Epithelial and Neural Cadherin in Mammalian Fertilization: Studies in the Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Gamete Handling
2.3. Protein Extraction and Western Immunoblotting
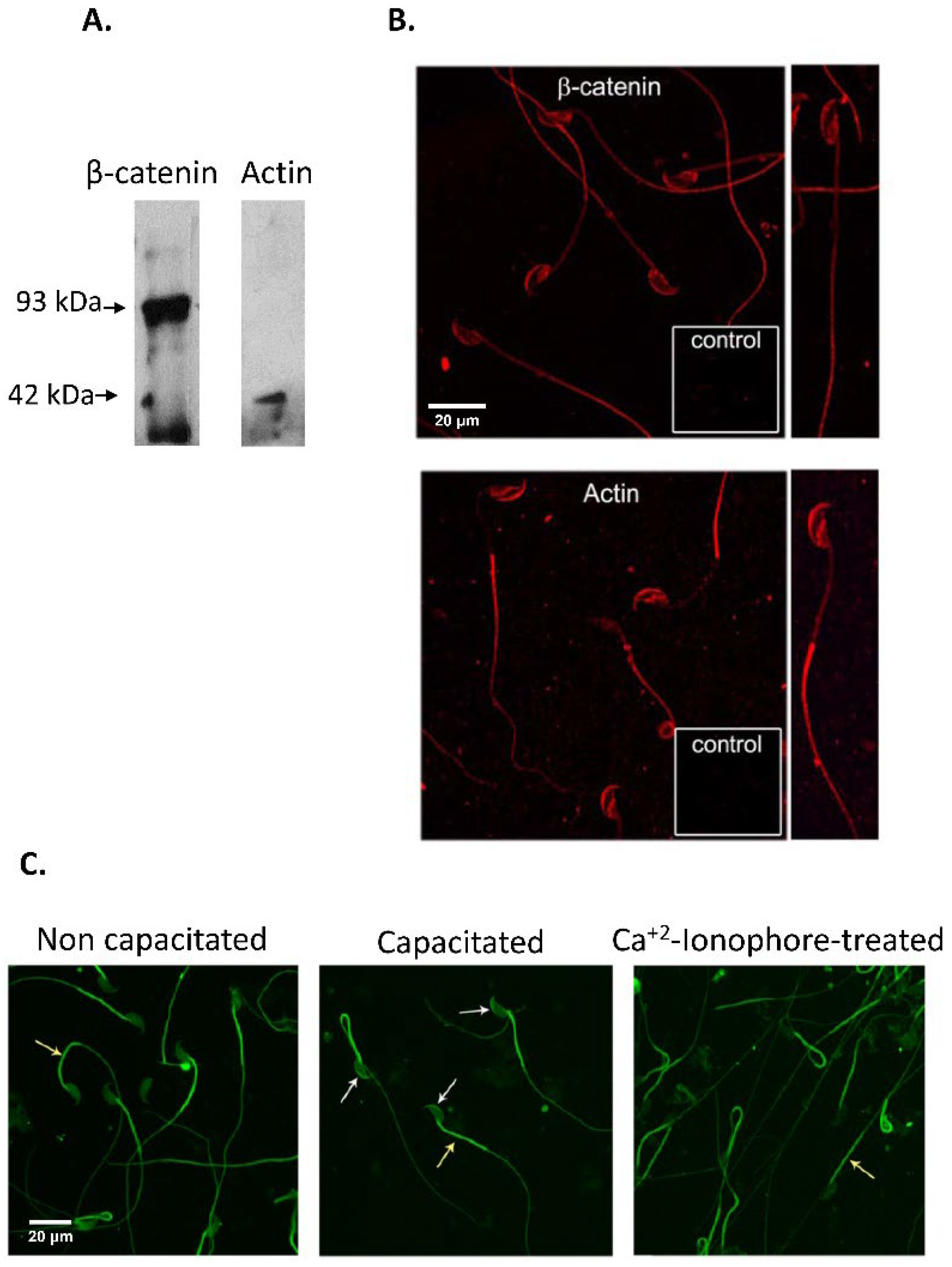
2.4. Sperm Capacitation and Acrosomal Exocytosis
2.5. Immunocytochemistry
2.6. Sperm-Oocyte Interaction Assays
2.6.1. IVF
2.6.2. Cumulus Penetration Assay
2.6.3. Sperm-Oolemma Binding and Fusion Assays
2.7. Statistical Analysis
3. Results
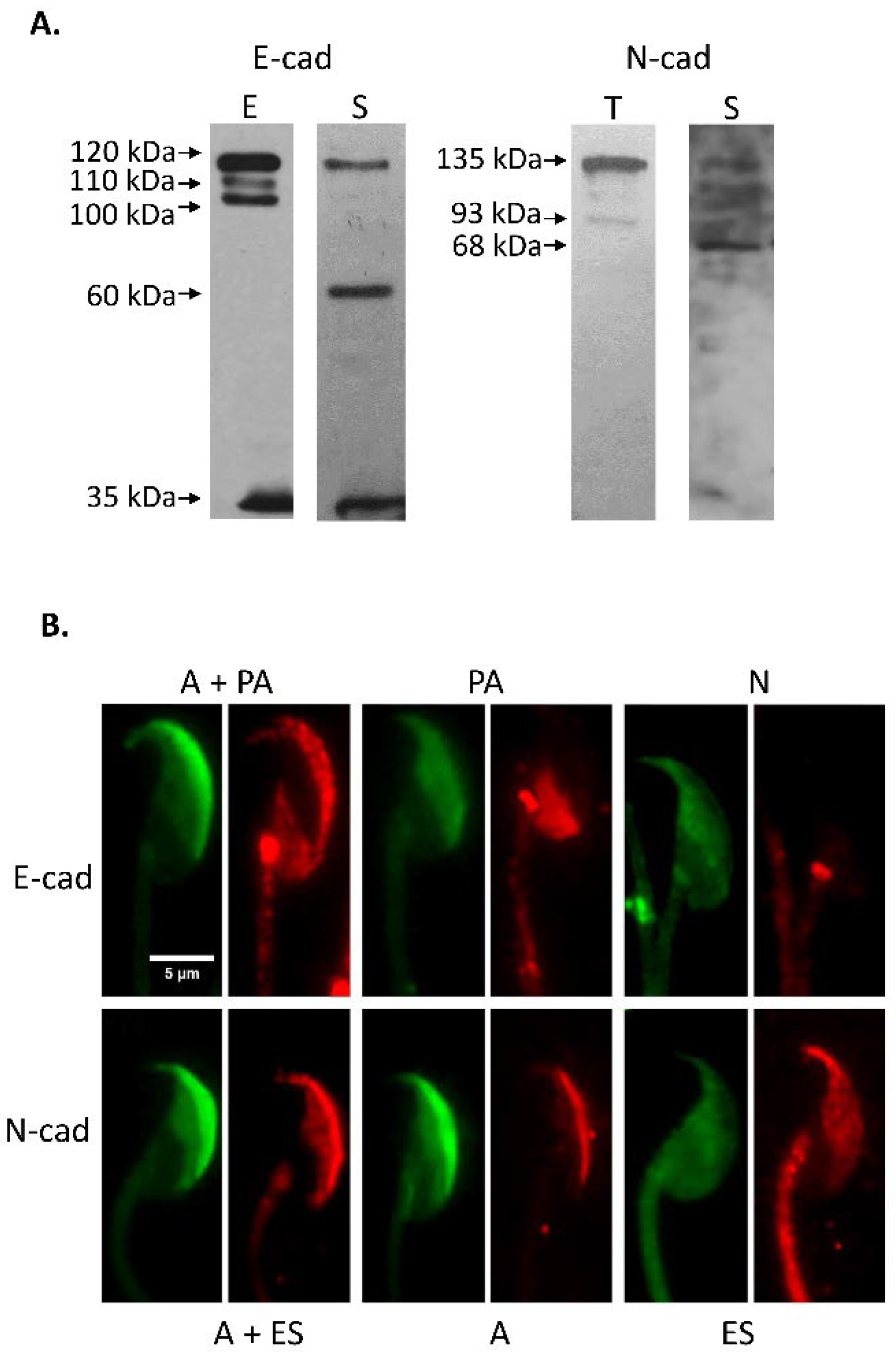
3.1. Immunodetection of E-cad and N-cad and Other Members of the Adherent Complex in Testis, Epididymis, and Cauda Epididymal Spermatozoa
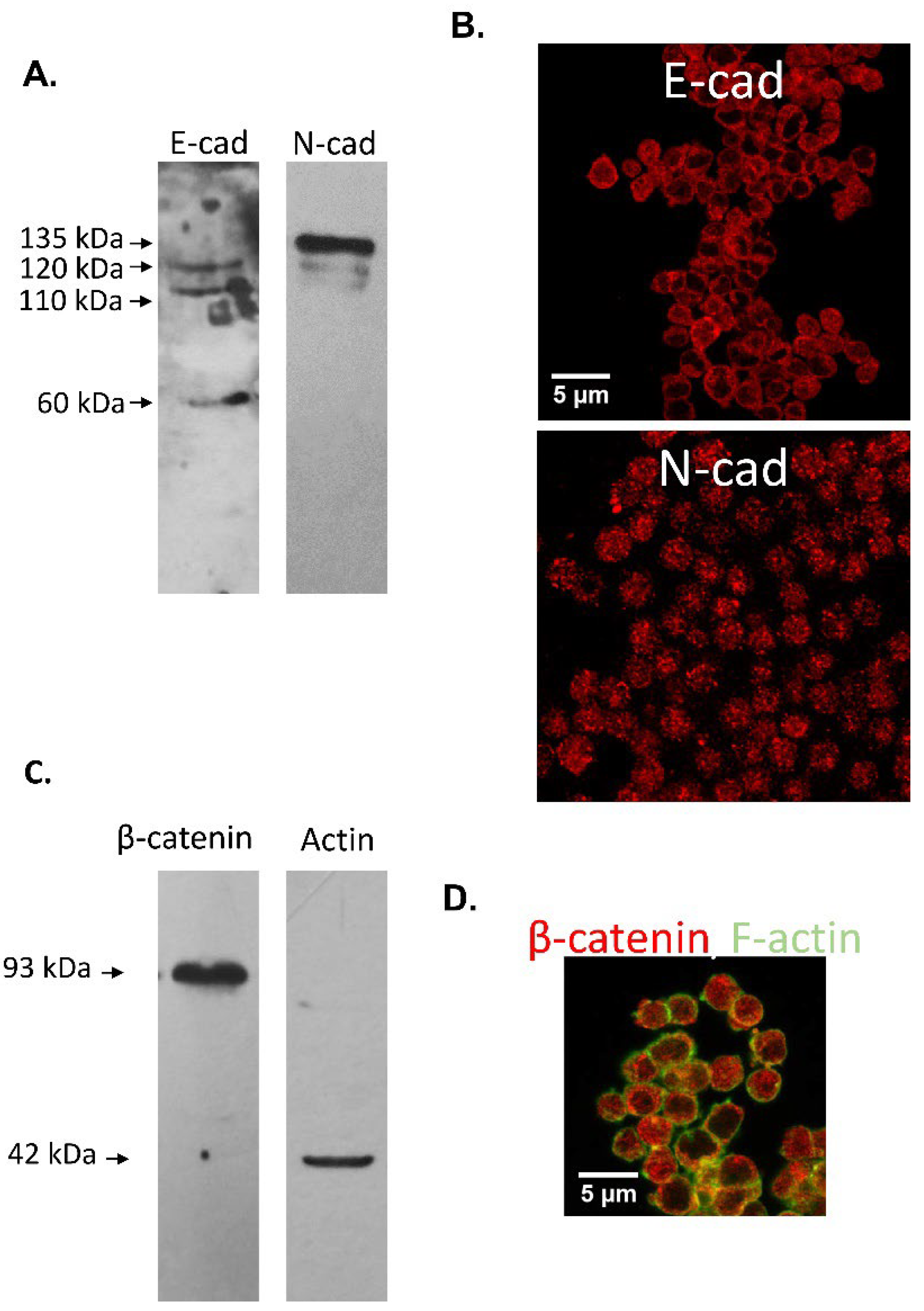
3.2. Immunodetection of E-cad and N-cad in Cumulus Oophorus Cells and Oocytes Recovered from Mature COCs
3.3. Effect of Anti-Cadherins Antibodies and Blocking Peptides upon Murine Fertilization
3.3.1. In Vitro Fertilization
3.3.2. Cumulus penetration and Sperm-Oocyte Interaction Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sullivan, R.; Légaré, C.; Lamontagne-Proulx, J.; Breton, S.; Soulet, D. Revisiting structure/functions of the human epididymis. Andrology 2019, 7, 748–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breton, S.; Nair, A.V.; Battistone, M.A. Epithelial dynamics in the epididymis: Role in the maturation, protection, and storage of spermatozoa. Andrology 2019, 7, 631–643. [Google Scholar] [CrossRef] [Green Version]
