Three Active Phytotoxic Compounds from the Leaves of Albizia richardiana (Voigt.) King and Prain for the Development of Bioherbicides to Control Weeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Samples
2.3. Extraction and Growth Assay with the Albizia Richardiana Extracts
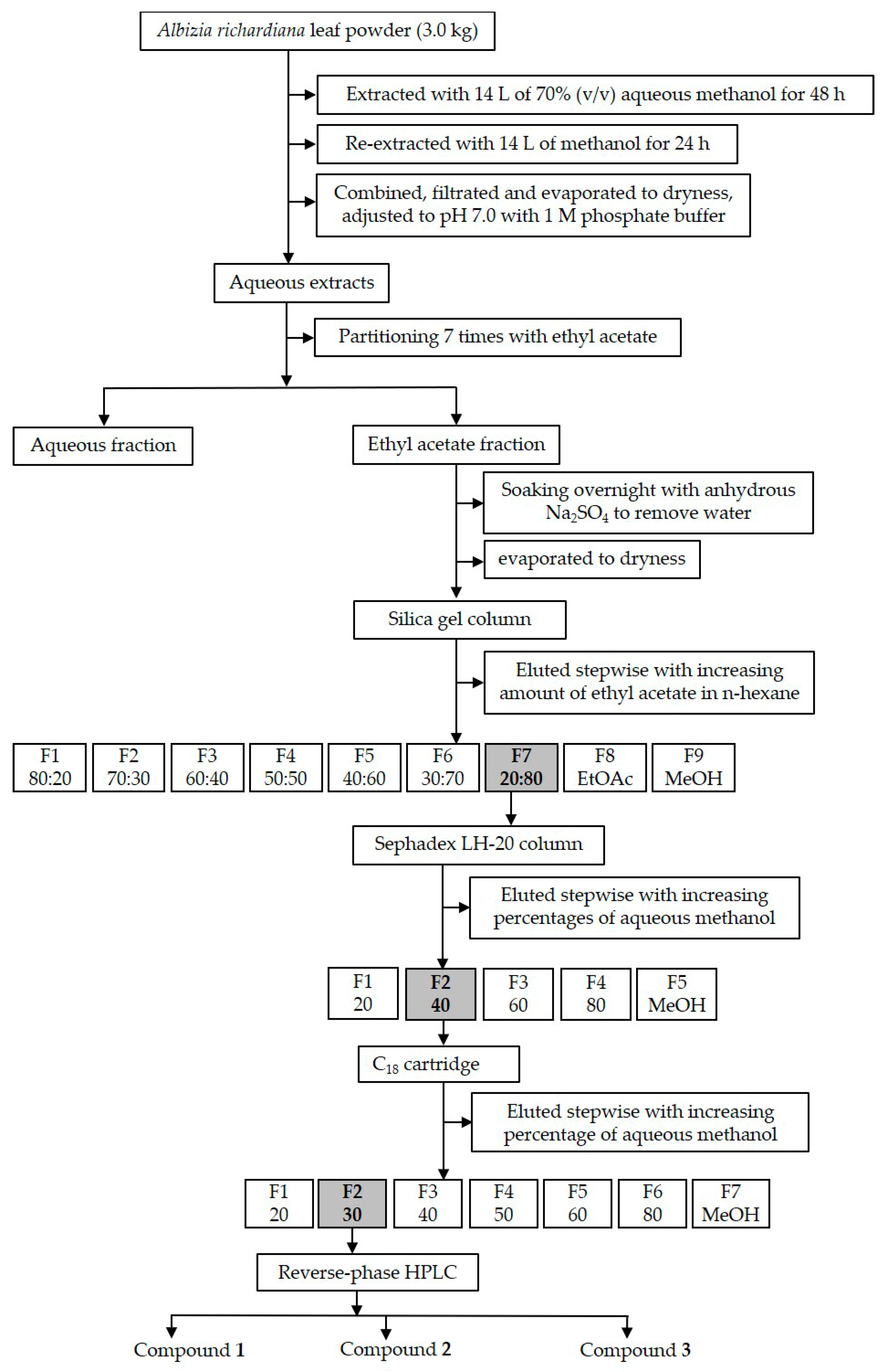
2.4. Purification of the Active Compounds
2.5. Growth Assay of the Characterized Compounds
2.6. Statistics
3. Results
3.1. Phytotoxic Effect of the Albizia Richardiana Extracts
3.2. Identification of Active Phytotoxic Compounds
3.3. Inhibitory Effects of the Allelopathic Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. La Situation Mondiale de L’alimentation et de L’agriculture–Changement Climatique, Agriculture et Securite Alimentaire; FAO: Rome, Italy, 2016; Volume 191. [Google Scholar]
- Tanentzap, A.J.; Lamb, A.; Walker, S.; Farmer, A. Resolving conflicts between agriculture and the natural environment. PLoS Biol. 2015, 13, 23–45. [Google Scholar] [CrossRef] [Green Version]
- Zimdahl, R.L. Fundamentals of Weed Science, 4th ed.; Academic Press: San Diego, CA, USA, 2013. [Google Scholar]
- Kaur, S.; Kaur, R.; Chauhan, B.S. Understanding crop-weed-fertilizer-water interactions and their implications for weed management in agricultural systems. Crop. Prot. 2018, 103, 65–72. [Google Scholar] [CrossRef]
- Jha, P.; Kumar, V.; Godara, R.K.; Chauhan, B.S. Weed management using crop competition in the United States: A review. Crop Prot. 2017, 95, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Gnanavel, I. Eco-friendly weed control options for sustainable agriculture. Sci. Int. 2015, 3, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Bajwa, A.A.; Akhter, M.J.; Iqbal, N.; Peerzada, A.M.; Hanif, Z.; Manalil, S.; Hashim, S.; Ali, H.H.; Kebaso, L.; Frimpong, D. Biology and management of Avena fatua and Avena ludoviciana: Two noxious weed species of agro-ecosystems. Environ. Sci. Pollut. Res. 2017, 24, 19465–19479. [Google Scholar] [CrossRef]
