Intestinal Region-Specific and Layer-Dependent Induction of TNFα in Rats with Streptozotocin-Induced Diabetes and after Insulin Replacement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Tissue Handling
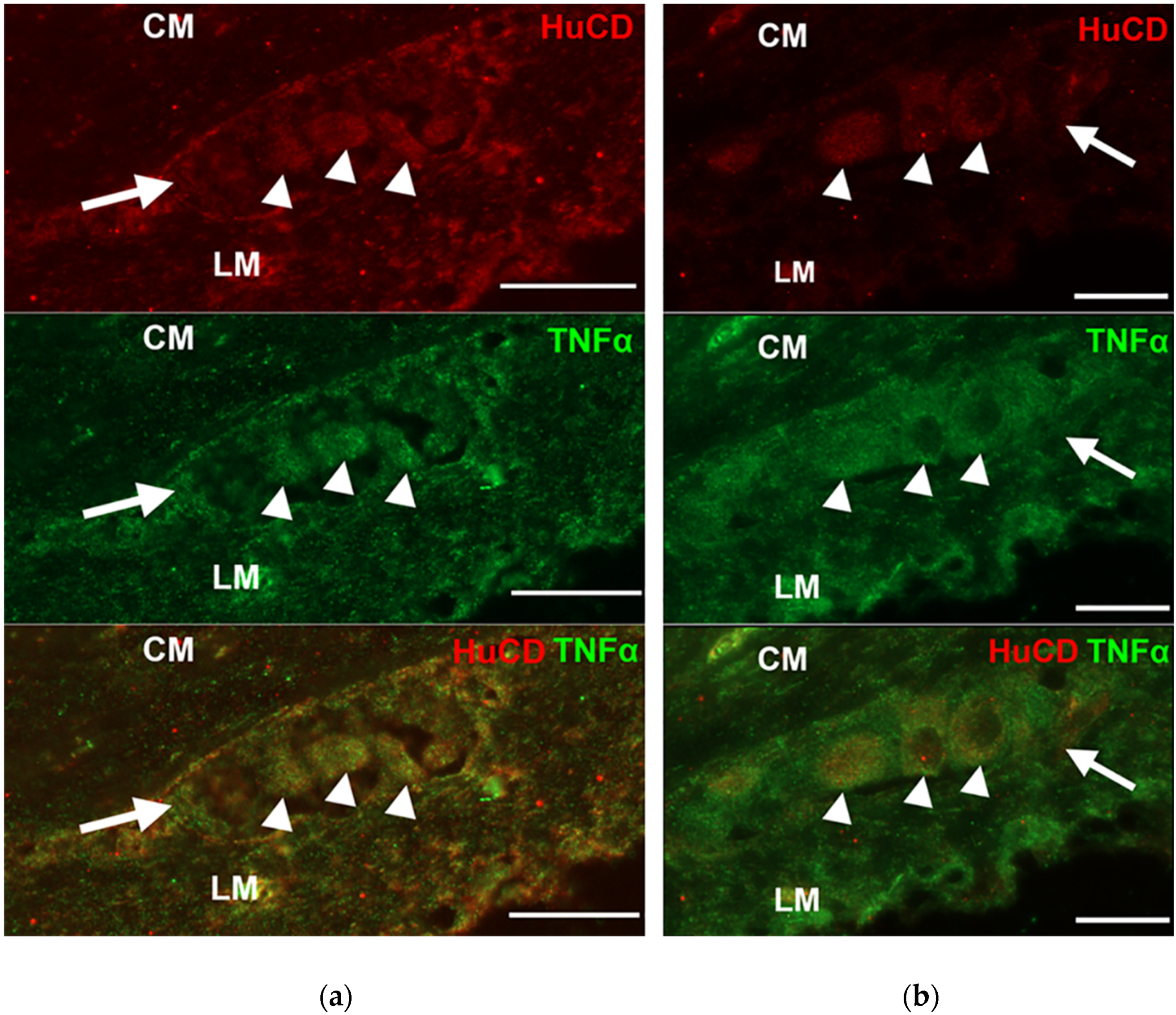
2.3. Fluorescent Immunohistochemistry
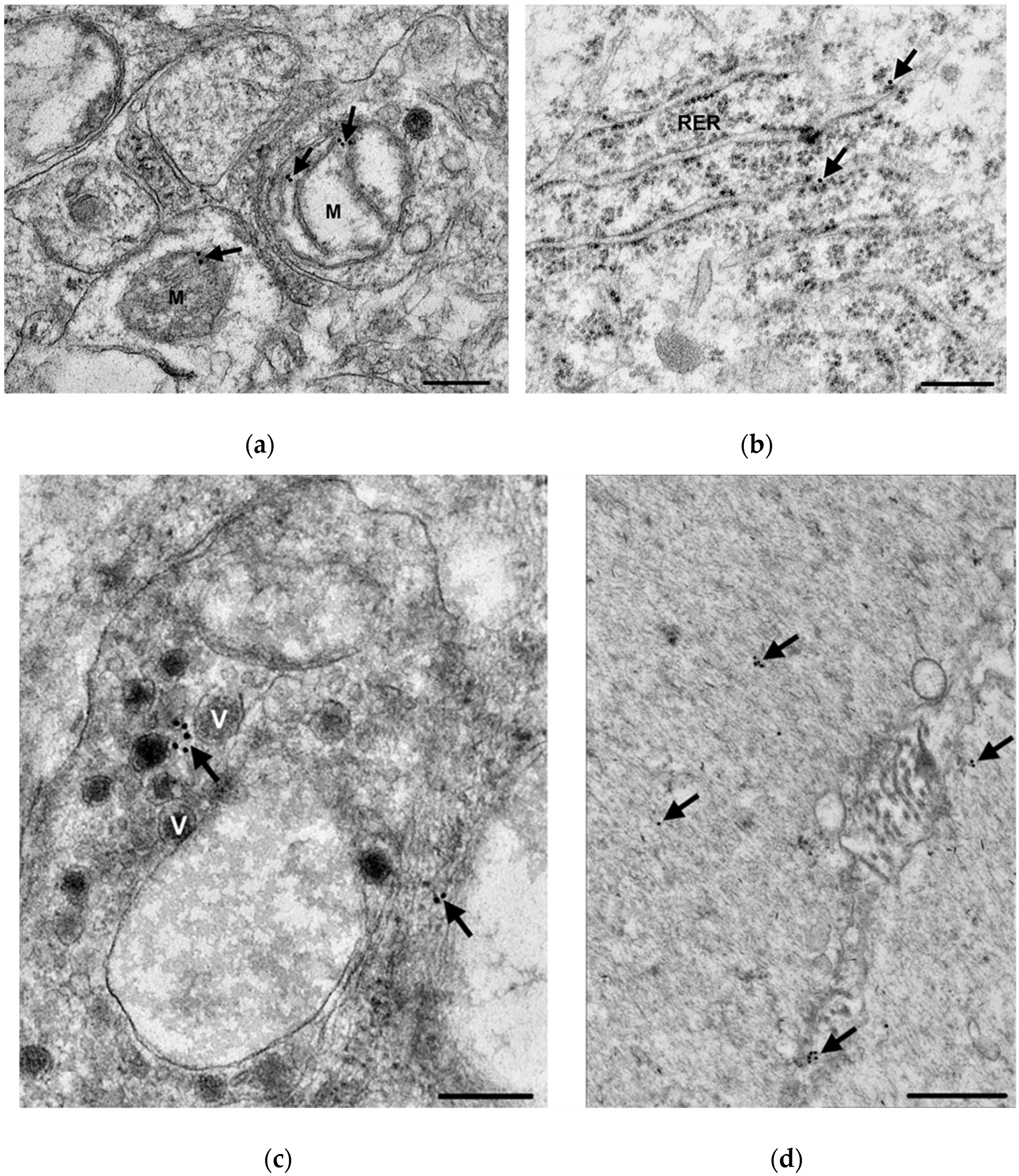
2.4. Quantitative Post-Embedding Immunohistochemistry
2.5. Measurement of Tissue TNFα Concentrations
2.6. Bradford Protein Micromethod for the Determination of Tissue Protein Content
2.7. Statistical Analysis
3. Results
3.1. Disease Characteristics of Experimental Animals
3.2. Presence of TNFα Immunoreactivity in the Intestinal Sections
3.3. Subcellular Localization and Quantification of TNFα Expression in Myenteric Ganglia
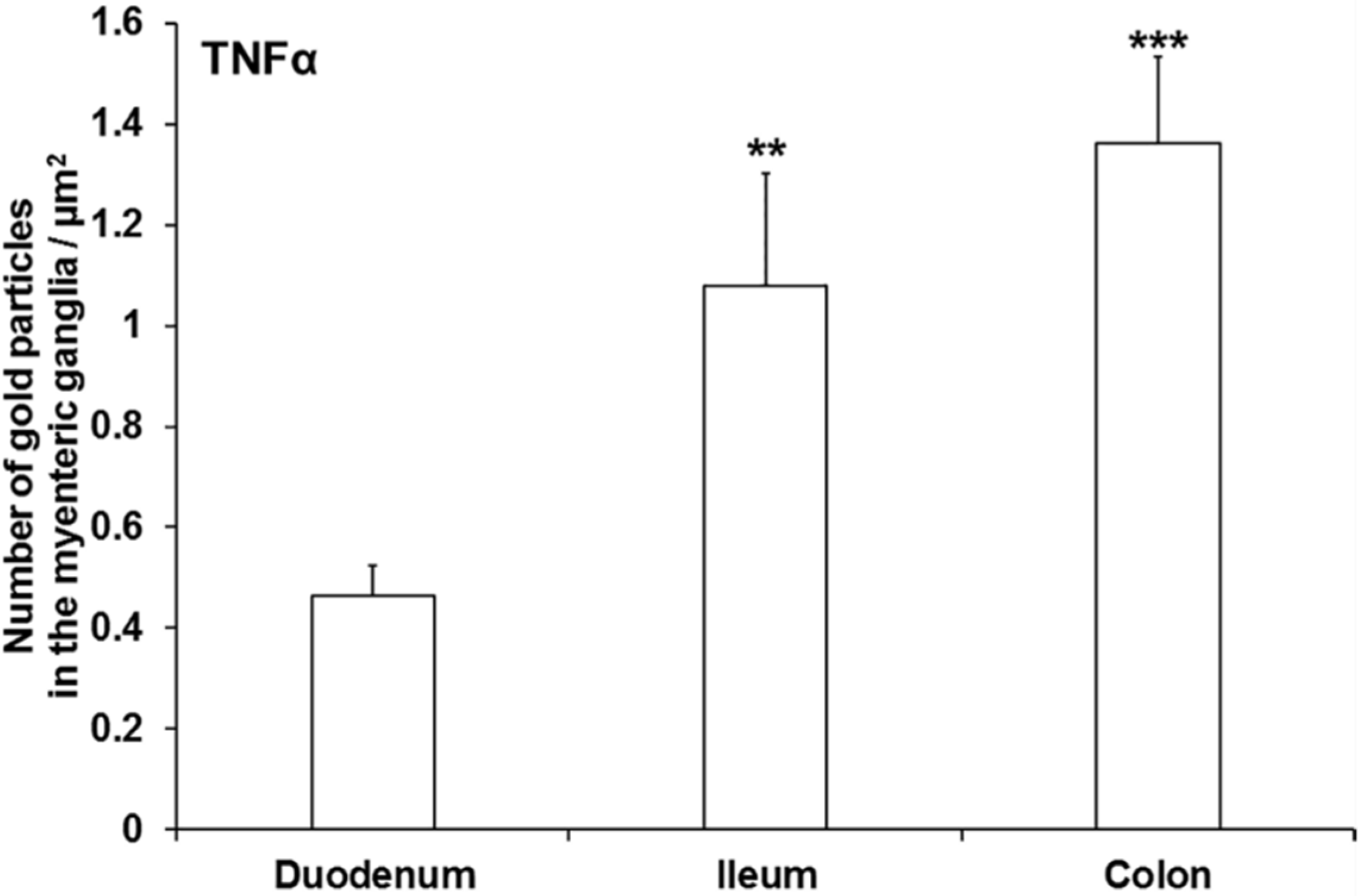
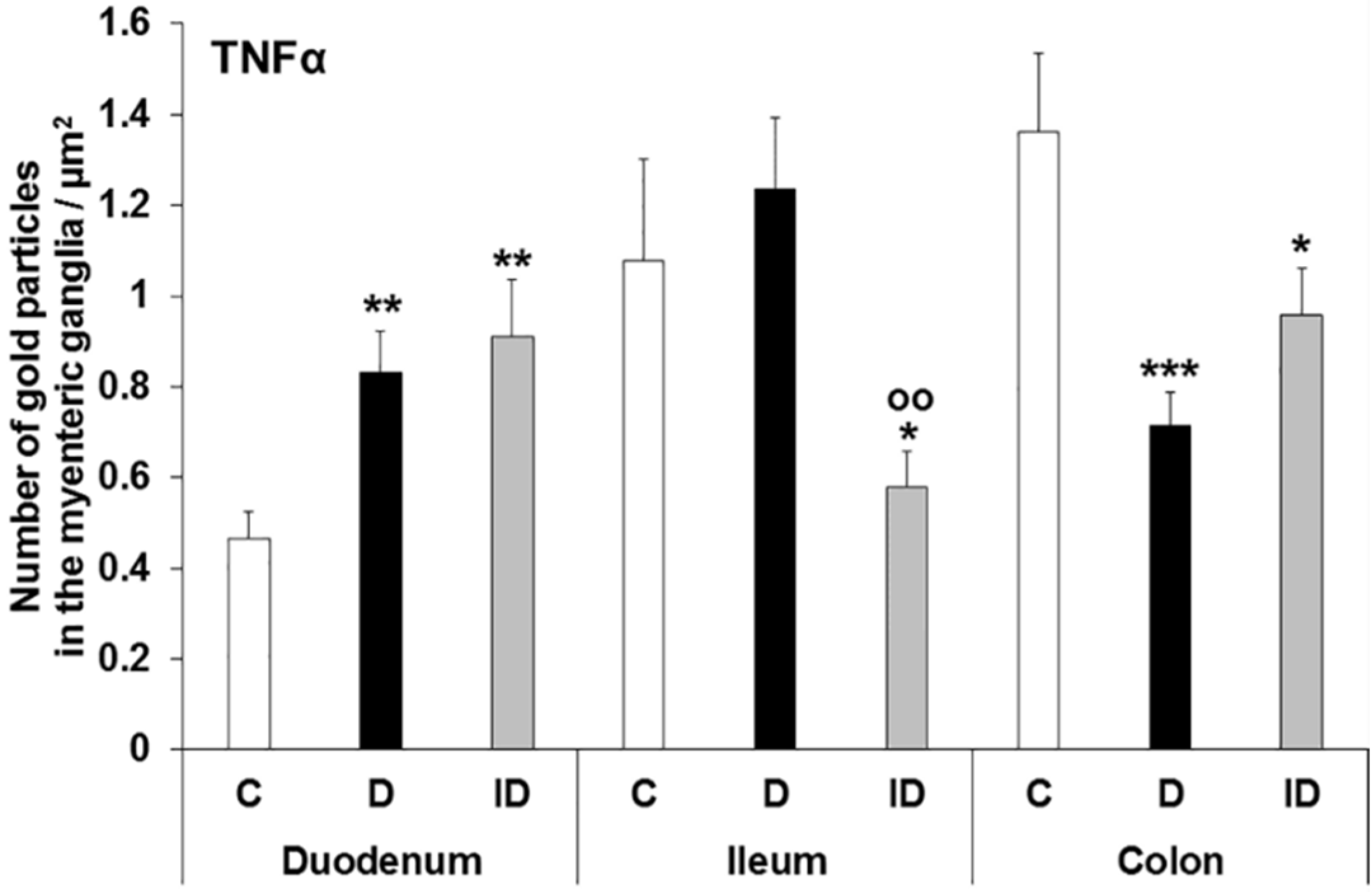
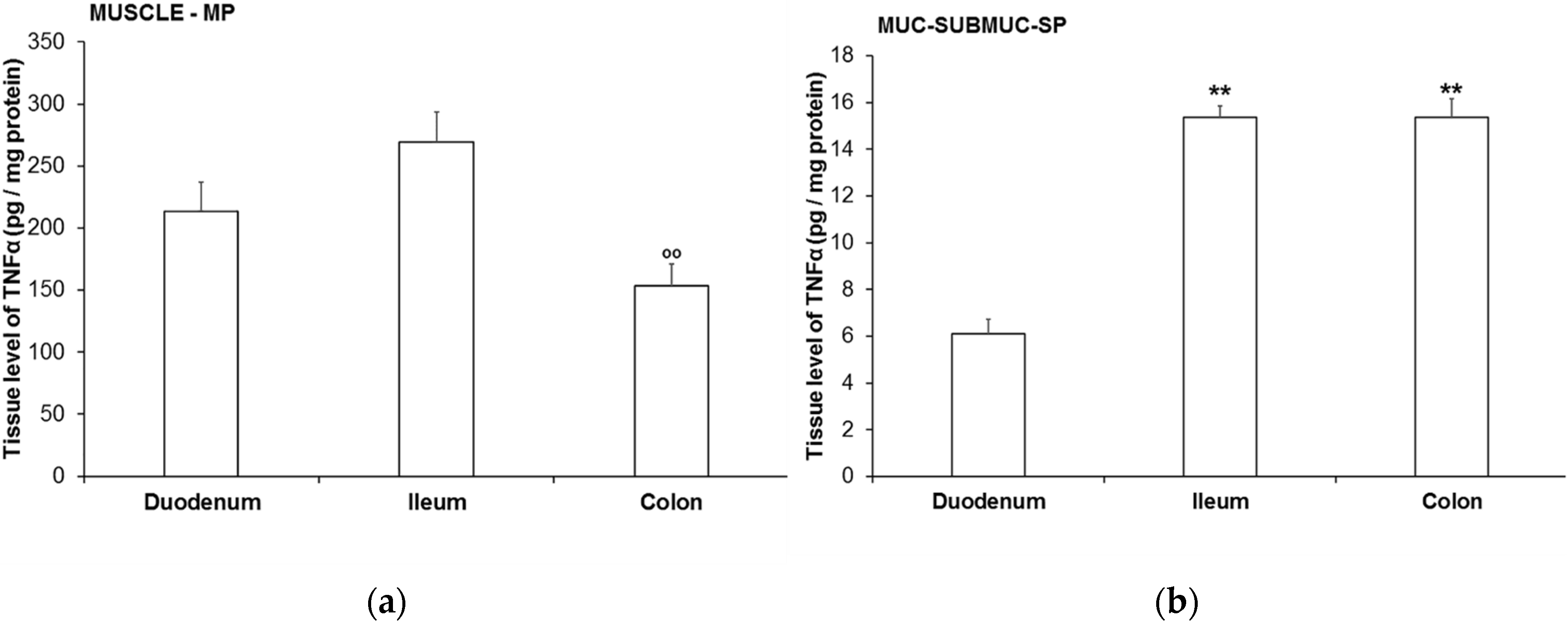
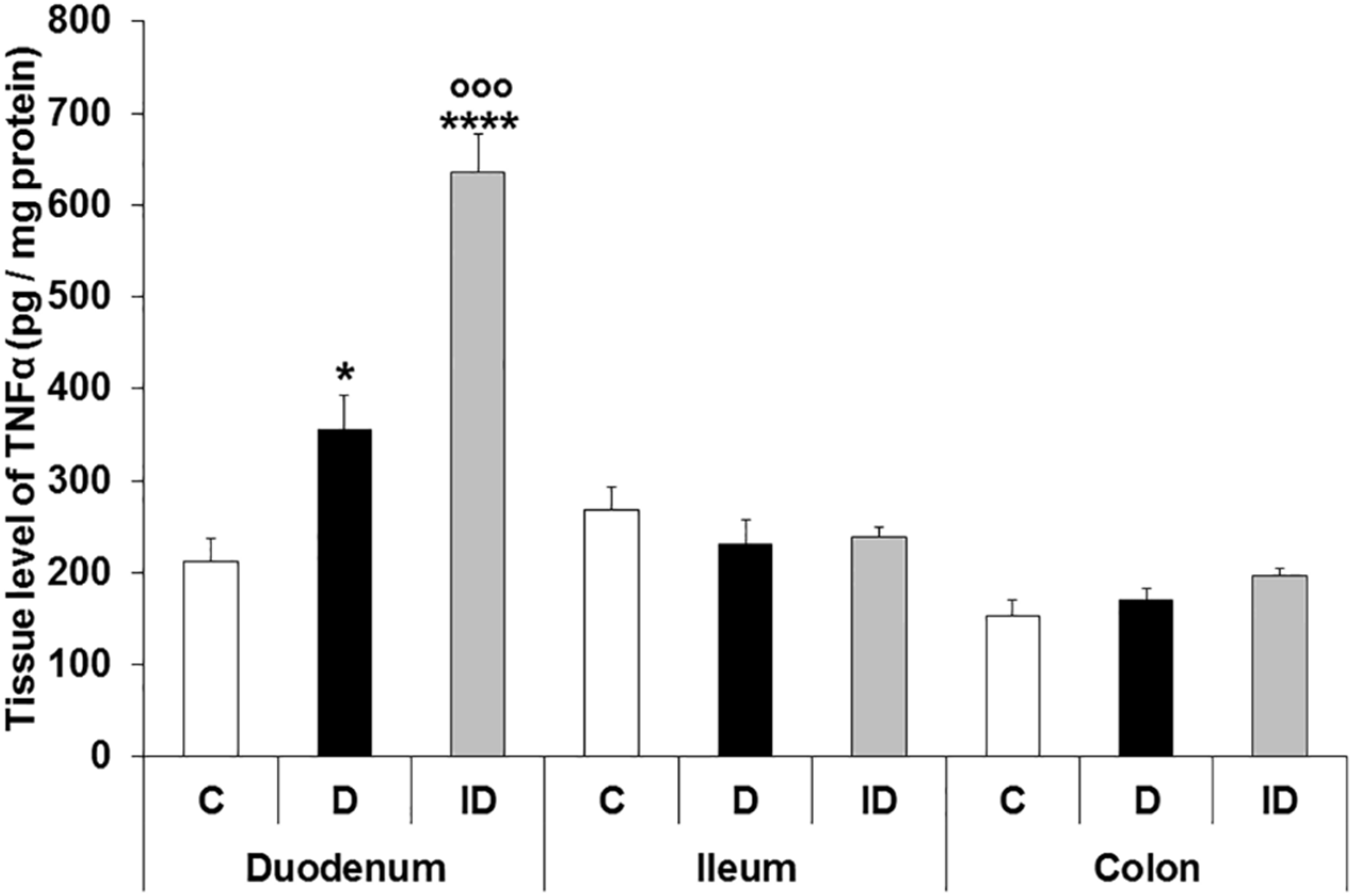
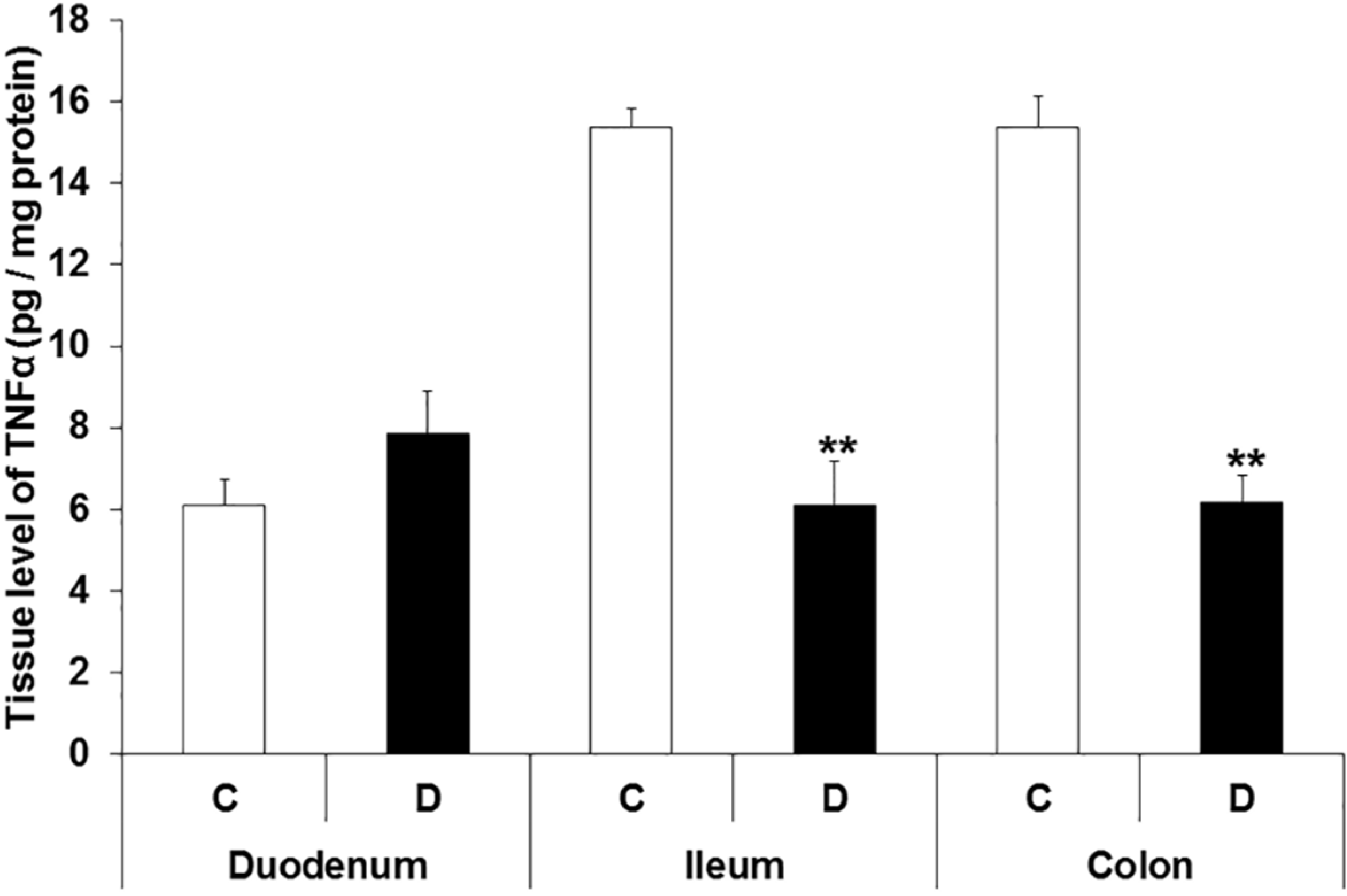
3.4. TNFα Levels in Different Intestinal Tissue Layers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burns, A.J.; Pachnis, V. Development of the enteric nervous system: Bringing together cells, signals and genes. Neurogastroenterol. Motil. 2009, 21, 100–102. [Google Scholar] [CrossRef]
- Brown, H.; Esterhazy, D. Intestinal immune compartmentalization: Implications of tissue specific determinants in health and disease. Mucosal Immunol. 2021, 1–12. [Google Scholar] [CrossRef]
- Wirth, R.; Bodi, N.; Maroti, G.; Bagyanszki, M.; Talapka, P.; Fekete, E.; Bagi, Z.; Kovacs, K.L. Regionally distinct alterations in the composition of the gut microbiota in rats with streptozotocin-induced diabetes. PLoS ONE 2014, 9, e110440. [Google Scholar] [CrossRef] [Green Version]
- Wirth, R.; Bódi, N.; Szalai, Z.; Chandrakumar, L.; Maróti, G.; Kovács, K.L.; Bagi, Z.; Mezei, D.; Balázs, J.; Bagyánszki, M. Perturbation of the mucosa-associated anaerobic gut microbiota in streptozotocin-induced diabetic rats. Acta Biol. Szeged. 2021, 65, 75–84. [Google Scholar] [CrossRef]
- Bodi, N.; Bagyanszki, M. Diabetic enteric neuropathy: Imbalance between oxidative and antioxidative mechanisms. In Diabetes: Oxidative Stress and Dietary Antioxidants, 2nd ed.; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 3, pp. 25–33. [Google Scholar]