- Björkgren, I.; Sipilä, P. The impact of epididymal proteins on sperm function. Reproduction 2019, 158, R155–R167. [Google Scholar] [CrossRef] [Green Version]
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377. [Google Scholar] [CrossRef]
- De Jonge, C. Biological basis for human capacitation-revisited. Hum. Reprod. Update 2017, 23, 289–299. [Google Scholar] [CrossRef]
- Puga Molina, L.C.; Luque, G.M.; Balestrini, P.A.; Marín-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular Basis of Human Sperm Capacitation. Front. Cell Dev. Biol. 2018, 27, 6–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagimachi, R. Fertilization studies and assisted fertilization in mammals: Their development and future. J. Reprod. Dev. 2012, 58, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.P. Sperm-egg interaction. Annu. Rev. Physiol. 2012, 74, 477–502. [Google Scholar] [CrossRef]
- Wassarman, P.M. Reproductive biology: Sperm protein finds its mate. Nature 2014, 508, 466–467. [Google Scholar] [CrossRef]
- Bianchi, E.; Wright, G.J. Sperm Meets Egg: The Genetics of Mammalian Fertilization. Annu. Rev. Genet. 2016, 50, 93–111. [Google Scholar] [CrossRef]
- Weigel Muñoz, M.; Carvajal, G.; Curci, L.; Gonzalez, S.N.; Cuasnicu, P.S. Relevance of CRISP proteins for epididymal physiology, fertilization, and fertility. Andrology 2019, 7, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Piprek, R.P.; Kloc, M.; Mizia, P.; Kubiak, J.Z. The Central Role of Cadherins in Gonad Development, Reproduction, and Fertility. Int. J. Mol. Sci. 2020, 21, 8264. [Google Scholar] [CrossRef] [PubMed]
- Gahlay, G.K.; Rajput, N. The enigmatic sperm proteins in mammalian fertilization: An overview. Biol. Reprod. 2020, 103, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Deneke, V.E.; Pauli, A. The Fertilization Enigma: How Sperm and Egg Fuse. Annu. Rev. Cell Dev. Biol. 2021, 37, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Wright, G.J. Find and fuse: Unsolved mysteries in sperm-egg recognition. PLoS Biol. 2020, 18, e3000953. [Google Scholar] [CrossRef]
- Angst, B.D.; Marcozzi, C.; Magee, A.I. The cadherin superfamily: Diversity in form and function. J. Cell Sci. 2001, 114, 629–641. [Google Scholar] [CrossRef]
- Takeichi, M. Functional correlation between cell adhesive properties and some cell surface proteins. J. Cell Biol. 1977, 75, 464–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Roy, F.; Berx, G. The cell-cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. 