High-Content C. elegans Screen Identifies Natural Compounds Impacting Mitochondria-Lipid Homeostasis and Promoting Healthspan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. C. elegans
2.2.1. C. elegans Strains and Maintenance
2.2.2. Compounds Treatment
2.2.3. Liquid Culture
2.2.4. Solid Medium
2.2.5. Multiwell Plates Preparation for Screening
2.2.6. Microscopy
2.2.7. Quantitative Analysis of Nematode’s Body Size and GFP Induction of gst-4
2.2.8. Fertility Assay
2.2.9. Heat Shock Assay
2.2.10. Toxicity Assay
2.2.11. Lifespan Analysis
2.2.12. Body Bend
2.2.13. Nile Red Staining
2.3. Drosophila Cells
High-Content-Screening Experiments in Drosophila Cells
2.4. Statistical Analysis
3. Results
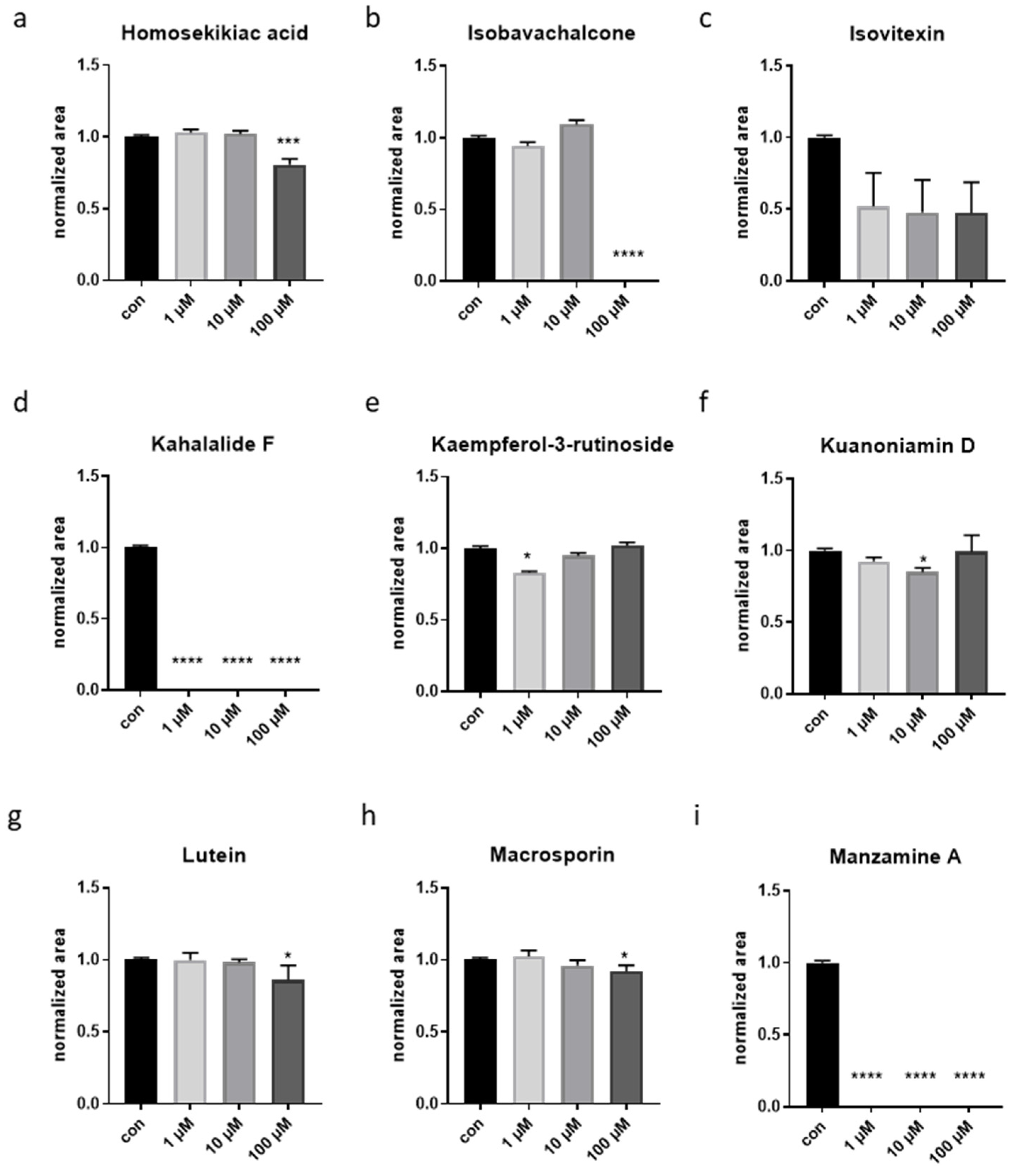
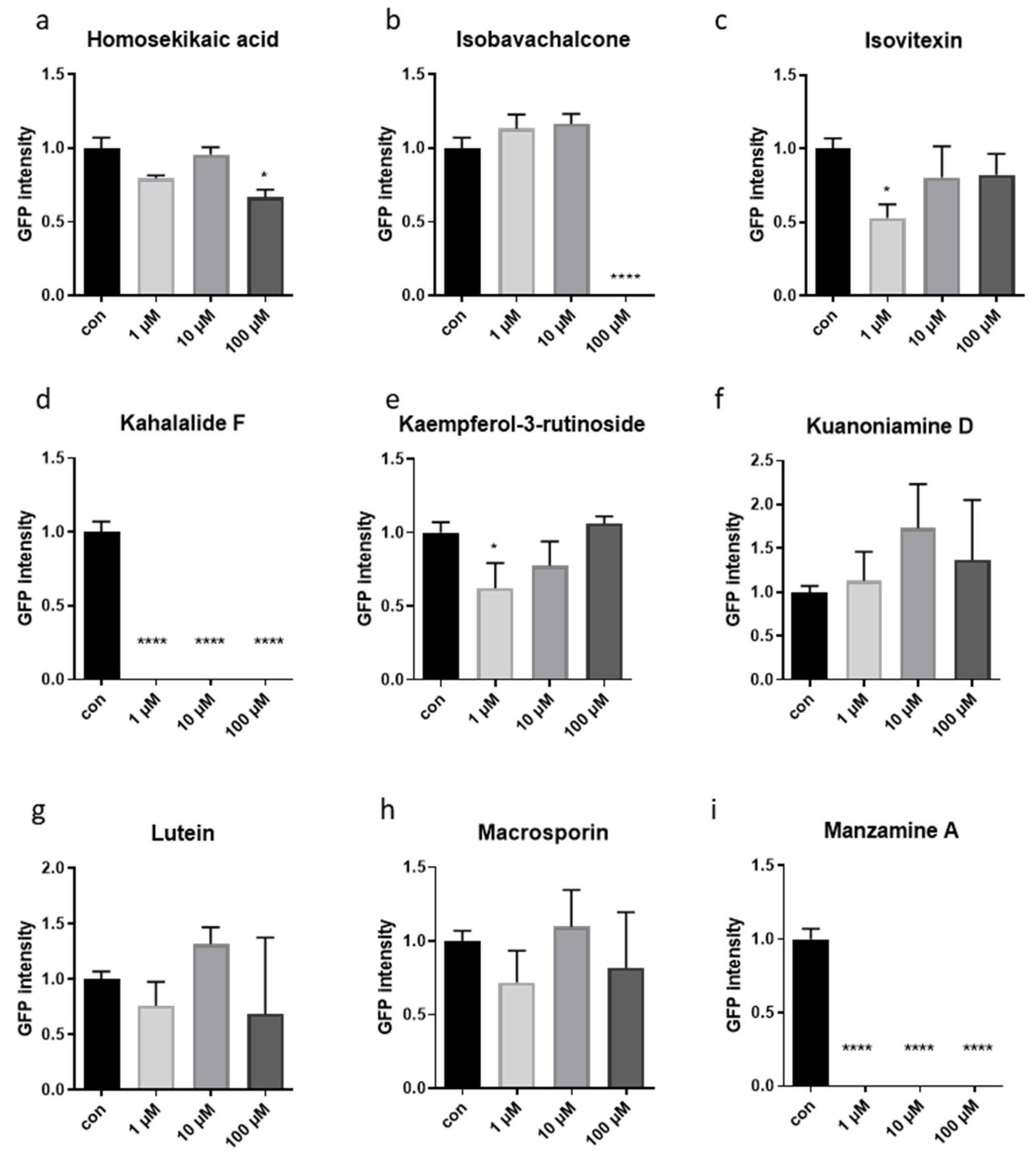
3.1. A Phenotype-Based Automated Screen Identified Four Natural Compounds with Potential Anti-Aging Effects
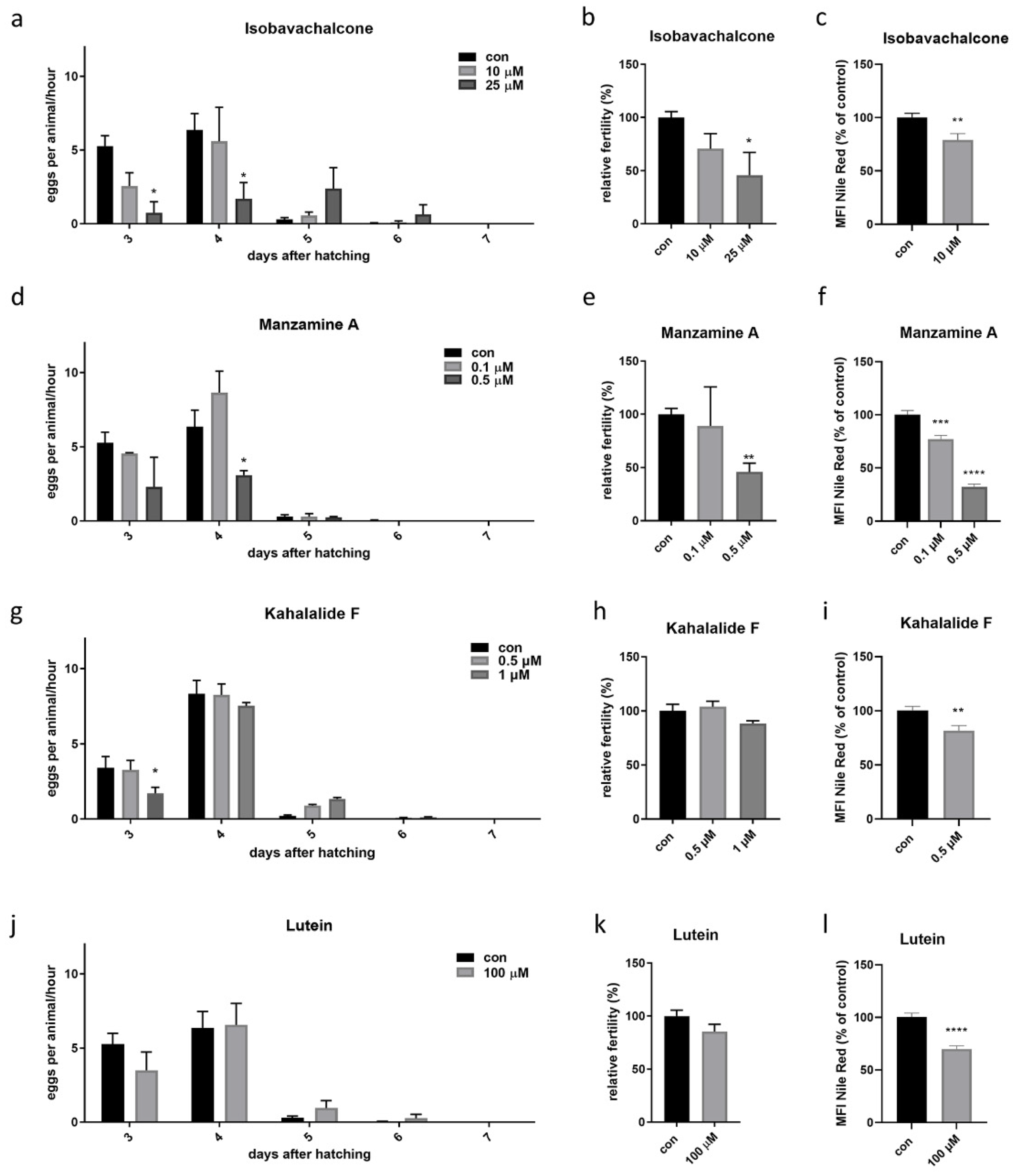
3.2. Validation of Four Compounds on Cellular and Animals’ Physiology
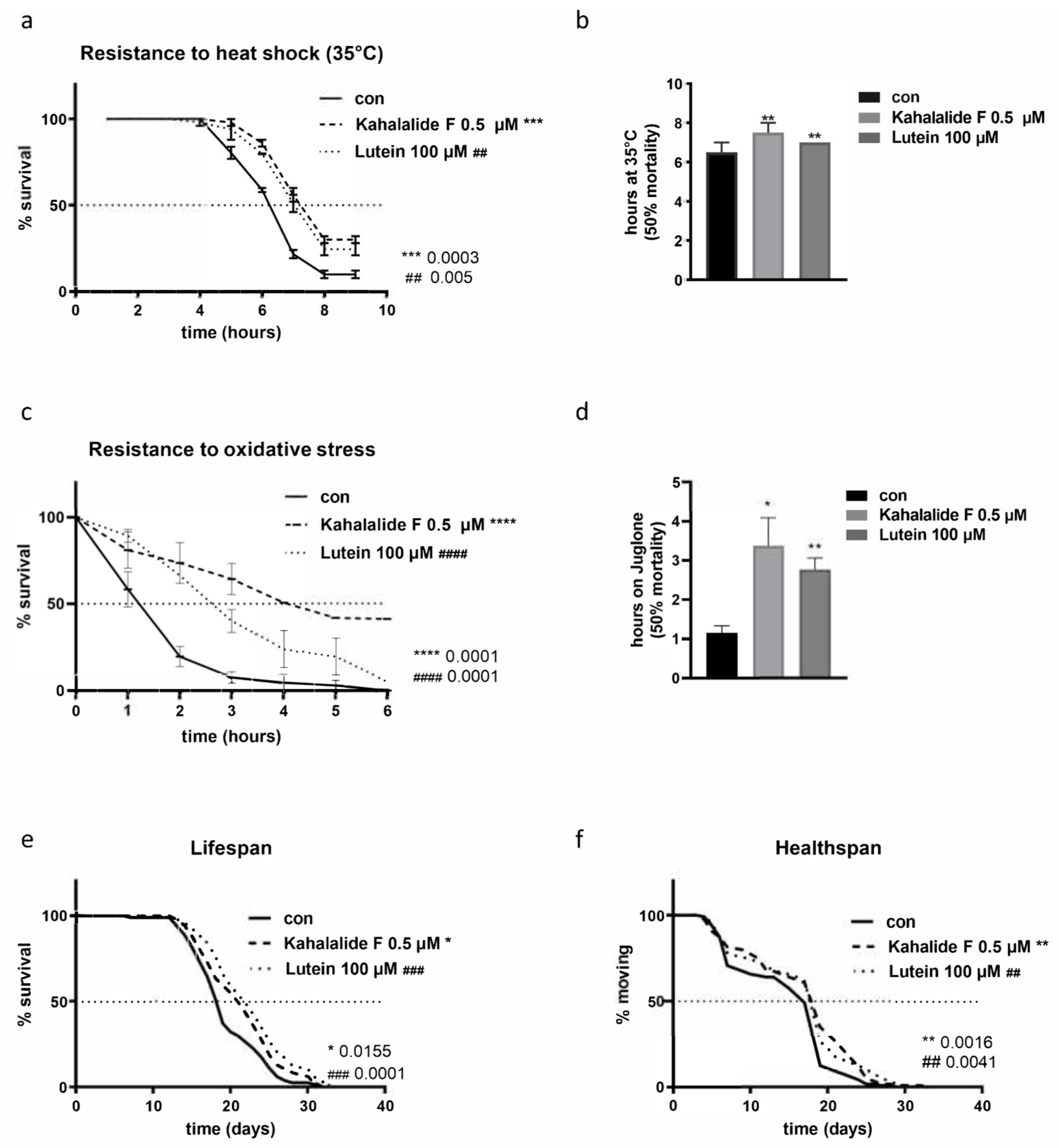
3.3. Kahalalide F and Lutein Promote Animals’ Healthspan