- Izbeki, F.; Wittman, T.; Rosztoczy, A.; Linke, N.; Bodi, N.; Fekete, E.; Bagyanszki, M. Immediate insulin treatment prevents gut motility alterations and loss of nitrergic neurons in the ileum and colon of rats with streptozotocin-induced diabetes. Diabetes Res. Clin. Pract. 2008, 80, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodi, N.; Szalai, Z.; Chandrakumar, L.; Bagyanszki, M. Region-dependent effects of diabetes and insulin-replacement on neuronal nitric oxide synthase- and heme oxygenase-immunoreactive submucous neurons. World J. Gastroenterol. 2017, 23, 7359–7368. [Google Scholar] [CrossRef] [PubMed]
- Yarandi, S.S.; Srinivasan, S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: Current status and future directions. Neurogastroenterol. Motil. 2014, 26, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Klinge, M.W.; Sutter, N.; Mark, E.B.; Haase, A.M.; Borghammer, P.; Schlageter, V.; Lund, S.; Fleischer, J.; Knudsen, K.; Drewes, A.M.; et al. Gastric Emptying Time and Volume of the Small Intestine as Objective Markers in Patients with Symptoms of Diabetic Enteropathy. J. Neurogastroenterol. Motil. 2021, 27, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Caputi, V.; Popov, J.; Giron, M.C. Gut Microbiota as a Mediator of Host Neuro-Immune Interactions: Implications in Neuroinflammatory Disorders. Mod. Trends Psychiatry 2021, 32, 40–57. [Google Scholar] [CrossRef]
- Chandrasekharan, B.; Jeppsson, S.; Pienkowski, S.; Belsham, D.D.; Sitaraman, S.V.; Merlin, D.; Kokkotou, E.; Nusrat, A.; Tansey, M.G.; Srinivasan, S. Tumor necrosis factor-neuropeptide Y cross talk regulates inflammation, epithelial barrier functions, and colonic motility. Inflamm. Bowel Dis. 2013, 19, 2535–2546. [Google Scholar] [CrossRef] [Green Version]
- Ruder, B.; Atreya, R.; Becker, C. Tumour Necrosis Factor Alpha in Intestinal Homeostasis and Gut Related Diseases. Int. J. Mol. Sci. 2019, 20, 1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanamee, E.S.; Faustman, D.L. Structural principles of tumor necrosis factor superfamily signaling. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B.; Kohr, W.J.; Hass, P.E.; Moffat, B.; Spencer, S.A.; Henzel, W.J.; Bringman, T.S.; Nedwin, G.E.; Goeddel, D.V.; Harkins, R.N. Human tumor necrosis factor. Production, purification, and characterization. J. Biol. Chem. 1985, 260, 2345–2354. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-alpha signaling in macrophages. Crit. Rev. Eukaryot Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchetti, L.; Klein, M.; Schlett, K.; Pfizenmaier, K.; Eisel, U.L. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J. Biol. Chem. 2004, 279, 32869–32881. [Google Scholar] [CrossRef] [Green Version]
- Coquenlorge, S.; Duchalais, E.; Chevalier, J.; Cossais, F.; Rolli-Derkinderen, M.; Neunlist, M. Modulation of lipopolysaccharide-induced neuronal response by activation of the enteric nervous system. J. Neuroinflammation 2014, 11, 202. [Google Scholar] [CrossRef] [Green Version]
- Barbara, J.A.; Van Ostade, X.; Lopez, A. Tumour necrosis factor-alpha (TNF-alpha): The good, the bad and potentially very effective. Immunol. Cell Biol. 1996, 74, 434–443. [Google Scholar] [CrossRef]
- Fischer, R.; Maier, O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: Role of TNF. Oxid. Med. Cell Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef] [Green Version]
- Nakaizumi, A.; Horie, T.; Kida, T.; Kurimoto, T.; Sugiyama, T.; Ikeda, T.; Oku, H. Nitric oxide potentiates TNF-alpha-induced neurotoxicity through suppression of NF-kappaB. Cell Mol. Neurobiol. 2012, 32, 95–106. [Google Scholar] [CrossRef]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.B.; Uchida, K.; Nakajima, H.; Yayama, T.; Hirai, T.; Watanabe, S.; Guerrero, A.R.; Kobayashi, S.; Ma, W.Y.; Liu, S.Y.; et al. Tumor necrosis factor-alpha antagonist reduces apoptosis of neurons and oligodendroglia in rat spinal cord injury. Spine 2011, 36, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Asensio, V.J.; Tolosa, L.; Gou-Fabregas, M.; Soler, R.M.; Llado, J.; Olmos, G. Tumor necrosis factor alpha and interferon gamma cooperatively induce oxidative stress and motoneuron death in rat spinal cord embryonic explants. Neuroscience 2009, 162, 959–971. [Google Scholar] [CrossRef]
- Kontermann, R.E.; Scheurich, P.; Pfizenmaier, K. Antagonists of TNF action: Clinical experience and new developments. Expert Opin. Drug Discov. 2009, 4, 279–292. [Google Scholar] [CrossRef]