- Lins, H.A.; Barros, A.P., Jr.; Silva, D.V.; Souza, M.F.; Freitas, M.A.M.; Soares, E.B.; Porto, M.A.F.; Mesquita, H.C.; Oliveira, F.S. Effectivity and selectivity of herbicides applied in pre-emergence in the sesame (Sesamum indicum L.) crop. Rev. Facul. Cienc. Agrarias UN Cuyo. 2020, 52, 1–12. [Google Scholar]
- Gianessi, L.P.; Reigner, N.P. The value of herbicides in US crop production. Weed Technol. 2007, 21, 559–566. [Google Scholar] [CrossRef]
- Fickett, N.D.; Boerboom, C.M.; Stoltenberg, D.E. Predicted corn yield loss due to weed competition prior to postemergence herbicide application on Wisconsin farms. Weed Technol. 2013, 27, 54–62. [Google Scholar] [CrossRef]
- Ghimire, N.; Woodward, R.T. Under- and over-use of pesticides: An international analysis. Ecol. Econ. 2013, 89, 73–81. [Google Scholar] [CrossRef]
- Baylis, A.D. Why glyphosate is a global herbicide: Strengths, weaknesses and prospects. Pest. Manag. Sci. 2000, 56, 299–308. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Parween, T.; Jan, S.; Mahmooduzzafar, S.; Fatma, T.; Siddiqui, Z.H. Selective effect of pesticides on plant—A review. Crit. Rev. Food Sci. Nut. 2016, 56, 160–179. [Google Scholar] [CrossRef]
- Farooq, N.; Abbas, T.; Tanveer, A.; Jabran, K. Allelopathy for weed management. In Co-Evolution of Secondary Metabolites; Springer: Cham, Switzerland, 2020; pp. 505–519. [Google Scholar]
- Heap, I. The International Survey of Herbicide Resistant Weeds. Available online: www.weedscience.org (accessed on 24 August 2021).
