Overexpression of Msx1 in Mouse Lung Leads to Loss of Pulmonary Vessels Following Vascular Hypoxic Injury
Abstract
:1. Introduction
2. Methods
2.1. Animal Model
2.2. Immunostaining for Human Paraffin-Embedded Sections
2.3. Measurement for Pulmonary Vessels
2.4. Isolectin Staining of Mouse Retina
2.5. Western Blotting of Mouse Lung
2.6. RNA Sequencing of Mouse Lung and Cultured Mouse Cells
2.7. Msx1 Plasmid and siRNA Transfections in Cultured Pulmonary Vascular Endothelial and Pulmonary Vascular Smooth Muscle Cells
2.8. Statistical Analysis
3. Results
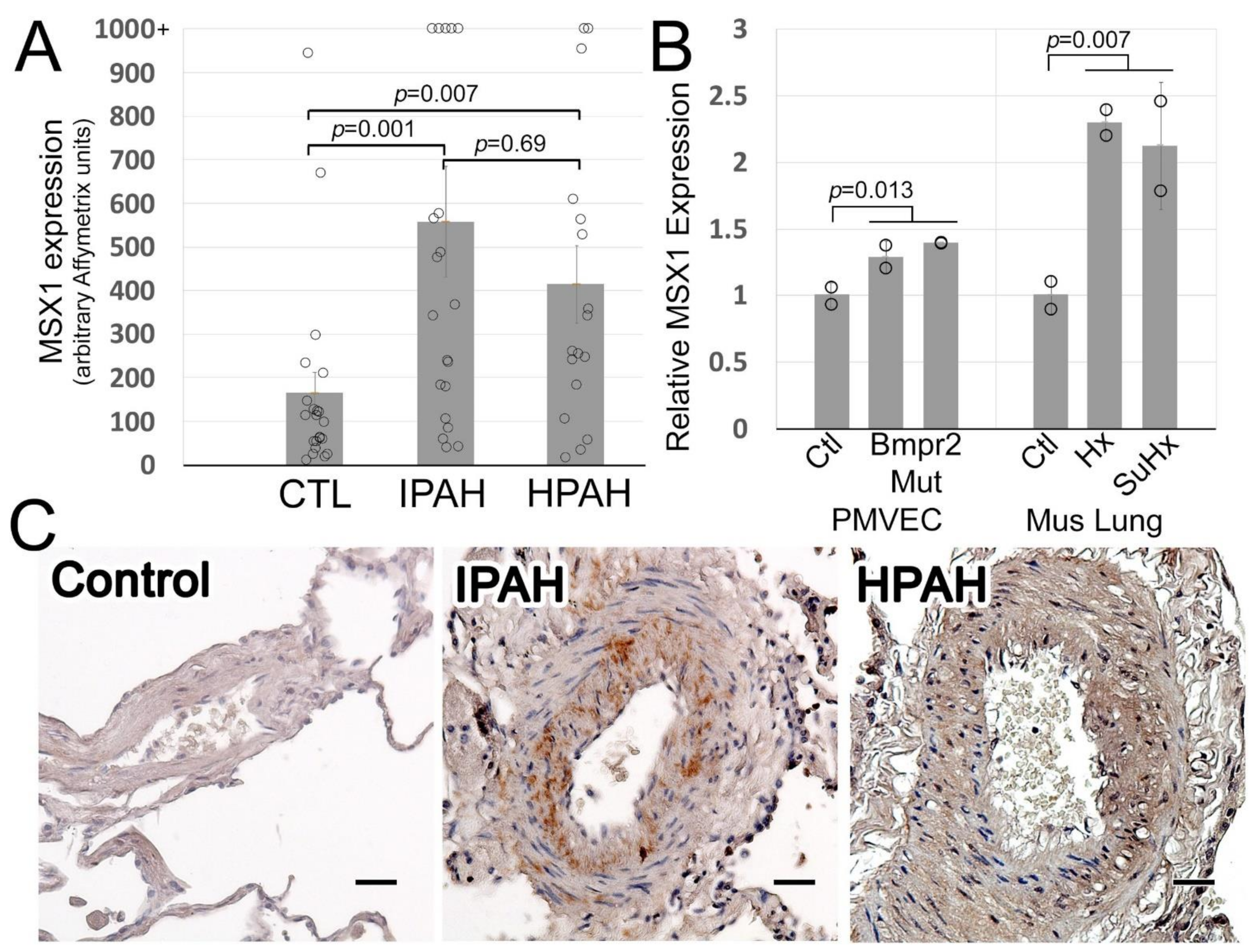
3.1. Msx1 Protein Expression Is Strikingly Increased in IPAH Pulmonary Arteries
3.2. MSX1 Expression Is Increased in Msx1OE Mice
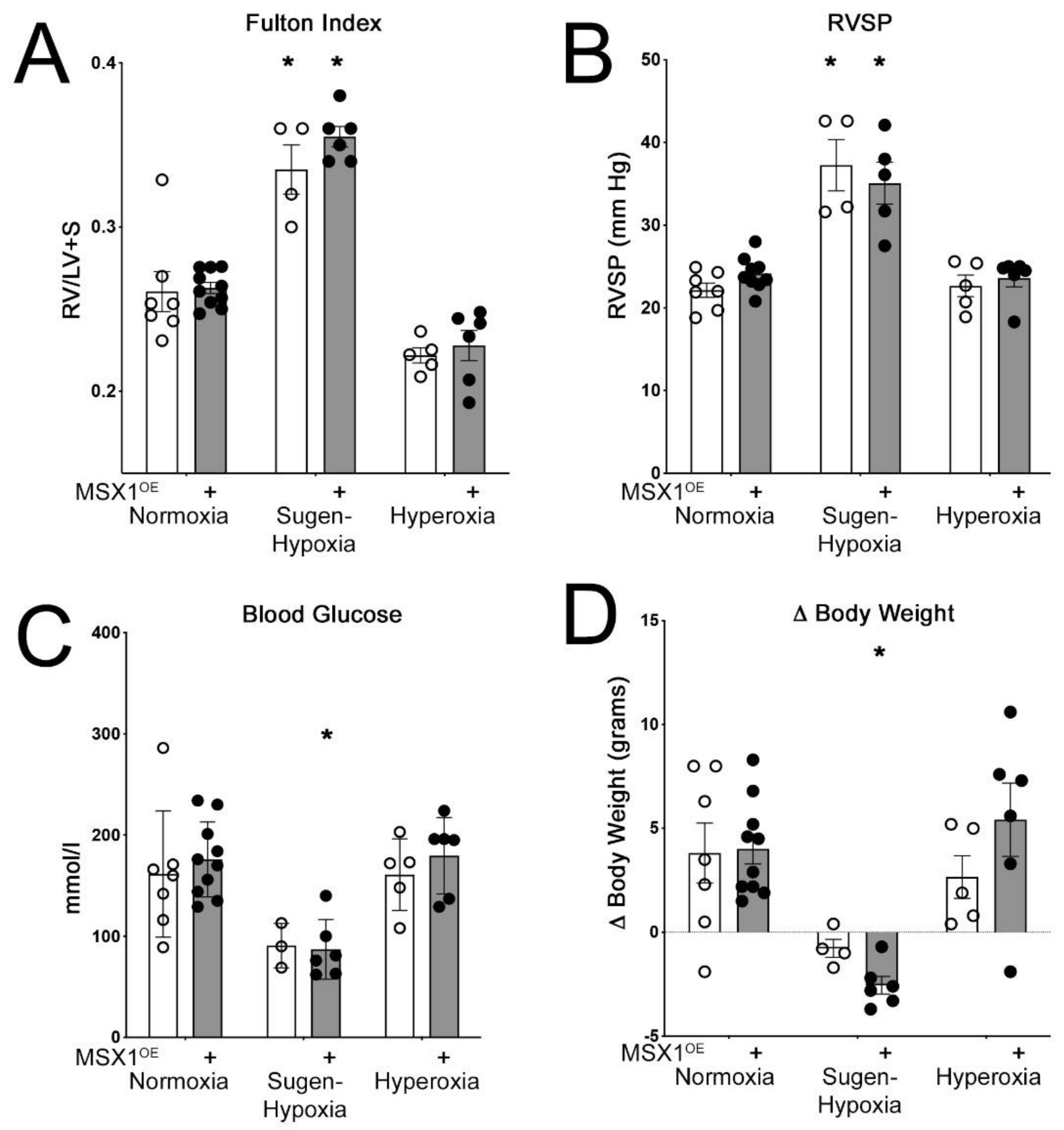
3.3. Hemodynamic Parameters in MSX1OE Mice Following Sugen Hypoxia or Hyperoxia Treatment
3.4. MSX1 Overexpression Leads to Loss of Capillaries in Mouse Retina and Following Sugen Hypoxia Treatment Resulted in Loss of Pulmonary Vessels in MSX1OE Mice
3.5. Gene Expression Differences in MSX1OE Mouse Lung Compared to Controls
3.6. MSX1 Gene Expression in PVECs and PVSMCs Are Involved in Cell Cycle, Cytoskeletal and Macromolecule Organization, and Programmed Cell Death
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Machado, R.F.; Gladwin, M.T. Pulmonary hypertension in hemolytic disorders: Pulmonary vascular disease: The global perspective. Chest 2010, 137, 30S–38S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachulla, E.; Gressin, V.; Guillevin, L.; Carpentier, P.; Diot, E.; Sibilia, J.; Kahan, A.; Cabane, J.; Francès, C.; Launay, D.; et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: A French nationwide prospective multicenter study. Arthritis Rheum. 2005, 52, 3792–3800. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, D.; St George, D.; Coleiro, B.; Knight, C.; Denton, C.P.; Davar, J.; Black, C.M.; Coghlan, J.G. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: Application of a registry approach. Ann. Rheum. Dis. 2003, 62, 1088–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, G.H.; Souza, R.; Salemi, V.M.; Jardim, C.V.; Gualandro, S.F. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur. Respir. J. 2012, 39, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Peacock, A.J.; Murphy, N.F.; McMurray, J.J.; Caballero, L.; Stewart, S. An epidemiological study of pulmonary arterial hypertension. Eur. Respir. J. 2007, 30, 104–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stacher, E.; Graham, B.B.; Hunt, J.M.; Gandjeva, A.; Groshong, S.D.; McLaughlin, V.V.; Jessup, M.; Grizzle, W.E.; Aldred, M.A.; Cool, C.D.; et al. Modern age pathology of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Sung, Y.K.; Yuan, K.; de Jesus Perez, V.A. Novel approaches to pulmonary arterial hypertension drug discovery. Expert Opin. Drug Discov. 