1. Introduction
The universal second messenger, 3′,5′-cyclic adenosine monophosphate (cAMP), was the first to be discovered in eukaryotic cells in 1958 by Earl Sutherland and colleagues [
1]. The cAMP signaling pathway is now considered to be a paradigm of intracellular signaling and is involved in a wide variety of cellular and physiological processes [
2,
3,
4,
5]. Activation of Gs protein-coupled receptors (GsPCRs) promotes activation of intracellular adenylyl cyclases (AC), leading to the generation of cAMP inside the cell from ATP. Signal termination occurs through the hydrolytic action of cAMP phosphodiesterase (PDE) enzymes and receptor desensitization. There are nine membrane-bound, and one soluble, isoforms of AC, whereas the PDE superfamily is made up of 21 genes, grouped into 11 diverse families (PDEs 1–11) [
6,
7].
The downstream signaling effects in response to cAMP production were originally attributed to activation of protein kinase A (PKA) isoforms [
8]. For example, gene transcription induced by cAMP is mediated through PKA phosphorylation of the cAMP response element-binding protein (CREB) transcription factor, amongst others [
9]. Subsequently, a new cAMP sensor, exchange protein directly activated by cAMP (EPAC) was identified, showing that the activation of the small GTPase, Rap1, by cAMP occurred independently of PKA [
10,
11]. Cyclic nucleotide-gated ion channels (HCN) and Popeye domain containing (POPDC) gene family are two additional recently discovered cAMP effectors [
12,
13,
14]. As effector proteins, PKA and EPAC are known to be anchored to specific intracellular sites by scaffold proteins, which act to maintain compartmentalization of cAMP signaling by anchoring PDEs adjacent to target effectors and, consequently, regulate the ability of cAMP to initiate downstream signaling events at discrete subcellular compartments or nanodomains [
15].
Evidence is emerging that compartmentalization of the two EPAC isoforms, EPAC1 and EPAC2, is key to tailoring their effector functions. For example, in vascular endothelial cells (VECs), VE-cadherin-based adhesion and cell permeability are mediated by PDE4D regulation of EPAC1 signaling complexes [
16]. In the first study to investigate the subcellular localization of EPAC1, two distinct populations of EPAC1 were observed [
17] in EPAC1-GFP transfected COS7 cells, where EPAC1 was localized mainly in and around the nucleus, along the nuclear membrane, but also in punctate structures identified to be the mitochondria in these cells [
17]. An increase in levels of EPAC1 at the nuclear membrane was observed in these cells following stimulation with forskolin and rolipram [
17]. Importantly, this was the first study to show that the EPAC1 membrane association was due to the EPAC1 Disheveled-EGL-plekstrin homology (DEP) domain [
17]. From this study, it was further determined that the localization of EPAC1 was cell cycle dependent, with EPAC1 being mainly associated with the nuclear membrane during metaphase, and then further associated with the mitotic spindle and centrosomes following the breakdown of the nuclear envelope [
17]. The localization of EPAC1 to the perinuclear region of cells has also been seen in several other cell lines, including COS1, human embryonic kidney (HEK293) cells and cancer cell lines, such as the human epidermal carcinoma (A431) and the human breast cancer (MCF7) cells [
17,
18,
19,
20,
21,
22,
23]. This is consistent with Rap1 fluorescence resonance energy transfer (FRET) activation studies that demonstrate activation of Rap1 in this region following elevations in intracellular cAMP [
21]. Inactive EPAC1 appears to be tethered to the nuclear envelope through interactions with the scaffold protein, Ran binding protein 2 (RanBP2) [
24,
25], which is a component of the nuclear pore complex, although the activation/inactivation cycle of EPAC1 at the nuclear/perinuclear region remains to be determined.
EPAC1 has also been observed, via confocal microscopy, total internal reflection fluorescence (TIRF) and FRET analysis, to translocate to the plasma membrane (PM) in HEK293 cells, at early time points following cAMP stimulation [
22]. Again, the EPAC1 DEP domain was found to be essential for EPAC1 translocation, but in cooperation with the catalytic region [
22], through interactions with the negatively charged phospholipid, phosphatidic acid (PA) [
26]. This results in the tethering of EPAC1 to the PM, allowing for Rap1 activation and facilitating cell adhesion [
26]. In addition, EPAC1 recruitment to the PM can occur independently of the DEP domain via interaction of the first 49 residues at the N-terminus of EPAC1 with the ezrin-radixin-moesin (ERM) family of actin-binding proteins [
27]. When active, ERM proteins, such as ezrin, can bind to EPAC1 and recruit the protein to PM in clusters, working with DEP-domain mediated translocation to control cell adhesion [
27]. EPAC2 has also been observed to localize at the PM, through interactions with PM-bound Ras, leading to localized Rap1 activation [
23,
28,
29].