- Qiao, Y.C.; Chen, Y.L.; Pan, Y.H.; Tian, F.; Xu, Y.; Zhang, X.X.; Zhao, H.L. The change of serum tumor necrosis factor alpha in patients with type 1 diabetes mellitus: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0176157. [Google Scholar] [CrossRef] [Green Version]
- Lechleitner, M.; Koch, T.; Herold, M.; Dzien, A.; Hoppichler, F. Tumour necrosis factor-alpha plasma level in patients with type 1 diabetes mellitus and its association with glycaemic control and cardiovascular risk factors. J. Intern. Med. 2000, 248, 67–76. [Google Scholar] [CrossRef]
- Alzamil, H. Elevated Serum TNF-alpha Is Related to Obesity in Type 2 Diabetes Mellitus and Is Associated with Glycemic Control and Insulin Resistance. J. Obes. 2020, 2020, 5076858. [Google Scholar] [CrossRef] [Green Version]
- Swaroop, J.J.; Rajarajeswari, D.; Naidu, J.N. Association of TNF-alpha with insulin resistance in type 2 diabetes mellitus. Indian J. Med. Res. 2012, 135, 127–130. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J. Cell Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef]
- Costagliola, C.; Romano, V.; De Tollis, M.; Aceto, F.; dell’Omo, R.; Romano, M.R.; Pedicino, C.; Semeraro, F. TNF-alpha levels in tears: A novel biomarker to assess the degree of diabetic retinopathy. Mediators Inflamm. 2013, 2013, 629529. [Google Scholar] [CrossRef] [PubMed]
- Donate-Correa, J.; Ferri, C.M.; Sanchez-Quintana, F.; Perez-Castro, A.; Gonzalez-Luis, A.; Martin-Nunez, E.; Mora-Fernandez, C.; Navarro-Gonzalez, J.F. Inflammatory Cytokines in Diabetic Kidney Disease: Pathophysiologic and Therapeutic Implications. Front Med. 2020, 7, 628289. [Google Scholar] [CrossRef]
- Purohit, S.; Sharma, A.; Zhi, W.; Bai, S.; Hopkins, D.; Steed, L.; Bode, B.; Anderson, S.W.; Reed, J.C.; Steed, R.D.; et al. Proteins of TNF-alpha and IL6 Pathways Are Elevated in Serum of Type-1 Diabetes Patients with Microalbuminuria. Front Immunol. 2018, 9, 154. [Google Scholar] [CrossRef] [Green Version]
- Navarro, J.F.; Mora-Fernandez, C. The role of TNF-alpha in diabetic nephropathy: Pathogenic and therapeutic implications. Cytokine Growth Factor Rev. 2006, 17, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Liang, R.; Huang, B.; Hou, J.; Yin, J.; Zhao, T.; Zhou, L.; Wu, R.; Qian, Y.; Wang, F. Tumor necrosis factor-alpha blockade ameliorates diabetic nephropathy in rats. Clin. Kidney J. 2021, 14, 301–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, H.E.; Darwiche, R.; Corbett, J.A.; Kay, T.W. Evidence that beta cell death in the nonobese diabetic mouse is Fas independent. J. Immunol. 1999, 163, 1562–1569. [Google Scholar]