2016, 11, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef]
- Humbert, M.; Sitbon, O.; Chaouat, A.; Bertocchi, M.; Habib, G.; Gressin, V.; Yaici, A.; Weitzenblum, E.; Cordier, J.F.; Chabot, F.; et al. Pulmonary arterial hypertension in France: Results from a national registry. Am. J. Respir. Crit. Care Med. 2006, 173, 1023–1030. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Keller, S.H.; Remillard, C.V.; Safrina, O.; Nicholson, A.; Zhang, S.L.; Jiang, W.; Vangala, N.; Landsberg, J.W.; Wang, J.Y.; et al. A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation 2009, 119, 2313–2322. [Google Scholar] [CrossRef] [Green Version]
- Remillard, C.V.; Tigno, D.D.; Platoshyn, O.; Burg, E.D.; Brevnova, E.E.; Conger, D.; Nicholson, A.; Rana, B.K.; Channick, R.N.; Rubin, L.J.; et al. Function of Kv1.5 channels and genetic variations of KCNA5 in patients with idiopathic pulmonary arterial hypertension. Am. J. Physiol. Cell Physiol. 2007, 292, C1837–1853. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Fessel, J.; West, J. Microarray studies in pulmonary arterial hypertension. Int. J. Clin. Pract. Suppl. 2011, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Austin, E.D.; Menon, S.; Hemnes, A.R.; Robinson, L.R.; Talati, M.; Fox, K.L.; Cogan, J.D.; Hamid, R.; Hedges, L.K.; Robbins, I.; et al. Idiopathic and heritable PAH perturb common molecular pathways, correlated with increased MSX1 expression. Pulm. Circ. 2011, 1, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catron, K.M.; Iler, N.; Abate, C. Nucleotides flanking a conserved TAAT core dictate the DNA binding specificity of three murine homeodomain proteins. Mol. Cell Biol. 1993, 13, 2354–2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catron, K.M.; Zhang, H.; Marshall, S.C.; Inostroza, J.A.; Wilson, J.M.; Abate, C. Transcriptional repression by Msx-1 does not require homeodomain DNA-binding sites. Mol. Cell Biol. 1995, 15, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Bendall, A.J.; Abate-Shen, C. Roles for Msx and Dlx homeoproteins in vertebrate development. Gene 2000, 247, 17–31. [Google Scholar] [CrossRef]