The distinct distributions of the EPAC1 and EPAC2 isoforms have also been studied in adult mouse cardiomyocytes using a novel fluorescent EPAC ligand, 8-[PharosΦ-cAMP]-2′-O-methyladenosine-3′,5′-cyclic monophosphate (Φ-O-Me-cAMP) [
30]. In these studies, Φ-O-Me-cAMP offered an advantage over EPAC1- and EPAC2-selective antibodies, which show a lack of specificity in immunolocalization studies [
30]. With Φ-O-Me-cAMP it was observed that endogenous EPAC2 levels were concentrated at T-tubules while endogenous EPAC1 was concentrated at perinuclear regions [
30]. These different localizations of EPAC1 and EPAC2 are no doubt important for their isoform-specific regulation in cells and consequently their diverse physiological functions.
Despite the emergence of Φ-O-Me-cAMP, there are still very few tools available to study EPAC1 localization in cells beyond transfection with labelled isoform. There is a commercially available monoclonal antibody (Ab), 5D3, which was first developed by the Bos laboratory but is limited in its usefulness because it acts as an allosteric agonist towards EPAC1 [
31]. 5D3 specifically activates EPAC1 by binding to an epitope in the EPAC1 CNBD that is normally hidden through interactions with the catalytic region [
31]. In addition, an EPAC2-specific monoclonal antibody, 5B1, has also been developed, with its epitope shown to be in a similar area of the EPAC2 CNBD as 5D3 [
31]. Whereas 5D3 can be used for immunoprecipitation and immunofluorescence studies, 5B1 is unable to precipitate EPAC2 from cells [
31]. Furthermore, in a study by Parnell et al., it was confirmed through IP studies that the 5D3 Ab selectively recognized the active conformation of EPAC1 in HEK293T cells transfected with FLAG tagged EPAC1, as well as the inactive form of the protein [
23]. The companies Abcam and Santa Cruz both sell EPAC1- and EPAC2-selective monoclonal and polyclonal antibodies, which have been used in several different studies. For example, a recombinant anti-EPAC1 antibody from Abcam (Cat. no. ab109415) has been used in Western blotting to detect EPAC1 levels in human retinal endothelial cells (RECs) following EPAC1-agonist treatment [
32]. While antibodies can be used for immunocytochemistry (ICC) or immunohistochemistry (IHC), current EPAC antibodies may not be useful for immunolocalization studies due to non-specific interactions; hence the mouse monoclonal EPAC1-A5 (Santa Cruz), goat polyclonal EPAC1-N16 (Abcam) and rabbit polyclonal anti-EPAC1 (Abcam) antibodies all gave a strong positive signal in immunocytochemistry of double knockout EPAC1 and EPAC2 mouse cardiomyocytes [
30]. Thus, the current available antibodies for ICC/IHC may not be specific enough for definite localization of EPAC isoforms in cells [
30].
An increasingly utilized alternative to antibody-based reagents are engineered proteins such as the peptide aptamer scaffolds, or Affimers, developed by Avacta Life Sciences (Wetherby, United Kingdom). These are small (12–15 kDa; 2–4 nm), monomeric, single-domain recombinant proteins of ~98 amino acids [
33]. Affimers are selected from a library in which two loops between adjacent pairs of β-strands at one end of the domain each contain a variable sequence of nine amino acids [
33,
34]. These two loops typically constitute the site by which they interact with their targets. Besides being small, Affimers have several other advantages over antibodies. They are structurally stable, even under the harshest conditions, and lack disulfide bridges. Affimers do not require post-translational modification, nor do they have cysteine residues, allowing efficient expression in E.coli. In addition, they have excellent thermal and chemical stabilities with a pH range of 2–13 and a melting temperature of 101 °C. In addition, they can be easily conjugated with reporter tags or drugs [
33]. Furthermore, the two variable loops provide versatile design opportunities as the two insert sequences can be randomized, allowing for identification of Affimers with desired affinity and specificity for specific targets that can be quickly generated using an established phage-display library (> 6 × 10
10) [
33].
In this study, we used the non-antibody Affimer technology to develop a selective probe that can recognize the cAMP sensor, EPAC1, for the purpose of biochemical and microscopy studies. Here, we describe the identification of EPAC1-selective Affimers from phage-display screens, their characterization in protein binding assays, further identification of an Affimer interaction site in the CNBD of EPAC1 and proof-of-principle use of an EPAC1 Affimer binder in co-immunoprecipitation and co-localization studies in transfected cells.