- Lee, L.F.; Xu, B.; Michie, S.A.; Beilhack, G.F.; Warganich, T.; Turley, S.; McDevitt, H.O. The role of TNF-alpha in the pathogenesis of type 1 diabetes in the nonobese diabetic mouse: Analysis of dendritic cell maturation. Proc. Natl. Acad. Sci. USA 2005, 102, 15995–16000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodi, N.; Talapka, P.; Poles, M.Z.; Hermesz, E.; Jancso, Z.; Katarova, Z.; Izbeki, F.; Wittmann, T.; Fekete, E.; Bagyanszki, M. Gut region-specific diabetic damage to the capillary endothelium adjacent to the myenteric plexus. Microcirculation 2012, 19, 316–326. [Google Scholar] [CrossRef]
- Chandrakumar, L.; Bagyanszki, M.; Szalai, Z.; Mezei, D.; Bodi, N. Diabetes-Related Induction of the Heme Oxygenase System and Enhanced Colocalization of Heme Oxygenase 1 and 2 with Neuronal Nitric Oxide Synthase in Myenteric Neurons of Different Intestinal Segments. Oxid. Med. Cell Longev. 2017, 2017, 1890512. [Google Scholar] [CrossRef] [PubMed]
- Bodi, N.; Mezei, D.; Chakraborty, P.; Szalai, Z.; Barta, B.P.; Balazs, J.; Razga, Z.; Hermesz, E.; Bagyanszki, M. Diabetes-related intestinal region-specific thickening of ganglionic basement membrane and regionally decreased matrix metalloproteinase 9 expression in myenteric ganglia. World J. Diabetes 2021, 12, 658–672. [Google Scholar] [CrossRef]
- Jancso, Z.; Bodi, N.; Borsos, B.; Fekete, E.; Hermesz, E. Gut region-specific accumulation of reactive oxygen species leads to regionally distinct activation of antioxidant and apoptotic marker molecules in rats with STZ-induced diabetes. Int. J. Biochem. Cell. Biol. 2015, 62, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Sanders, L.M.; Henderson, C.E.; Hong, M.Y.; Barhoumi, R.; Burghardt, R.C.; Carroll, R.J.; Turner, N.D.; Chapkin, R.S.; Lupton, J.R. Pro-oxidant environment of the colon compared to the small intestine may contribute to greater cancer susceptibility. Cancer Lett. 2004, 208, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, Y.; Namkoong, S.; Lee, J. Esculetin inhibits the inflammatory response by inducing heme oxygenase-1 in cocultured macrophages and adipocytes. Food Funct. 