- Zhang, H.; Catron, K.M.; Abate-Shen, C. A role for the Msx-1 homeodomain in transcriptional regulation: Residues in the N-terminal arm mediate TATA binding protein interaction and transcriptional repression. Proc. Natl. Acad. Sci. USA 1996, 93, 1764–1769. [Google Scholar] [CrossRef] [Green Version]
- Lopes, M.; Goupille, O.; Saint Cloment, C.; Lallemand, Y.; Cumano, A.; Robert, B. Msx genes define a population of mural cell precursors required for head blood vessel maturation. Development 2011, 138, 3055–3066. [Google Scholar] [CrossRef] [Green Version]
- Petit, S.; Meary, F.; Pibouin, L.; Jeanny, J.C.; Fernandes, I.; Poliard, A.; Hotton, D.; Berdal, A.; Babajko, S. Autoregulatory loop of Msx1 expression involving its antisense transcripts. J. Cell Physiol. 2009, 220, 303–310. [Google Scholar] [CrossRef]
- Blin-Wakkach, C.; Lezot, F.; Ghoul-Mazgar, S.; Hotton, D.; Monteiro, S.; Teillaud, C.; Pibouin, L.; Orestes-Cardoso, S.; Papagerakis, P.; Macdougall, M.; et al. Endogenous Msx1 antisense transcript: In vivo and in vitro evidences, structure, and potential involvement in skeleton development in mammals. Proc. Natl. Acad. Sci. USA 2001, 98, 7336–7341. [Google Scholar] [CrossRef] [Green Version]
- Vandersmissen, I.; Craps, S.; Depypere, M.; Coppiello, G.; van Gastel, N.; Maes, F.; Carmeliet, G.; Schrooten, J.; Jones, E.A.; Umans, L.; et al. Endothelial Msx1 transduces hemodynamic changes into an arteriogenic remodeling response. J. Cell Biol. 2015, 210, 1239–1256. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Yuan, G.; Ye, W.; Cho, K.W.; Chen, Y. An atypical canonical bone morphogenetic protein (BMP) signaling pathway regulates Msh homeobox 1 (Msx1) expression during odontogenesis. J. Biol. Chem. 2014, 289, 31492–31502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyono, M.; Shibuya, M. Bone morphogenetic protein 4 mediates apoptosis of capillary endothelial cells during rat pupillary membrane regression. Mol. Cell Biol. 2003, 23, 4627–4636. [Google Scholar] [CrossRef] [Green Version]
- Binato, R.; Alvarez Martinez, C.E.; Pizzatti, L.; Robert, B.; Abdelhay, E. SMAD 8 binding to mice Msx1 basal promoter is required for transcriptional activation. Biochem. J. 2006, 393, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cines, D.B.; Pollak, E.S.; Buck, C.A.; Loscalzo, J.; Zimmerman, G.A.; McEver, R.P.; Pober, J.S.; Wick, T.M.; Konkle, B.A.; Schwartz, B.S.; et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998, 91, 3527–3561. [Google Scholar] [PubMed]