2. Materials and Methods
2.1. Materials
Acrylamide/Bis-acrylamide, 30% (v/v) solution, Ammonium-15N chloride, Ampicillin sodium salt, Adenosine 3′,5′-cyclic monophosphate (cAMP), Adenosine 5′-triphosphate disodium salt hydrate (ATP) 3,3′,5,5′-Tetramethylbenzidine Liquid Substrate, Benzamidine Sepharose 4Fast Flow (High sub), cOmplete™ Protease inhibitor cocktail, Forskolin, Helmanex™ III, ISOGRO®-15N Powder, Kanamycin sulfate, L-Glutathione reduced, N, N, N′, N′-tetramethylethylenediamine (TEMED), Pefabloc® SC, Poly-D-Lysine hydrobromide (PDL), PreScission® Protease, SYPRO® Orange Protein Gel Stain and Thrombin Protease were from Sigma-Aldrich (Dorset, UK). 5,7-Dibromo-6-fluoro-3,4-dihydro-2-methyl-1(2H)-quinolinecarboxaldehyde (CE3F4) was from Tocris (Bristol, UK). Alfa Aesar™ Ponceau S, BL21 Star (DE3) One Shot Chemically Competent E.coli, Biotium Covergrip Coverslip Sealant, Bovine Serum Albumin (BSA), DL-1,4-Dithiothreitol (DTT), Dimethyl Sulfoxide (DMSO), EZ-Link™ Sulfo-NHS-LC-Biotin, Isopropyl-β-D-thio-galactopyranoside (IPTG), Lipofectamine 3000 Transfection Reagent, Lipofectamine™ LTX Reagent with PLUS™ Reagent, PageBlue Protein staining solution, Phosphate-Buffered Saline (PBS) Tablets (10 mM sodium phosphate, 2.68 mM potassium chloride, 140 mM sodium chloride, pH 7.45), Prolong Glass Antifade Mountant and SuperSignal™ West Pico chemiluminescent substrate were purchased from Thermo Fisher Scientific (Waltham, MA, USA). 8-(2-[7-Nitro-4-benzofurazanyl]aminoethylthio) adenosine-3′,5′-cyclic monophosphate (8-NBD-cAMP) and 8-pCPT-2′-O-Me-cAMP (D-007) were purchased from Biolog LSI (Bremen, Germany). 5-alpha-Competent E.coli, BL21 (DE3) Competent E.coli, Protein G Agarose Beads, Protein G Magnetic Beads, Prestained protein standard Marker Broad Range (11–190 kDa) and RIPA Buffer (10×) were from New England Biolabs (Hertfordshire, UK). His-Tag (27E8) (Magnetic Bead Conjugate) and His-Tag (27E8) (Sepharose® Bead Conjugate) were purchased from Cell Signaling Technology (Danves), MA, USA). Nitrocellulose membranes were purchased from Bio-Rad Laboratories Ltd. (Hertforshire, UK).
2.2. Protein Purification
Colonies from stock plates of transformed E.coli bacteria expressing recombinant EPAC1ΔDEP, EPAC1ΔDEP mutants (L273W, D276R, R279L and F300D; provided by Professor Holger Rehmann, Hochschule Flensburg, Germany), EPAC2ΔDEP, or EPAC1-CNBD were used to inoculate LB-Broth supplemented with 100 µg/mL ampicillin (or kanamycin for EPAC1ΔDEP mutants) and left to grow overnight with shaking (200 RPM) at 37 °C. These pre-cultures were then diluted 1:20 in LB-Broth supplemented with 100 µg/mL appropriate antibiotic and then incubated for a further four hours (37 °C with shaking) until an optical density reading at 600 nm (OD
600) reached two (Biochrom WPA S1200+ Visible Spectrophometer). To induce the expression of GST-tagged proteins, fresh isopropyl-β-D-thio-galactopyranoside (IPTG) was added to a final concentration of 100 µM and then the cultures were left to grow O/N at 19 °C with shaking. To extract GST-fusion protein, pellets were resuspended in 25 mL of lysis buffer (50mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 5% (
v/v) glycerol) per pellet, supplemented with 0.5 mg/mL lysozyme, 0.1% (
v/v) Triton-X 100 and cOmplete Protease inhibitor cocktail (1 tablet/50 mL)). Cells were then lysed by sonication (SONICS Vibra-Cell™ VCX 130 Ultrasonic Liquid Processor, SONICS) for three minutes (15 s on, 15 s off) at 65% amplitude on ice. Bulk protein purification was then carried out using a previously devised protocol [
35] for batch absorption of GST-tagged protein to pre-equilibrated glutathione Sepharose 4B beads (GS4B). The GST-tag was removed from purified proteins by adding 80 U of thrombin and incubating for four hours at 4 °C in a sealed column. Pefabloc was added to the collected eluate to a final concentration of 1 mM to block further thrombin activity. For further purification of EPAC1 proteins, and to remove any degraded, low-molecular-weight cleaved forms of EPAC1, the combined cleaved protein fractions were loaded onto a gel filtration column (HiLoad 16/600 Superdex 200 pg, ÄKTA, GE Healthcare) equilibrated with GFC buffer (PBS; 150 mM NaCl, 2.5% (
v/v) glycerol). The fractions of ÄKTA purified EPAC1ΔDEP protein were combined and analyzed by SDS-PAGE and PageBlue staining or Ponceau-S staining. Protein concentrations were determined using A
280 aliquots were stored at −80 °C.