2014, 5, 2371–2377. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, S.; Sung, J.; Yang, J.; Choi, Y.; Jeong, H.S.; Lee, J. Nobiletin Attenuates the Inflammatory Response Through Heme Oxygenase-1 Induction in the Crosstalk Between Adipocytes and Macrophages. J. Med. Food 2017, 20, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Hsiao, L.D.; Cho, R.L.; Yang, C.M. CO-Releasing Molecule-2 Induces Nrf2/ARE-Dependent Heme Oxygenase-1 Expression Suppressing TNF-alpha-Induced Pulmonary Inflammation. J. Clin. Med. 2019, 8, 436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeev, E.; Vucic, D. Intracellular regulation of TNF activity in health and disease. Cytokine 2018, 101, 26–32. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Wang, H.; Ma, Y.; Zhao, X.; Zhang, X.; Yang, H.; Qian, J.; Li, J. Saccharomyces boulardii alleviates ulcerative colitis carcinogenesis in mice by reducing TNF-alpha and IL-6 levels and functions and by rebalancing intestinal microbiota. BMC Microbiol. 2019, 19, 246. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Y.; Chen, X.; Zhao, Y.; Wu, Y.; Li, Y.; Wang, X.; Chen, H.; Xiang, C. Induction of Intestinal Th17 Cells by Flagellins from Segmented Filamentous Bacteria. Front. Immunol. 2019, 10, 2750. [Google Scholar] [CrossRef]
- Locantore, P.; Del Gatto, V.; Gelli, S.; Paragliola, R.M.; Pontecorvi, A. The Interplay between Immune System and Microbiota in Osteoporosis. Mediat. Inflamm. 2020, 2020, 3686749. [Google Scholar] [CrossRef]
- Tian, F.; Han, B.; Duan, M. Serum tumor necrosis factor-α interleukin -6 and galctin-3 concentrations in children with Mycoplasma pneumoniae pneumonia. Zhongguo Dang Dai Er Ke Za Zhi 2014, 16, 1001–1004. [Google Scholar]
- Wang, Y.; Zhang, Y.; Lu, W.; Wang, L. Serum Tumor Necrosis Factor-alpha and Interferon-gamma Levels in Pediatric Mycoplasma pneumoniae Pneumonia: A Systematic Review and Meta-Analysis. Can. Respir. J. 2018, 2018, 8354892. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Fan, L.; Wang, Y.; Huang, L.; Wang, M.; Zhu, C.; Hao, C.; Ji, W.; Liang, H.; Yan, Y.; et al. High co-expression of TNF-alpha and CARDS toxin is a good predictor for refractory Mycoplasma pneumoniae pneumonia. Mol. Med. 2019, 25, 38. [Google Scholar] [CrossRef] [PubMed]
