., Rochais, F., & Guillet, B. (2021). Comparison of a New 68Ga-Radiolabelled PET Imaging Agent sCD146 and RGD Peptide for In Vivo Evaluation of Angiogenesis in Mouse Model of Myocardial Infarction. Cells, 10(9), 2305. https://doi.org/10.3390/cells10092305