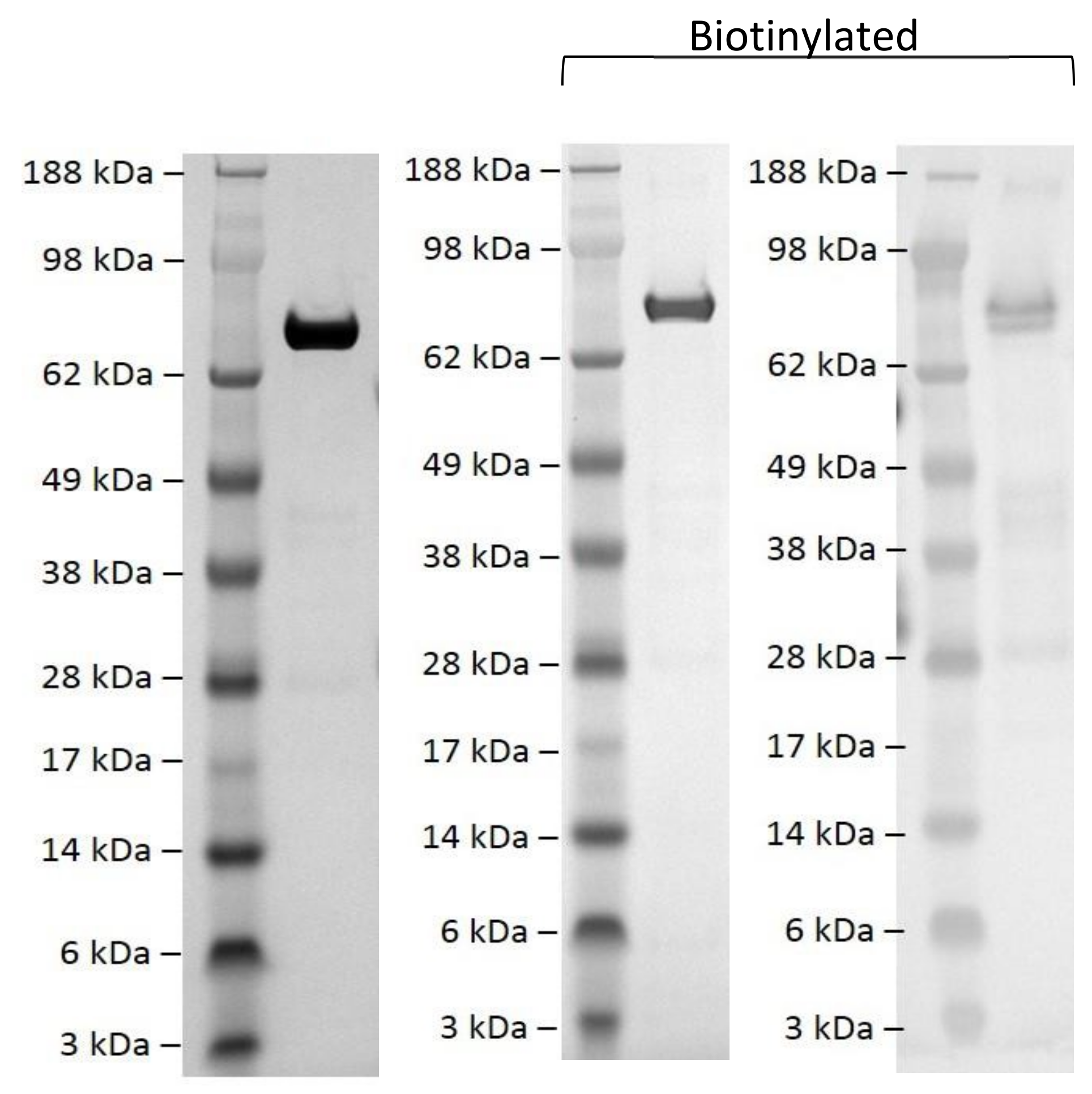
2.3. Biotinylation of EPAC1ΔDEP Protein
Purified EPAC1ΔDEP was biotinylated using amine reactive Biotinylation reagent, EZ-Link™ Sulfo-NHS-LC-biotin (Thermo Fisher Scientific, Waltham, MA, USA) in a 10-fold molar excess for 2 h on ice. Excess biotin was removed by diafiltration into PBS, pH 7.4. Successful biotinylation was confirmed using Western blotting developed with Streptavidin-HRP (ab7403; Abcam, Cambridge, UK).
2.4. Phage-Display Screens
Phage screens were performed using a Type II Affimer library, T2[9_9]ph4v1 (Avacta Life Sciences, Wetherby, United Kingdom). Up to three rounds of panning were performed during each screen. During two selections, EPAC1 was immobilized alone (S01, S04) and additional selections were performed with either 10 µM D-007 (EPAC1-agonist) (S02, S05) or 10 µM CE34F (EPAC1-antagonist) (S03, S06) in the buffer (PBS, pH 7.4). All phage selections consisted of three panning rounds. Bound phage were used to infect E.coli cells and then plated. The Affimer protein coding regions from each phage screen were subcloned into a vector containing both HA and His6 tags (pEtLECTRA cHAH6) and transformed into E.coli. 90 positive colonies were taken from pans two and three of each of the six phage selections (S01-S03; S04-S06; 1080 in total). Each colony was grown overnight and the resulting candidate Affimer proteins were purified by immobilized metal affinity chromatography. The concentrations of the purified Affimer proteins were normalized to 2.5 µg/mL for use during primary screening.