- Boutin, C.; Strandberg, B.; Carpenter, D.; Mathiassen, S.K.; Thomas, P.J. Herbicide impact on non-target plant reproduction: What are the toxicological and ecological implications? Environ. Pollut. 2014, 185, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Jabran, K.; Farooq, M. Implications of potential allelopathic crops in agricultural systems. In Allelopathy; Springer: Berlin/Heidelberg, Germany, 2013; pp. 349–385. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Weidenhamer, J.D.; Callaway, R.M. Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J. Chem. Ecol. 2010, 36, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, A.A.; Chauhan, B.S.; Farooq, M.; Shabbir, A.; Adkins, S.W. What do we really know about alien plant invasion? A review of the invasion mechanism of one of the world’s worst weeds. Planta 2016, 244, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, B.K.; Hwang, M.H.; Sacks, E.J.; Yu, C.Y.; Ki, S.H.; Chung, I.M. Screening of allelochemicals in Miscanthus sacchariflorus extracts and assessment of their effects on germination and seedling growth of common weeds. Plants 2020, 9, 1313. [Google Scholar] [CrossRef]
- Einhellig, F.A. Mechanism of action of allelochemicals in Allelopathy. Allelopathy 1994, 582, 96–116. [Google Scholar]
- Sun, Q.; Heilmann, J.; Konig, B. Natural phenolic metabolites with anti-angiogenic properties—A review from the chemical point of view. Beilstein J. Org. Chem. 2015, 11, 249–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladhari, A.; Haouala, R.; Dellagreca, M. New dammarane triterpene from Cleome arabica L. Chem. Nat. Compd. 2014, 50, 684–686. [Google Scholar] [CrossRef]
- Gaaliche, B.; Ladhari, A.; Medeiros, A.G.; de Mimoun, M.B.; Hajlaoui, M.R. Relationship between phytochemical profiles and phytotoxic proprieties of Tunisian fig leaf cultivars. S. Afr. J. Bot. 2017, 112, 322–328. [Google Scholar] [CrossRef]
- Qian, H.; Xu, J.; Lu, T.; Zhang, Q.; Qu, Q.; Yang, Z.; Pan, X. Responses of unicellular alga Chlorella pyrenoidosa to allelochemical linoleic acid. Sci. Total Environ. 2018, 625, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.I.; Pueyo, Y.; Pellissier, F.; Ramos, J.; Espinosa-Ruiz, A.; Millery, A.; Alados, C.L. Phytotoxic effects of volatile and water-soluble chemicals of Artemisia herba alba. J. Arid Environ. 2018, 151, 1–8. [Google Scholar] [CrossRef]
- Mecina, G.F.; Santos, V.H.M.; Andrade, A.R.; Dokkedal, A.L.; Saldanha, L.L.; Silva, R.M.G. Phytotoxicity of Tridax procumbens. L. S. Afr. J. Bot. 2016, 102, 130–136. [Google Scholar] [CrossRef]
- Hazrati, H.; Saharkhiz, M.J.; Moein, M.; Khoshghalb, H. Phytotoxic effects of several essential oils on two weed species and tomato. Biocatal. Agric. Biotechnol. 2018, 13, 204–212. [Google Scholar] [CrossRef]
- Daniel, J.M.; Gillian, K.B.; Joseph, T.M.; Pauline, Y.L. Molecular phylogeny of Acacia Mill. (Mimosoideae: Leguminosae): Evidence for major clades and informal classification. Taxon 2010, 59, 7–19. [Google Scholar]
- Azad, S.; Paul, N.K.; Matin, A. Do pre-sowing treatments affect seed germination in Albizia richardiana and Lagerstroemia speciosa? Front. Agric. China 2010, 4, 181–184. [Google Scholar] [CrossRef]
- Salam, A.; Akhter, K.; Rahman, M.A.; Chowdhury, M.H.; Mridha, M.N.A.; Chowdhury, F.H. CCB preservative treatment of rajkoroi (Albizia richardiana King & Prain) wood by soaking method. Eco-Friendly Agril. J. 2019, 1, 74–77. [Google Scholar]