3.4. Lutein Pro-Health Effects Are Mediated by nlg-1 Induction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, W.F.; Tolliday, N. Cell-based assays for high-throughput screening. Mol. Biotechnol. 2010, 45, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Zlitni, S.; Blanchard, J.E.; Brown, E.D. High-throughput screening of model bacteria. Methods Mol. Biol. 2009, 486, 13–27. [Google Scholar] [PubMed]
- Zimmermann, A.; Hofer, S.; Pendl, T.; Kainz, K.; Madeo, F.; Carmona-Gutierrez, D. Yeast as a tool to identify anti-aging compounds. FEMS Yeast Res. 2018, 18, foy020. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Ni, J.; Ruggiero, A.; Walshe, K.; Rogers, M.S.; Chattopadhyay, N.; Glicksman, M.A.; Rogers, J.T. A high-throughput drug screen targeted to the 5’untranslated region of Alzheimer amyloid precursor protein mRNA. J. Biomol. Screen. 2006, 11, 469–480. [Google Scholar] [CrossRef] [Green Version]
- Crowe, A.; Ballatore, C.; Hyde, E.; Trojanowski, J.Q.; Lee, V.M.-Y. High throughput screening for small molecule inhibitors of heparin-induced tau fibril formation. Biochem. Biophys. Res. Commun. 2007, 358, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Case, A.; Ni, J.; Yeh, L.-A.; Stein, R.L. Development of a mechanism-based assay for tissue transglutaminase--results of a high-throughput screen and discovery of inhibitors. Anal. Biochem. 2005, 338, 237–244. [Google Scholar] [CrossRef]