2.5. Orthogonal Screening of Identified Affimer Clones
HA-tagged Affimers from the 90 selected clones from phage-display screens were screened in multiple independent bead-based multiplex binding assays using a flow cytometer Intellicyt iQue instrument to confirm their interaction with biotinylated EPAC1ΔDEP. The iQue screening platform is a bead-based flow system for quick analysis of clones against a multiplexed target set. Biotinylated EPAC1ΔDEP and mIgG2b (positive control) were immobilized to streptavidin-functionalized beads, in the presence of 10 µM D-007 (EPAC1-agonist) or 10 µM CE34F (EPAC1-antagonist) in the final assay solution. The HA-tagged Affimers interacting with bead-bound EPAC1ΔDEP were detected using anti HA-tag antibodies with an AlexaFluor 488 conjugate. Similarly, the immobilized mIgG2b positive control was detected with and anti-mIgG2b HA tagged Affimer (G12) followed by Alexa-fluor-conjugated anti-HA-tag antibodies. Affimers found to interact with immobilized EPAC1ΔDEP were further tested for cross-reactivity by testing their interaction with bead-immobilized human and dog C-reactive protein (CRP) and/or anti-carcinoembryonic antigen (CEA). Affimers displaying no cross-reactivity were sequenced and used in follow-on assays.
2.6. Dot Blotting
Different concentrations of recombinant EPAC1ΔDEP (GST-tag free) were spotted onto nitrocellulose membranes. Antibody/Affimer interactions were then detected by far-Western blotting. The membranes were blocked for one hour at RT with 5% (w/v) “Marvel” milk (antibody blot) or BSA (Affimer blot) in 1 × TBST followed by a two-hour incubation at RT in either 4.5 nM (1:1000) of 5D3 antibody in 5% (w/v) milk in 1 × TBST, or 45 nM of Affimer diluted in 5% (w/v) BSA in 1 × TBST. Membranes were then incubated for one hour at RT with an appropriate secondary antibody (anti-mouse for 5D3 antibody or His-tag for Affimer) and the protein signal was detected with ECL (SuperSignal™ West Pico chemiluminescent substrate; Thermo Fisher Scientific, Waltham, MA, USA) and visualized using a Fusion FX imaging system.
2.7. Microscale Thermophoresis (MST)
The Monolith NT™ Protein Labelling Kit Red-NHS containing the red fluorescent dye NT-647 was used to label recombinant EPAC1-CNBD protein according to the manufacturer’s protocol. Equal volumes of labelled protein were then added to Monolith NT.115 capillaries (Nanotemper technologies) and then measured at LED/excitation set at 80–100% and MST power of 60% (high setting) on the MST instrument. The normalized fluorescence (Fnorm (%)) from MO.Affinity Analysis software version 2.2.4 (Nanotemper technologies) was normalized as a fold change with the relative fluorescence associated with the lowest concentration of antibody set to 1 (Arbitrary Units: AU). The pEC50 was determined by nonlinear curve fitting analysis (Log (agonist) vs. response (three parameters)) GraphPad Prism.
2.8. Protein Interaction ELISA Assays
GST-tagged recombinant proteins (1 µg/100 µL/well) were added to Pierce™ glutathione coated plates (15,140; Thermo Fisher Scientific), and incubated overnight at 4 °C. 100 µL/well of 0.002–0.250 µM 5D3 or 5B1 antibody, or 0.004–4 µM of Affimer was then added and incubated for one hour at RT. 100 µL/well of 1:10,000 anti-mouse or anti-his-tag HRP-conjugated secondary antibodies were then added to 5D3/5B1 antibody or Affimer wells, respectively, and incubated for a further one hour at RT. Recombinant protein alone was used as a control. Each experiment was performed in triplicates and between each incubation step, the wells were washed three times with wash buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl and 0.05% (v/v) Tween-20 buffer). Protein, antibodies and Affimers were diluted in assay buffer (10 mM Tris-HCl pH 7.4 and 150 mM NaCl buffer). Plates were kept covered during all incubation steps. Finally, 100 µL/well of 3, 3′,5,5′-Tetramethylbenzidine Liquid (ELISA) Substrate, Supersensitive was added to each well and incubated for 30 min at RT. Detection of color change was measured at wavelength 655 nm using a FLUOstar Omega Microplate reader (BMG Labtech, Aylesbury, Bucks, UK). All data were normalized as a fold change where the lowest concentration of antibody or Affimer was set to 1 (arbitrary units).