- Odelberg, S.J.; Kollhoff, A.; Keating, M.T. Dedifferentiation of mammalian myotubes induced by msx1. Cell 2000, 103, 1099–1109. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Lee, H.; Price, S.M.; Shen, M.M.; Abate-Shen, C. Msx homeobox genes inhibit differentiation through upregulation of cyclin D1. Development 2001, 128, 2373–2384. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, K.; Kim, S.; Lee, J.H. Msx1 gene overexpression induces G1 phase cell arrest in human ovarian cancer cell line OVCAR3. Biochem. Biophys. Res. Commun. 2001, 281, 1234–1240. [Google Scholar] [CrossRef]
- Son, M.J.; Rho, S.B.; Kim, K.; Oh, M.; Son, C.; Song, S.Y.; Park, K. Homeoprotein Msx1-PIASy Interaction Inhibits Angiogenesis. Cells 2020, 9, 1854. [Google Scholar] [CrossRef]
- Park, K.; Kim, K.; Rho, S.B.; Choi, K.; Kim, D.; Oh, S.H.; Park, J.; Lee, S.H.; Lee, J.H. Homeobox Msx1 interacts with p53 tumor suppressor and inhibits tumor growth by inducing apoptosis. Cancer Res. 2005, 65, 749–757. [Google Scholar]
- Nagel, S.; Ehrentraut, S.; Meyer, C.; Kaufmann, M.; Drexler, H.G.; MacLeod, R.A. Repressed BMP signaling reactivates NKL homeobox gene MSX1 in a T-ALL subset. Leuk. Lymphoma 2015, 56, 480–491. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakamura, S.; Nishida, W.; Sobue, K. Bone morphogenetic protein-induced MSX1 and MSX2 inhibit myocardin-dependent smooth muscle gene transcription. Mol. Cell Biol. 2006, 26, 9456–9470. [Google Scholar] [CrossRef] [Green Version]
- Happé, C.; Kurakula, K.; Sun, X.Q.; da Silva Goncalves Bos, D.; Rol, N.; Guignabert, C.; Tu, L.; Schalij, I.; Wiesmeijer, K.C.; Tura-Ceide, O.; et al. The BMP Receptor 2 in Pulmonary Arterial Hypertension: When and Where the Animal Model Matches the Patient. Cells 2020, 9, 1422. [Google Scholar] [CrossRef]
- West, J.; Fagan, K.; Steudel, W.; Fouty, B.; Lane, K.; Harral, J.; Hoedt-Miller, M.; Tada, Y.; Ozimek, J.; Tuder, R.; et al. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ. Res. 2004, 94, 1109–1114. (In eng) [Google Scholar] [CrossRef] [Green Version]
- West, J.D.; Chen, X.; Ping, L.; Gladson, S.; Hamid, R.; Lloyd, J.E.; Talati, M. Adverse effects of BMPR2 suppression in macrophages in animal models of pulmonary hypertension. Pulm. Circ. 2019, 10, 2045894019856483. [Google Scholar] [CrossRef] [Green Version]
- Fessel, J.P.; Flynn, C.R.; Robinson, L.J.; Penner, N.L.; Gladson, S.; Kang, C.J.; Wasserman, D.H.; Hemnes, A.R.; West, J.D. Hyperoxia synergizes with mutant bone morphogenic protein receptor 2 to cause metabolic stress, oxidant injury, and pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2013, 49, 778–787. [Google Scholar] [CrossRef] [Green Version]