- Brenner, T.; Yamin, A.; Abramsky, O.; Gallily, R. Stimulation of tumor necrosis factor-alpha production by mycoplasmas and inhibition by dexamethasone in cultured astrocytes. Brain Res. 1993, 608, 273–279. [Google Scholar] [CrossRef]
- Trueeb, B.S.; Braun, R.O.; Auray, G.; Kuhnert, P.; Summerfield, A. Differential innate immune responses induced by Mycoplasma hyopneumoniae and Mycoplasma hyorhinis in various types of antigen presenting cells. Vet. Microbiol. 2020, 240, 108541. [Google Scholar] [CrossRef] [PubMed]
- Burgueno, J.F.; Barba, A.; Eyre, E.; Romero, C.; Neunlist, M.; Fernandez, E. TLR2 and TLR9 modulate enteric nervous system inflammatory responses to lipopolysaccharide. J. Neuroinflammation 2016, 13, 187. [Google Scholar] [CrossRef] [Green Version]
- Yoo, B.B.; Mazmanian, S.K. The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity 2017, 46, 910–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuffre, M.; Moretti, R.; Campisciano, G.; da Silveira, A.B.M.; Monda, V.M.; Comar, M.; Di Bella, S.; Antonello, R.M.; Luzzati, R.; Croce, L.S. You Talking to Me? Says the Enteric Nervous System (ENS) to the Microbe. How Intestinal Microbes Interact with the ENS. J. Clin. Med. 2020, 9, 3705. [Google Scholar] [CrossRef]
- Gough, P.; Myles, I.A. Tumor Necrosis Factor Receptors: Pleiotropic Signaling Complexes and Their Differential Effects. Front. Immunol. 2020, 11, 585880. [Google Scholar] [CrossRef]
- Bu, L.; Cao, X.; Zhang, Z.; Wu, H.; Guo, R.; Ma, M. Decreased secretion of tumor necrosis factor-alpha attenuates macrophages-induced insulin resistance in skeletal muscle. Life Sci. 2020, 244, 117304. [Google Scholar] [CrossRef]
- Clark, I.; Atwood, C.; Bowen, R.; Paz-Filho, G.; Vissel, B. Tumor necrosis factor-induced cerebral insulin resistance in Alzheimer’s disease links numerous treatment rationales. Pharmacol. Rev. 2012, 64, 1004–1026. [Google Scholar] [CrossRef] [Green Version]