- Pedro, L.; Padrós, J.; Beaudet, L.; Schubert, H.-D.; Gillardon, F.; Dahan, S. Development of a high-throughput AlphaScreen assay measuring full-length LRRK2(G2019S) kinase activity using moesin protein substrate. Anal. Biochem. 2010, 404, 45–51. [Google Scholar] [CrossRef]
- Hansen, M.; Hsu, A.-L.; Dillin, A.; Kenyon, C. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 2005, 1, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Curran, S.P.; Ruvkun, G. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 2007, 3, e56. [Google Scholar] [CrossRef] [Green Version]
- Maglioni, S.; Mello, D.F.; Schiavi, A.; Meyer, J.; Ventura, N. Mitochondrial bioenergetic changes during development as an indicator of C. elegans health-span. Aging 2019, 11, 6535–6554. [Google Scholar] [CrossRef]
- Maglioni, S.; Arsalan, N.; Ventura, N. C. elegans screening strategies to identify pro-longevity interventions. Mech. Ageing Dev. 2016, 157, 60–69. [Google Scholar] [CrossRef]
- Mondal, S.; Hegarty, E.; Martin, C.; Gökçe, S.K.; Ghorashian, N.; Ben-Yakar, A. Large-scale microfluidics providing high-resolution and high-throughput screening of Caenorhabditis elegans poly-glutamine aggregation model. Nat. Commun. 2016, 7, 13023. [Google Scholar] [CrossRef] [PubMed]