- Alon, T.; Hemo, I.; Itin, A.; Pe’er, J.; Stone, J.; Keshet, E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med. 1995, 1, 1024–1028. [Google Scholar] [CrossRef]
- Ivanova, E.; Toychiev, A.H.; Yee, C.W.; Sagdullaev, B.T. Optimized protocol for retinal wholemount preparation for imaging and immunohistochemistry. J. Vis. Exp. 2013, 82, e51018. [Google Scholar] [CrossRef]
- Tual-Chalot, S.; Allinson, K.R.; Fruttiger, M.; Arthur, H.M. Whole mount immunofluorescent staining of the neonatal mouse retina to investigate angiogenesis in vivo. J. Vis. Exp. 2013, 77, e50546. [Google Scholar] [CrossRef] [Green Version]
- Jat, P.S.; Noble, M.D.; Ataliotis, P.; Tanaka, Y.; Yannoutsos, N.; Larsen, L.; Kioussis, D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc. Natl. Acad. Sci. USA 1991, 88, 5096–5100. [Google Scholar] [CrossRef] [Green Version]
- Pozzi, A.; Moberg, P.E.; Miles, L.A.; Wagner, S.; Soloway, P.; Gardner, H.A. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 2202–2207. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo-Garcia, J.L.; Arias, T.; Rojas, Y.; Garcia-Ruiz, V.; Santos, A.; Martin-Puig, S.; Ruiz-Cabello, J. Metabolic Reprogramming in the Heart and Lung in a Murine Model of Pulmonary Arterial Hypertension. Front. Cardiovasc. Med. 2018, 5, 110. [Google Scholar] [CrossRef]
- Hellström, M.; Phng, L.K.; Hofmann, J.J.; Wallgard, E.; Coultas, L.; Lindblom, P.; Alva, J.; Nilsson, A.K.; Karlsson, L.; Gaiano, N.; et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 2007, 445, 776–780. [Google Scholar] [CrossRef]
- Austin, E.D.; Cogan, J.D.; West, J.D.; Hedges, L.K.; Hamid, R.; Dawson, E.P.; Wheeler, L.A.; Parl, F.F.; Loyd, J.E.; Phillips, J.A. Alterations in oestrogen metabolism: Implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur. Respir. J. 2009, 34, 1093–1099. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Chen, C.; Cheng, G.; Liang, L.; Yao, X.; Yang, G.; You, P.; Shou, X. Peroxisome proliferator-activated receptor γ attenuates serotonin-induced pulmonary artery smooth muscle cell proliferation and apoptosis inhibition involving ERK1/2 pathway. Microvasc. Res. 2015, 100, 17–24. [Google Scholar] [CrossRef]
- Ding, K.; Liu, W.Y.; Zeng, Q.; Hou, F.; Xu, J.Z.; Yang, Z. Msx1-modulated muscle satellite cells retain a primitive state and exhibit an enhanced capacity for osteogenic differentiation. Exp. Cell Res. 2017, 352, 84–94. [Google Scholar] [CrossRef]