- Al Faruq, M.A.; Zaman, S.; Katoh, M. Perceptions of local people toward community development and forest conservation in Bangladesh: The case of Sal forests. J. For. Plan. 2017, 22, 1. [Google Scholar]
- Rahman, M.M.; Das, A.K.; Asaduzzaman, M.; Biswas, S.K.; Hannan, M.O. Physical and mechanical properties of Raj Koroi (Albizia richardiana) plywood. Afr. J. Wood Sci. For. 2013, 2, 98–103. [Google Scholar]
- Xinrong, Y.; Anmin, C.; Fang, S.; Bingyi, F.; Jinlin, Q.; Yingfu, M.; Quan, L.; Yuan, G.; Shuqian, W.; Werner, H. Encyclopedic Reference of Traditional Chinese Medicine; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Islam, M.N.; Tasnim, H.; Arshad, L.; Haque, A.; Tareq, S.M.; Kamal, A.T.M.M.; Rahman, M.; Reza, A.S.M.A.; Chowdhury, K.A.A.; Tareq, A.M. Stem extract of Albizia richardiana exhibits potent antioxidant, cytotoxic, antimicrobial, anti-inflammatory and thrombolytic effects through in vitro approach. Clin. Phytosci. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity and growth inhibitory substances from Albizia richardiana (Voigt.) King & Prain. Appl. Sci. 2021, 11, 1455. [Google Scholar]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef]
- Marino, S.D.; Borbone, N.; Zollo, F.; Ianaro, A.; Meglio, P.D.; Iorizzi, M. Megastigmane and phenolic components from Laurus nobilis L. leaves and their inhibitory effects on nitric oxide production. J. Agric. Food Chem. 2004, 52, 7525–7531. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Chin, Y.W.; Lim, S.W.; Kim, Y.C.; Kim, J. Norisoprenoids and hepatoprotective flavone glycosides from the aerial parts of Beta vulgaris var. cicla. Arch. Pharm. Res. 2004, 27, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, H.; Hosoe, S.; Ueda, K.; Sasaki, M.; Kim, J. First synthesis of (±)-robinlin. Biosci. Biotechnol. Biochem. 2004, 68, 1961–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxicity of the novel compound 3-hydroxy-4-oxo-β-dehydroionol and compound 3-oxo-α-ionone from Albizia richardiana (Voigt.) King & Prain. Environ. Technol. Innov. 2021, 23, 101779. [Google Scholar]
- Islam, M.S.; Zaman, F.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic potential of Chrysopogon aciculatus (Retz.) Trin. (Poaceae). Weed Biol. Manag. 2019, 19, 51–58. [Google Scholar] [CrossRef]
- Hossen, K.; Das, K.R.; Okada, S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathic potential and active substances from Wedelia chinensis (Osbeck). Foods 2020, 9, 1591. [Google Scholar] [CrossRef]
- Hossen, K.; Kato-Noguchi, H. Determination of allelopathic properties of Acacia catechu (L.f.) Willd. Not. Bot. Horti Agrobot. Cluj Napoca 2020, 48, 2050–2059. [Google Scholar] [CrossRef]
- Rob, M.; Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity and identification of phytotoxic substances from Schumannianthus dichotomus. Plants 2020, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Grisi, P.U.; Gualtieri, S.C.J.; Ranal, M.A.; Santana, D.G. Allelopathic interference of Sapindus saponaria root and mature leaf aqueous extracts on diaspore germination and seedling growth of Lactuca sativa and Allium cepa. Braz. J. Bot. 2012, 35, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Imatomi, M.; Novaes, P.; Gualtieri, S.C.J. Interspecific variation in the allelopathic potential of the family Myrtaceae. Acta Bot. Bras. 2013, 27, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Walsh, D.; Sanderson, D.; Hall, L.M.; Mugo, S.; Hills, M.J. Allelopathic effects of camelina (Camelina sativa) and canola (Brassica napus) on wild oat, flax and radish. Allelopathy J. 2014, 33, 83–96. [Google Scholar]