- Maglioni, S.; Arsalan, N.; Franchi, L.; Hurd, A.; Opipari, A.W.; Glick, G.D.; Ventura, N. An automated phenotype-based microscopy screen to identify pro-longevity interventions acting through mitochondria in C. elegans. Biochim. Biophys. Acta 2015, 1847, 1469–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrascheck, M.; Ye, X.; Buck, L.B. A high-throughput screen for chemicals that increase the lifespan of Caenorhabditis elegans. Ann. N. Y. Acad. Sci. 2009, 1170, 698–701. [Google Scholar] [CrossRef]
- Petrascheck, M.; Ye, X.; Buck, L.B. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature 2007, 450, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Lucanic, M.; Garrett, T.; Yu, I.; Calahorro, F.; Shahmirzadi, A.A.; Miller, A.; Gill, M.S.; Hughes, R.E.; Holden-Dye, L.; Lithgow, G.J. Chemical activation of a food deprivation signal extends lifespan. Aging Cell 2016, 15, 832–841. [Google Scholar] [CrossRef]
- Ye, X.; Linton, J.M.; Schork, N.J.; Buck, L.B.; Petrascheck, M. A pharmacological network for lifespan extension in Caenorhabditis elegans. Aging Cell 2014, 13, 206–215. [Google Scholar] [CrossRef]
- Bulterijs, S.; Braeckman, B.P. Phenotypic Screening in C. elegans as a Tool for the Discovery of New Geroprotective Drugs. Pharmaceuticals 2020, 13, 164. [Google Scholar] [CrossRef]