2.9. Thermal Shift Assays (TSA)
Thermal shift assays were carried out in Thermo Fisher Scientific™ Abgene 1.2 mL Polypropylene 96-well storage plates (10243223; Thermo Fisher Scientific, Waltham, MA, USA), using a modified protocol based on the methods described by Huynh and Partch [
36]. All dilutions were prepared in assay buffer (50 mM Tris-HCl pH 7.5, 50 mM NaCl, 2.5% (
v/v) glycerol and 5 mM DTT). 1–2 µg/well of GST-tagged recombinant protein was combined with 20 × SYPRO Orange dye and added to wells. Plates were sealed with a sheet of optically clear adhesive (MicroAmp™ Optical Adhesive Film; Applied Biosystems) and mixed for 15 min on an orbital shaker at RT before incubating overnight at 4 °C. The fluorescence readout of SYPRO Orange dye was then measured using an Applied Biosystems StepOnePlus Real-time PCR Instrument (System Version 2.2.3; Thermo Fisher Scientific) with excitation wavelength 470 nm and emission wavelength 570 nm over a temperature range from 11 to 80 °C ramped in 0.5 °C increments with plateau times of 30 s. The melting temperature (Tm) (°C) was derived from calculating the first derivative of the fluorescence emission as a function of temperature (−dF/dT) (Equation (1)) where Tm is the lowest first derivative value. For multiple experimental replicates, the mean Tm was calculated with standard error of the mean.
Equation (1). First Derivative Calculations.
2.10. Cell Culture
COS1 cells were grown in Dulbecco’s modified Eagle’s medium (without added glutamine) (DMEM), 10% (v/v) fetal bovine serum, 2 mM glutaMAX, and 100 units/mL and 100 μg/mL penicillin and streptomycin, respectively. Cultures were incubated at 37 °C, 5% (v/v) CO2. U20S cell lines stably expressing pBabe-Flag-Epac1 (HS 1-881) (EPAC1 U2OS), pBabe-Flag-Epac2 (EPAC2 U2OS) or vector alone (Control U2OS), were gifts from Professor Holger Rehmann (Hochschule Flensburg, Germany). U2OS cells were grown in Dulbecco’s modified Eagle’s medium (without added glutamine) (DMEM), 10% (v/v) fetal bovine serum, 2 mM glutaMAX, and 100 units/mL and 100 μg/mL penicillin and streptomycin, respectively and incubated at 37 °C, 5% (v/v) CO2. Puromycin (2 mg/L (w/v)) was added to growth medium to maintain stable expression of EPAC1 and EPAC2 U2OS cell lines only.
2.11. SDS-PAGE and Immunoblotting
Equal amounts of recombinant proteins or cell lysates were separated on 8, 10, 12 or 12.5% (w/v) SDS-PAGE gels, depending on protein size, by electrophoresis. Lysates were then wet transferred from gels to nitrocellulose membranes using Mini Trans-Blot® electrophoretic transfer cell (Bio-Rad) and 1x transfer buffer (250 mM Tris Base, 1.92 M Glycine) for one hour and 30 min at 80 V. Membranes were then blocked in 5% (w/v) “Marvel” milk powder in 1 × TBST (100 mM Tris-HCl, pH 7.4, 59 mM NaCl, 0.1% (w/v) Tween-20) for one hour at RT. Primary antibodies diluted in 1 × TBST were incubated overnight at 4 °C followed by a one hour incubation at RT with the secondary antibody diluted in 1 × TBST. Protein signals were detected using ECL (SuperSignal™ West Pico chemiluminescent substrate) and visualized using Fusion FX imaging software (Vilber Lourmat, France).
2.12. Immunoprecipitation (IP)
U2OS cells were grown to 100% confluency in 6-well plates (9.6 cm2 per well) and then placed on ice and washed with 1 mL/well ice-cold PBS. Cells were then lysed with 0.5 mL/well lysis buffer (1x RIPA buffer supplemented with 1 × complete Protease inhibitor cocktail). Cell lysates were subjected to immunoblotting with 5D3 mAb (1.35 or 2.6 µg), 5B1 mAb (2.5 µg), rabbit IgG (4 µg) antibodies or unbound Affimer either alone (2.6 µg) and incubated with rotation for 30 min at 4 °C. Protein G Agarose or α-His Tag Sepharose® beads were then added and incubated with rotation for one hour at 4 °C. Beads were then collected by centrifugation and heated in 1x sample buffer for five minutes at 95 °C. The input and immunoprecipitated samples were then analyzed by Western blotting.