- Zucker, M.M.; Wujak, L.; Gungl, A.; Didiasova, M.; Kosanovic, D.; Petrovic, A.; Klepetko, W.; Schermuly, R.T.; Kwapiszewska, G.; Schaefer, L.; et al. LRP1 promotes synthetic phenotype of pulmonary artery smooth muscle cells in pulmonary hypertension. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1604–1616. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Frid, M.G.; Graham, B.B.; Tuder, R.M. Dynamic and diverse changes in the functional properties of vascular smooth muscle cells in pulmonary hypertension. Cardiovasc. Res. 2018, 114, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yuan, Y.; Li, L.; Fan, J.; Li, C.; Peng, W.; Ren, G. Homeobox protein MSX1 inhibits the growth and metastasis of breast cancer cells and is frequently silenced by promoter methylation. Int. J. Mol. Med. 2018, 41, 2986–2996. [Google Scholar] [CrossRef] [Green Version]
- Tríbulo, C.; Aybar, M.J.; Sánchez, S.S.; Mayor, R. A balance between the anti-apoptotic activity of Slug and the apoptotic activity of msx1 is required for the proper development of the neural crest. Dev. Biol. 2004, 275, 325–342. [Google Scholar] [CrossRef] [Green Version]
- Trousse, F.; Esteve, P.; Bovolenta, P. Bmp4 mediates apoptotic cell death in the developing chick eye. J. Neurosci. 2001, 21, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lee, P.L.; Lee, C.I.; Wei, S.Y.; Lim, S.H.; Lin, T.E.; Chien, S.; Chiu, J.J. BMP receptor-integrin interaction mediates responses of vascular endothelial Smad1/5 and proliferation to disturbed flow. J. Thromb. Haemost. 2013, 11, 741–755. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
West, J.; Rathinasabapathy, A.; Chen, X.; Shay, S.; Gladson, S.; Talati, M. Overexpression of Msx1 in Mouse Lung Leads to Loss of Pulmonary Vessels Following Vascular Hypoxic Injury. Cells 2021, 10, 2306. https://doi.org/10.3390/cells10092306
West J, Rathinasabapathy A, Chen X, Shay S, Gladson S, Talati M. Overexpression of Msx1 in Mouse Lung Leads to Loss of Pulmonary Vessels Following Vascular Hypoxic Injury. Cells. 2021; 10(9):2306. https://doi.org/10.3390/cells10092306
Chicago/Turabian StyleWest, James, Anandharajan Rathinasabapathy, Xinping Chen, Sheila Shay, Shanti Gladson, and Megha Talati. 2021. "Overexpression of Msx1 in Mouse Lung Leads to Loss of Pulmonary Vessels Following Vascular Hypoxic Injury" Cells 10, no. 9: 2306. https://doi.org/10.3390/cells10092306
APA StyleWest, J., Rathinasabapathy, A., Chen, X., Shay, S., Gladson, S., & Talati, M. (2021). Overexpression of Msx1 in Mouse Lung Leads to Loss of Pulmonary Vessels Following Vascular Hypoxic Injury. Cells, 10(9), 2306. https://doi.org/10.3390/cells10092306