- Rea, S.L.; Ventura, N.; Johnson, T.E. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 2007, 5, e259. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.I.; Pincus, Z.; Slack, F.J. Longevity and stress in Caenorhabditis elegans. Aging 2011, 3, 733–753. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Hwang, W.; Jung, J.; Park, S.; Cabatbat, J.J.T.; Kim, P.-J.; Lee, S.-J.V. Inverse correlation between longevity and developmental rate among wild C. elegans strains. Aging 2016, 8, 986–999. [Google Scholar] [CrossRef] [Green Version]
- Munoz, M.J.; Riddle, D.L. Positive selection of Caenorhabditis elegans mutants with increased stress resistance and longevity. Genetics 2003, 163, 171–180. [Google Scholar] [CrossRef]
- Maglioni, S.; Schiavi, A.; Runci, A.; Shaik, A.; Ventura, N. Mitochondrial stress extends lifespan in C. elegans through neuronal hormesis. Exp. Geronto. 2014, 56, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Ji, F.; Breen, P.; Sewell, A.; Han, M.; Sadreyev, R.; Ruvkun, G. Mitochondrial Dysfunction in C. elegans Activates Mitochondrial Relocalization and Nuclear Hormone Receptor-Dependent Detoxification Genes. Cell Metab. 2019, 29, 1182–1191.e4. [Google Scholar] [CrossRef] [PubMed]
- Stiernagle, T. Maintenance of C. elegans; WormBook. 2006; 1–11. Available online: http://www.wormbook.org (accessed on 25 December 2021).