- Bari, I.N.; Kato-Noguchi, H.; Iwasaki, A.; Suenaga, K. Allelopathic potency and an active substance from Anredera cordifolia (Tenore) Steenis. Plants 2019, 8, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magiero, E.C.; Assmann, J.M.; Marchese, J.A.; Capelin, D.; Paladini, M.V.; Trezzi, M.M. Efeito alelopático de Artemisia annua L. na germinação e desenvolvimento inicial deplântulas de alface (Lactuca sativa L.) e leiteiro (Euphorbia heterophylla L.). Rev. Bras. Plantas Med. 2009, 11, 317–324. [Google Scholar] [CrossRef]
- Scognamiglio, M.; D’Abrosca, B.; Esposito, A.; Pacifico, S.; Monaco, P.; Fiorentino, A. Plant growth inhibitors: Allelopathic role or phytotoxic effects? Focus on Mediterranean biomes. Phytochem. Rev. 2013, 12, 803–830. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Sánchez-Moreiras, A.; González, L. Ecophysiological approach in allelopathy. Crit. Rev. Plant. Sci. 1999, 18, 577–608. [Google Scholar] [CrossRef]
- Javaid, A.; Anjum, T. Control of Parthenium hysterophorus L., by aqueous extracts of allelopathic grasses. Pakistan J. Bot. 2006, 38, 139–145. [Google Scholar]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Islam, A.K.M.M.; Kato-Noguchi, H. Plant growth inhibitory activity of medicinal plant Hyptis suaveolens: Could allelopathy be a cause? Emirates J. Food Agric. 2013, 25, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Franco, D.M.; Silva, E.M.; Saldanha, L.L.; Adachi, S.A.; Schley, T.R.; Rodrigues, T.M.; Dokkedal, A.L.; Nogueira, F.T.S.; Rolim de Almeida, L.F. Flavonoids modify root growth and modulate expression of short-root and HD-ZIP III. J. Plant Physiol. 2015, 188, 89–95. [Google Scholar] [CrossRef]
- Levizou, E.F.I.; Karageorgou, P.; Psaras, G.K.; Manetas, Y. Inhibitory effects of water-soluble leaf leachates from Dittrichia viscosa on lettuce root growth, statocyte development and graviperception. Flora—Morphol. Distrib. Funct. Ecol. Plants. 2002, 197, 152–157. [Google Scholar] [CrossRef] [Green Version]
- Piyatida, P.; Kato-Noguchi, H. Screening of allelopathic activity of eleven Thai medicinal plants on seedling growth of five test plant species. Asian J. Plant Sci. 2010, 9, 486–491. [Google Scholar] [CrossRef]
- Morikawa, C.I.O.; Miyaura, R.; de Lourdes Tapia y Figueroa, M.; Rengifo Salgado, E.L.; Fujii, Y. Screening of 170 Peruvian plant species for allelopathic activity by using the sandwich method. Weed Biol. Manag. 2012, 12, 1–11. [Google Scholar] [CrossRef]
- Rob, M.M.; Hossen, K.; Khatun, M.R.; Iwasaki, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Identification and application of bioactive compounds from Garcinia xanthochymus Hook. for weed management. Appl. Sci. 2021, 11, 2264. [Google Scholar] [CrossRef]
- Yan, X.; Lynch, J.P.; Beebe, S.E. Genetic variation for phosphorus efficiency of common bean in contrasting soil types: I. vegetative response. Crop. Sci. 1995, 35, 1086–1093. [Google Scholar] [CrossRef]
- Schneider, R.; Razungles, A.; Augier, C.; Baumes, R. Monoterpenic and norisoprenoidic glycoconjugates of Vitis vinifera L. cv. Melon B. as precursors of odorants in Muscadet wines. J. Chromatogr. A 2001, 936, 145–157. [Google Scholar]
- Aubert, C.; Ambid, C.; Baumes, R.; Gunata, Z. Investigation of bound aroma constituents of yellow-fleshed nectarines (Prunus persica L. Cv. Springbright). Changes in bound aroma profile during maturation. J. Agric. Food Chem. 2003, 51, 6280–6286. [Google Scholar] [CrossRef]