2.13. Immunofluorescent Confocal Microscopy
COS1 cells were seeded to at least 90% confluency onto PDL-coated 25 mm diameter No 1.5H high-precision glass coverslips and allowed to adhere overnight in growth medium (37 °C, 5% (v/v) CO2). Cells were then transiently co-transfected with 780A-mCherry DNA and EPAC1-FLAG or EPAC1-(K212R)-FLAG. Cells were then transfected with X-tremeGENE 360 (XTG360) Transfection Reagent (Sigma-Aldrich) as per the manufacturer’s protocols. To check the transfection efficiency wide-field imaging was carried out on FLoid™ Cell Imaging Station (Thermo Fisher Scientific 4471136), normal white light and red or green fluorescence were used. After 48 h, growth medium containing transfection reagents was replaced with fresh medium and coverslips were incubated for 30 min at 37 °C, 5% (v/v) CO2. Cells were then stimulated or unstimulated with 100 µM of 007 and incubated for 15 min at 37 °C, 5% (v/v) CO2 before fixation with 2 mL/well of 4% (v/v) paraformaldehyde (prepared in PBS) for 15 min at RT. Cells were then quenched to remove free aldehydes with 2 mL/well of filter sterilized (Whatman Puradisc 25 mm syringe filter 0.2 µm) 50 mM NH4Cl (prepared in PBS) for 10 min at RT. Cells were then permeabilized with 2 mL/well of 0.1% (v/v) Triton-x 100 in PBS for 10 min at RT. Cells were washed three times with PBS between each incubation. Cells were then blocked for one hour at RT in blocking buffer (1% (w/v) BSA, 0.3% (v/v) Triton-X 100 in PBS, filtered (Whatman Puradisc 25 mm syringe filter 0.2 µm)). Primary and secondary antibodies (α-FLAG and goat α-mouse IgG H + L (Alexa Fluor 488)) were prepared in blocking buffer and cells were incubated for one hour at RT. Coverslips were mounted onto Fisherbrand™ microscopic glass slides (12383118; Thermo Fisher Scientific) using Prolong™ Glass Antifade Mountant, sealed with Biotium covergrip coverslip sealant and stored at 4 °C. Samples were analyzed using a Leica HC PL APO C52 63x water objective on a Leica TCS SP8 STED 3 × confocal microscope. Samples were excited using a Supercontinuum White Light Laser at 488 and 580 nm for Alexa Fluor 488 and mCherry, respectively, using a Leica HyD1 hybrid detector with detection windows of 500–550 nm for Alexa Fluor 488 and 590–650 nm for mCherry.
2.14. Data Analysis
Calculations were performed using Microsoft Excel 2016 for Windows (Microsoft Software) and graphs were made using GraphPad Prism version 5.2 or 8.4.2 for Windows (GraphPad Software LLC, San Diego, CA, USA). Unless otherwise stated, data values are represented as the mean ± standard error of the mean (SEM) or the mean ± standard deviation (SD). The density of protein bands from Western blots was quantified using ImageJ/Fiji version 1.53c (National Institute of Health, Bethesda, Maryland, USA).
To compare two sets of means with a minimum of three experimental repeats, p-values were obtained from unpaired t-tests using GraphPad Prism version 5.2/9.0.0 for Windows. Analysis of variance (ANOVA) was used for data sets with three or more sets of means, with a minimum of three experimental repeats. p-values were from one-way ANOVA with Dunnett’s multiple comparison test using GraphPad Prism versions 5.2/9.0.0.
Confocal images were acquired using the Leica Application Suit X software version 3.0.15 and saved in the LIF format and analyzed using ImageJ/Fiji. Z-stacks were taken with a zoom factor of 1.3 (2048 × 2048 nm). Deconvolution was performed using Huygens Professional software using default settings apart from 0.01% quality change threshold and images were saved in the ICS image format and analyzed using ImajeJ/Fiji. Scale bars were added to microscopy images using the ImageJ Microscopy Scale plugin (National Institute of Health, Bethesda, Maryland, USA). Using ImageJ/Fiji, Z-stacks were analyzed using the Stacks Z-project maximum intensity projection method. Brightness and contrast adjustments post-acquisition were performed using ImageJ/Fiji.
4. Discussion
To provide a target to generate EPAC1-selective Affimers for phage display, a purification protocol for recombinant EPAC1ΔDEP protein was optimized to obtain protein that was at least 90% pure. In general, the optimized protocol produced approximately 1 mg EPAC1ΔDEP per liter of culture. Purified EPAC1ΔDEP was biotinylated and used as a target in phage-display screens to identify candidate EPAC1-selective Affimers that were subjected to final functional assessment using a fluorescent-based protein interaction screen. Phage-display screens were carried out in the presence of the EPAC-selective agonists, 007, or the antagonist, CE3F4, and sequencing of interacting phage revealed nine unique Affimers, five of which were established as being EPAC1-positive binders in orthogonal screens. Despite the presence of 007 or CE3F4 during phage-display screening, the isolated Affimers did not appear to discriminate between the ligand bound and ligand-free forms of EPAC1.