- Gosai, S.J.; Kwak, J.H.; Luke, C.J.; Long, O.S.; King, D.E.; Kovatch, K.J.; Johnston, P.A.; Shun, T.Y.; Lazo, S.J.; Perlmutter, D.H.; et al. Automated high-content live animal drug screening using C. elegans expressing the aggregation prone serpin alpha1-antitrypsin Z. PLoS ONE 2010, 5, e15460. [Google Scholar]
- Han, S.K.; Lee, D.; Lee, H.; Kim, D.; Son, H.; Yang, J.-S.; Lee, S.-J.V.; Kim, S. OASIS 2: Online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 2016, 7, 56147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staab, T.A.; Evgrafov, O.; Knowles, J.A.; Sieburth, D. Regulation of synaptic nlg-1/neuroligin abundance by the skn-1/Nrf stress response pathway protects against oxidative stress. PLoS Genet. 2014, 10, e1004100. [Google Scholar] [CrossRef] [PubMed]
- Ventura, N.; Rea, S.L.; Schiavi, A.; Torgovnick, A.; Testi, R.; Johnson, T.E. p53/CEP-1 increases or decreases lifespan, depending on level of mitochondrial bioenergetic stress. Aging Cell 2009, 8, 380–393. [Google Scholar] [CrossRef] [Green Version]
- Hart, A.C. Behavior in WormBook, The C. elegans Research Community. 2006. Available online: http://www.wormbook.org/index.html (accessed on 25 December 2021).
- Tschapalda, K.; Zhang, Y.-Q.; Liu, L.; Golovnina, K.; Schlemper, T.; Eichmann, T.O.; Lal-Nag, M.; Sreenivasan, U.; McLenithan, J.; Ziegler, S.; et al. A Class of Diacylglycerol Acyltransferase 1 Inhibitors Identified by a Combination of Phenotypic High-throughput Screening, Genomics, and Genetics. EBioMedicine 2016, 8, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Stringer, C.; Wang, T.; Michaelos, M.; Pachitariu, M. Cellpose: A generalist algorithm for cellular segmentation. Nat. Methods 2021, 18, 100–106. [Google Scholar] [CrossRef]
- McQuin, C.; Goodman, A.; Chernyshev, V.; Kamentsky, L.; Cimini, B.A.; Karhohs, K.W.; Doan, M.; Ding, L.; Rafelski, S.M.; Thirstrup, D.; et al. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol. 2018, 16, e2005970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stöter, M.; Janosch, A.; Barsacchi, R.; Bickle, M. CellProfiler and KNIME: Open-Source Tools for High-Content Screening. Methods Mol. Biol. 2019, 1953, 43–60. [Google Scholar]
- Ventura, N.; Rea, S.L. Caenorhabditis elegans mitochondrial mutants as an investigative tool to study human neurodegenerative diseases associated with mitochondrial dysfunction. Biotechnol. J. 2007, 2, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, A.; Torgovnick, A.; Kell, A.; Megalou, E.; Castelein, N.; Guccini, I.; Marzocchella, L.; Gelino, S.; Hansen, M.; Malisan, F.; et al. Autophagy induction extends lifespan and reduces lipid content in response to frataxin silencing in C. elegans. Exp. Gerontol. 2013, 48, 191–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashrafi, K.; Chang, F.Y.; Watts, J.; Fraser, A.G.; Kamath, R.S.; Ahringer, J.; Ruvkun, G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 2003, 421, 268–272. [Google Scholar] [CrossRef]
- Wahba, A.E.; Peng, J.; Kudrimoti, S.; Tekwani, B.L.; Hamann, M.T. Structure-activity relationship studies of manzamine A: Amidation of positions 6 and 8 of the beta-carboline moiety. Bioorg. Med. Chem. 2009, 17, 7775–7782. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Chen, W.D.; Wang, Y.D. DAF-16/FOXO Transcription Factor in Aging and Longevity. Front. Pharmacol. 2017, 8, 548. [Google Scholar] [CrossRef]
- Lan, J.; Rollins, J.A.; Zang, X.; Wu, D.; Zou, L.; Wang, Z.; Ye, C.; Wu, Z.; Kapahi, P.; Rogers, A.N.; et al. Translational Regulation of Non-autonomous Mitochondrial Stress Response Promotes Longevity. Cell Rep. 2019, 28, 1050–1062.e6. [Google Scholar] [CrossRef] [Green Version]
- Robida-Stubbs, S.; Glover-Cutter, K.; Lamming, D.W.; Mizunuma, M.; Narasimhan, S.D.; Neumann-Haefelin, E.; Sabatini, D.M.; Blackwell, T.K. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012, 15, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Bennett, C.; Wende, H.V.; Simko, M.; Klum, S.; Barfield, S.; Choi, H.; Pineda, V.V.; Kaeberlein, M. Activation of the mitochondrial unfolded protein response does not predict longevity in Caenorhabditis elegans. Nat. Commun. 2014, 5, 3483. [Google Scholar] [CrossRef]