- Hubert Alem, H.; Ojeda, H.; Rigou, P.; Schneider, R.; Torregrosa, L. The reduction of plant sink/source does not systematically improve the metabolic composition of Vitis vinifera white fruit. Food Chem. 2021, 345, 128825. [Google Scholar] [CrossRef] [PubMed]
- Herderich, M.; Neubert, C.; Winterhalter, P.; Schreier, P. Identification of C13-norisoprenoid flavour precursors in starfruit (Averrhoa carambola L.). Flavour Fragr. J. 1992, 7, 179–185. [Google Scholar] [CrossRef]
- Samanta, S.K.; Kandimalla, R.; Gogoia, B.; Dutta, K.N.; Choudhury, P.; Deb, P.K.; Devi, R.; Pal, B.K.; Talukdar, N.C. Phytochemical portfolio and anticancer activity of Murraya koenigii and its primary active component, mahanine. Pharmacol. Res. 2018, 129, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Hadipour, E.; Taleghani, K.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological effects of red beetroot and betalains: A review. Phytoth. Res. 2020, 34, 1847–1867. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.Y.; Ma, Y.X.; Wang, H.; Chen, M. Studies on chemical constituents of stems of Herpetospermum pedunculosum. China J. Chin. Mater. Medica. 2020, 45, 2571–2577. [Google Scholar]
- Tian, F.; Chang, C.-J.; Grutzner, J.B.; Nichols, D.E.; McLaughlin, J.L. Robinlin: A novel bioactive homo-monoterpene from Robinia psedoacacis L. (Fabaceae). Bioorg. Med. Chem. Lett. 2001, 11, 2603–2606. [Google Scholar] [CrossRef]
- DellaGreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Temussi, F.; Zarrelli, A. New dimeric phenanthrenoids from the rhizomes of Juncus acutus. Structure determination and antialgal activity. Tetrahedron 2003, 59, 2317–2324. [Google Scholar] [CrossRef]
- Islam, M.S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Evaluation of phytotoxic potential and identification of phytotoxic compounds in Rumex maritimus. Plant Biosys. 2017, 152, 804–809. [Google Scholar] [CrossRef]






| Tested Species | I50 Values (mg Dry Weight Equivalent Extract/mL) | ||
|---|---|---|---|
| Shoot | Root | ||
| Dicot | Lactuca sativa (lettuce) | 14.0 | 11.0 |
| Monocot | Lolium multiflorum (Italian ryegrass) | 32.0 | 28.0 |
| Test Plant | Compound 1 | Compound 2 | Compound 3 | |
|---|---|---|---|---|
| (mg/mL) | ||||
| Lepidium sativum (Cress) | Shoot | 0.4133 | 0.2432 | 0.1671 |
| Root | 0.2845 | 0.1552 | 0.0827 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossen, K.; Ozaki, K.; Teruya, T.; Kato-Noguchi, H. Three Active Phytotoxic Compounds from the Leaves of Albizia richardiana (Voigt.) King and Prain for the Development of Bioherbicides to Control Weeds. Cells 2021, 10, 2385. https://doi.org/10.3390/cells10092385
Hossen K, Ozaki K, Teruya T, Kato-Noguchi H. Three Active Phytotoxic Compounds from the Leaves of Albizia richardiana (Voigt.) King and Prain for the Development of Bioherbicides to Control Weeds. Cells. 2021; 10(9):2385. https://doi.org/10.3390/cells10092385
Chicago/Turabian StyleHossen, Kawsar, Kaori Ozaki, Toshiaki Teruya, and Hisashi Kato-Noguchi. 2021. "Three Active Phytotoxic Compounds from the Leaves of Albizia richardiana (Voigt.) King and Prain for the Development of Bioherbicides to Control Weeds" Cells 10, no. 9: 2385. https://doi.org/10.3390/cells10092385
APA StyleHossen, K., Ozaki, K., Teruya, T., & Kato-Noguchi, H. (2021). Three Active Phytotoxic Compounds from the Leaves of Albizia richardiana (Voigt.) King and Prain for the Development of Bioherbicides to Control Weeds. Cells, 10(9), 2385. https://doi.org/10.3390/cells10092385