- Rask-Madsen, C.; Dominguez, H.; Ihlemann, N.; Hermann, T.; Kober, L.; Torp-Pedersen, C. Tumor necrosis factor-alpha inhibits insulin’s stimulating effect on glucose uptake and endothelium-dependent vasodilation in humans. Circulation 2003, 108, 1815–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halse, R.; Pearson, S.L.; McCormack, J.G.; Yeaman, S.J.; Taylor, R. Effects of tumor necrosis factor-alpha on insulin action in cultured human muscle cells. Diabetes 2001, 50, 1102–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, B.A.; Fidalgo, S.; Wang, C.; Parmar, L.; Mandona, K.; Panossian, A.; Flint, M.S.; Ranson, R.N.; Saffrey, M.J.; Yeoman, M.S. The TNF-alpha antagonist etanercept reverses age-related decreases in colonic SERT expression and faecal output in mice. Sci. Rep. 2017, 7, 42754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Body Weight (g) ± SEM | Blood Glucose Concentration (mmol/L) ± SEM | |||
|---|---|---|---|---|
| Initial | Final | Initial | Final (Average) | |
| Controls (n = 5) | 224.4 ± 7.13 | 504 ± 15.65 a | 5.96 ± 0.34 | 5.81 ± 0.22 |
| Diabetics (n = 5) | 228.4 ± 7.19 | 373.4 ± 12.51 ab | 6.2 ± 0.25 | 24.19 ± 0.61 ab |
| Insulin-treated diabetics (n = 4) | 251.5 ± 4.35 | 481.5 ± 13.4 ac | 6.65 ± 0.18 | 9.48 ± 0.14 abc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bódi, N.; Chandrakumar, L.; al Doghmi, A.; Mezei, D.; Szalai, Z.; Barta, B.P.; Balázs, J.; Bagyánszki, M. Intestinal Region-Specific and Layer-Dependent Induction of TNFα in Rats with Streptozotocin-Induced Diabetes and after Insulin Replacement. Cells 2021, 10, 2410. https://doi.org/10.3390/cells10092410
Bódi N, Chandrakumar L, al Doghmi A, Mezei D, Szalai Z, Barta BP, Balázs J, Bagyánszki M. Intestinal Region-Specific and Layer-Dependent Induction of TNFα in Rats with Streptozotocin-Induced Diabetes and after Insulin Replacement. Cells. 2021; 10(9):2410. https://doi.org/10.3390/cells10092410
Chicago/Turabian StyleBódi, Nikolett, Lalitha Chandrakumar, Afnan al Doghmi, Diána Mezei, Zita Szalai, Bence Pál Barta, János Balázs, and Mária Bagyánszki. 2021. "Intestinal Region-Specific and Layer-Dependent Induction of TNFα in Rats with Streptozotocin-Induced Diabetes and after Insulin Replacement" Cells 10, no. 9: 2410. https://doi.org/10.3390/cells10092410
APA StyleBódi, N., Chandrakumar, L., al Doghmi, A., Mezei, D., Szalai, Z., Barta, B. P., Balázs, J., & Bagyánszki, M. (2021). Intestinal Region-Specific and Layer-Dependent Induction of TNFα in Rats with Streptozotocin-Induced Diabetes and after Insulin Replacement. Cells, 10(9), 2410. https://doi.org/10.3390/cells10092410