- Durieux, J.; Wolff, S.; Dillin, A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 2011, 144, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Dean, C.; Dresbach, T. Neuroligins and neurexins: Linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006, 29, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Maglioni, S.; Schiavi, A.; Melcher, M.; Brinkmann, V.; Luo, Z.; Raimundo, N.; Laromaine, A.; Meyer, J.N.; Distelmaier, F.; Ventura, N. Lutein restores synaptic functionality in a C. elegans model for mitochondrial complex I deficiency. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Munkacsy, E.; Rea, S.L. The paradox of mitochondrial dysfunction and extended longevity. Exp. Gerontol. 2014, 56, 221–233. [Google Scholar] [CrossRef] [Green Version]
- Ventura, N.; Rea, S.L.; Testi, R. Long-lived C. elegans mitochondrial mutants as a model for human mitochondrial-associated diseases. Exp. Gerontol. 2006, 41, 974–991. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Moreno, J.; Rodriguez, H.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Mares, J. Lutein and Zeaxanthin Isomers in Eye Health and Disease. Annu. Rev. Nutr. 2016, 36, 571–602. [Google Scholar] [CrossRef] [Green Version]
- Santocono, M.; Zurria, M.; Berrettini, M.; Fedeli, D.; Falcioni, G. Influence of astaxanthin, zeaxanthin and lutein on DNA damage and repair in UVA-irradiated cells. J. Photochem. Photobiol. B 2006, 85, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Sujak, A.; Gabrielska, J.; Grudziński, W.; Borc, R.; Mazurek, P.; Gruszecki, W.I. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch. Biochem. Biophys. 1999, 371, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar]
- Swanson, H.M.; Smith, J.R., Jr.; Gong, X.; Rubin, L.P. Lutein, but not other carotenoids, selectively inhibits breast cancer cell growth through several molecular mechanisms. FASEB J. 2016, 30 (Suppl. S1), 34.2. [Google Scholar]
- Liu, R.; Wang, T.; Zhang, B.; Qin, L.; Wu, C.; Li, Q.; Ma, L. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 56, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Kiko, T.; Nakagawa, K.; Tsuduki, T.; Suzuki, T.; Arai, H.; Miyazawa, T. Significance of lutein in red blood cells of Alzheimer’s disease patients. J. Alzheimers Dis. 2012, 28, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Rosen, R.; Jordan, A.; Hu, D.N. Management of Ocular Diseases Using Lutein and Zeaxanthin: What Have We Learned from Experimental Animal Studies? J. Ophthalmol. 2015, 2015, 523027. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, R.; Devaraj, S.N.; Padma, V.V. Lutein protects HT-29 cells against Deoxynivalenol-induced oxidative stress and apoptosis: Prevention of NF-kappaB nuclear localization and down regulation of NF-kappaB and Cyclo-Oxygenase-2 expression. Free Radic. Biol. Med. 2010, 49, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.-H.; Ohgami, K.; Shiratori, K.; Suzuki, Y.; Hirano, T.; Koyama, Y.; Yoshida, K.; Ilieva, I.; Iseki, K.; Ohno, S. Inhibitory effects of lutein on endotoxin-induced uveitis in Lewis rats. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2562–2568. [Google Scholar] [CrossRef]
- Ashino, T.; Yamanaka, R.; Yamamoto, M.; Shimokawa, H.; Sekikawa, K.; Iwakura, Y.; Shioda, S.; Numazawa, S.; Yoshida, T. Negative feedback regulation of lipopolysaccharide-induced inducible nitric oxide synthase gene expression by heme oxygenase-1 induction in macrophages. Mol. Immunol. 2008, 45, 2106–2115. [Google Scholar] [CrossRef]
- Cruz, L.J.; Luque-Ortega, J.R.; Rivas, L.; Albericio, F. Kahalalide F an antitumor depsipeptide in clinical trials, and its analogues as effective antileishmanial agents. Mol. Pharm. 2009, 6, 813–824. [Google Scholar] [CrossRef]