The binding characteristics of the five Affimers, named 380A, 414A, 604A, 748A and 780A, were further characterized for their ability to interact with EPAC1, using different solid and solution phase in vitro assays. From these assays, Affimers 780A, 380A and 414A were identified using dot blots and ELISA assays as the top three binders that showed selectivity for recombinant EPAC1ΔDEP and EPAC1-CNBD protein, over EPAC2, although their relative binding affinities were much lower than the pre-existing EPAC1 mAb, 5D3. In addition, from these experiments, it was clear that Affimer 780A interacted equally well with the active and inactive conformations of EPAC1, as provoked by ligand binding. Affimer A780 therefore presents technical advantages over mAb 5D3 in that it is not conformation selective, whereas 5D3 favors interaction with the active conformation of EPAC1.
The potential interaction sites of Affimer 780A on EPAC1 were first investigated using ELISA and TSA assays to assess interaction of the Affimer with four CNBD mutants of recombinant EPAC1ΔDEP (L273W, D276R, R279L and F300D), which were originally used to identify the binding epitope for mAb 5D3 in the EPAC1 CNBD [
31]. ELISA assays revealed that the binding of Affimer 780A was significantly reduced by D276R and R279L mutants, compared to WT EPAC1, whereas the binding efficacy of mAb 5D3 was significantly impaired by mutants L273W and D276R. Recombinant F300D was found to be in a non-native form by TSA. Thus, although Affimer 780A and mAb 5D3 interact within the same discrete region within the EPAC1 CNBD, it appears that their binding mode are subtly different, that, and the comparative size differences between 780A and mAb 5D3, may go some way to explain the differences in conformational selectivity between the two affinity agents.
Having determined that Affimer 780A is an affective binder for EPAC1, in cell interaction assays were carried out next. Using immunoprecipitation assays, the top three Affimer binders were all found to selectively precipitate EPAC1 protein from U2OS cells expressing EPAC1, but not EPAC2. Moreover, in agreement with in vitro studies, the Affimers did not show preferential interaction with the active form of EPAC1, in comparison with mAb 5D3. Next confocal microscopy experiments were used to visualize the expression of a protein chimera of Affimer 780A with fluorescent mCherry protein in COS1 cells. From these experiments it was found that Affimer 780A localized to the nuclei of COS1 cells, whether or not EPAC1 was pharmacologically activated by treatment of cells with 007. Furthermore, Affimer 780A was observed to co-localize with FLAG-tagged EPAC1 and a mis-targeted mutant of EPAC1, K212R, predominantly at punctate loci throughout the cytosolic regions of COS1 cells, following 007 treatment.
Recombinant protein scaffolds, such as Affimers, can and have been used to observe direct target binding. Affimers are not only small proteins (12–14 kDa), allowing for good penetration of cells, but can also be easily labelled with fluorescent molecules at specific sites [
37,
38]. This offers improved resolution, providing a more confident assessment of the localization of an Affimer with its specific target, such as EPAC1 in the case of Affimer 780A. Through confocal microscopy studies, Affimer 780A was shown to localize with overexpressed EPAC1, with or without 007 stimulation, further demonstrating that the Affimer does not favor the active or inactive conformations of EPAC1, unlike mAb 5D3, which interacts selectively with active EPAC1 [
23]. In addition, the co-localization of Affimer 780A with a mis-targeting EPAC1 mutant (K212R) confirms that the Affimer binds to EPAC1 in cells regardless of location. Furthermore, the subcellular co-localization of the Affimer with EPAC1 predominantly in the nuclear and perinuclear regions here agree with previous studies, using several different cell types, providing further confidence that Affimer 780A interacts with EPAC1 in cells. For example, in COS-7 cells transfected with GFP-tagged EPAC1 protein, fluorescent confocal microscopy showed an association of EPAC1 with the nucleus and along the nuclear membrane [
17]. In addition, using an EPAC1 FRET sensor, where EPAC1 was sandwiched between two different fluorescent proteins, active EPAC1 was observed to co-localize to membranes and the cytosol, but particularly at the nuclear envelope and perinuclear region in four different cell lines tested, including HEK293 and NIE-115 neuroblastoma cell lines [
39]. Moreover, in an immunocytochemistry (ICC) study, using transfected FLAG-tagged EPAC1 in COS1 cells, the immunoreactivity of EPAC1, was also seen to associate distinctly with the perinuclear region of COS1 cells [
18]. Using both live cell fluorescent confocal experiments and FRET imaging of HEK293 cells, it was also observed that active EPAC1 associates with the nuclear envelope, nucleus, PM, endomembranes and cytosol [
22]. In this case, however, when cells were stimulated with 007, there was a redistribution of a small subfraction of EPAC1 to the PM [
22], which is consistent with what was observed here. Finally, HEK293T cells overexpressing EPAC1-FLAG and immunostained with mAB 5D3 also showed that active EPAC1 associated with the perinuclear region of cells [
23]. The fact that the localization of Affimer 780A-mCherry in COS1 cells observed in this project agree with results from the studies described above, suggests that Affimer 780A has the potential to be used as a probe for microscopy studies involving EPAC1.