- Negi, B.; Kumar, D.; Rawat, D.S. Marine Peptides as Anticancer Agents: A Remedy to Mankind by Nature. Curr. Protein Pept. Sci. 2017, 18, 885–904. [Google Scholar] [CrossRef]
- Lee, Y.; Phat, C.; Hong, S.C. Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides 2017, 95, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Waters, A.L.; Valeriote, F.A.; Hamann, M.T. An efficient and cost-effective approach to kahalalide F N-terminal modifications using a nuisance algal bloom of Bryopsis pennata. Biochim. Biophys. Acta 2015, 1850, 1849–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Rocha, M.; Bonay, P.; Avila, J. The antitumoral compound Kahalalide F acts on cell lysosomes. Cancer Lett. 1996, 99, 43–50. [Google Scholar] [CrossRef]
- Suárez, Y.; González, L.; Cuadrado, A.; Berciano, M.; Lafarga, M.; Muñoz, A. Kahalalide F, a new marine-derived compound, induces oncosis in human prostate and breast cancer cells. Mol. Cancer Ther. 2003, 2, 863–872. [Google Scholar]
- Baranov, S.V.; Jauhari, A.; Carlisle, D.L.; Friedlander, R.M. Two hit mitochondrial-driven model of synapse loss in neurodegeneration. Neurobiol. Dis. 2021, 158, 105451. [Google Scholar] [CrossRef]
- Binotti, B.; Jahn, R.; Perez-Lara, A. An overview of the synaptic vesicle lipid composition. Arch. Biochem. Biophys. 2021, 709, 108966. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]



| Days after Hatching | ||||||
|---|---|---|---|---|---|---|
| 3 | 4 | 6 | ||||
| Compound (Concentration) | Phenotype | GFP | Phenotype | GFP | Phenotype | GFP |
| DMSO (0.25%) control | GA | GA and progeny | Starved | |||
| Homosekikaic acid (10 µM) | ||||||
| Homosekikaic acid (100 µM) | ||||||
| Isobavachalcone (10 µM) | ||||||
| Isobavachalcone (25 µM) | ||||||
| Isobavachalcone (50 µM) | ||||||
| Isovitexin (1 µM) | ||||||
| Isovitexin (10 µM) | ||||||
| Isovitexin (100 µM) | ||||||
| Kaempferol-3-rutiniside (1 µM) | ||||||
| Kaempferol-3-rutiniside (10 µM) | ||||||
| Kaempferol-3-rutinoside (100 µM) | ||||||
| Kahalalide F (0.1 µM) | ||||||
| Kahalalide F (0.5 µM) | ||||||
| Kahalalide F (1 µM) | ||||||
| Kuanoniamine D (50 µM) | ||||||
| Kuanoniamine D (100 µM) | ||||||
| Lutein (1 µM) | ||||||
| Lutein (10 µM) | ||||||
| Lutein (100 µM) | ||||||
| Macrosporin (10 µM) | ||||||
| Macrosporin (100 µM) | ||||||
| Manzamine A (0.1 µM) | ||||||
| Manzamine A (0.5 µM) | ||||||
| Manzamine A (1 µM) | ||||||
| Color legend: Phenotype | ||||||
| Similar to DMSO control | ||||||
| Slightly slower development, leaner or paler than control (L3–L4) | ||||||
| Quite slower than control (L2–L3) | ||||||
| Sick or arrested development | ||||||
| Color legend: GFP expression | ||||||
| Increased signal compared to control | ||||||
| Similar to DMSO control | ||||||
| Decreased signal compared to control | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maglioni, S.; Arsalan, N.; Hamacher, A.; Afshar, S.; Schiavi, A.; Beller, M.; Ventura, N. High-Content C. elegans Screen Identifies Natural Compounds Impacting Mitochondria-Lipid Homeostasis and Promoting Healthspan. Cells 2022, 11, 100. https://doi.org/10.3390/cells11010100
Maglioni S, Arsalan N, Hamacher A, Afshar S, Schiavi A, Beller M, Ventura N. High-Content C. elegans Screen Identifies Natural Compounds Impacting Mitochondria-Lipid Homeostasis and Promoting Healthspan. Cells. 2022; 11(1):100. https://doi.org/10.3390/cells11010100
Chicago/Turabian StyleMaglioni, Silvia, Nayna Arsalan, Anna Hamacher, Shiwa Afshar, Alfonso Schiavi, Mathias Beller, and Natascia Ventura. 2022. "High-Content C. elegans Screen Identifies Natural Compounds Impacting Mitochondria-Lipid Homeostasis and Promoting Healthspan" Cells 11, no. 1: 100. https://doi.org/10.3390/cells11010100
APA StyleMaglioni, S., Arsalan, N., Hamacher, A., Afshar, S., Schiavi, A., Beller, M., & Ventura, N. (2022). High-Content C. elegans Screen Identifies Natural Compounds Impacting Mitochondria-Lipid Homeostasis and Promoting Healthspan. Cells, 11(1), 100. https://doi.org/10.3390/cells11010100