2008, 65, 3756–3788. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, B.; Sato, Y.; Kameya, T.; Okayasu, I.; Mukai, K. Differential expression of N-cadherin and E-cadherin in normal human tissues. Arch. Histol. Cytol. 2006, 69, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Gumbiner, B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 622–634. [Google Scholar] [CrossRef]
- Hazan, R.B.; Qiao, R.; Keren, R.; Badano, I.; Suyama, K. Cadherin switch in tumor progression. Ann. N. Y. Acad. Sci. 2004, 1014, 155–163. [Google Scholar] [CrossRef]
- Halbleib, J.M.; Nelson, W.J. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006, 20, 3199–3214. [Google Scholar] [CrossRef] [Green Version]
- Takeichi, M. Self-organization of animal tissues: Cadherin-mediated processes. Dev. Cell 2011, 21, 24–26. [Google Scholar] [CrossRef] [Green Version]
- Arslan, F.N.; Eckert, J.; Schmidt, T.; Heisenberg, C.P. Holding it together: When cadherin meets cadherin. Biophys. J. 2021, 120, 4182–4192. [Google Scholar] [CrossRef]
- Gupta, S.; Yap, A.S. How adherens junctions move cells during collective migration. Fac. Rev. 2021, 10, 56. [Google Scholar] [CrossRef]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Niewiadomska, Z.; Dworecka-Kaszak, B.; Ngosa Toka, F.; Jurka, P. Role of Cadherins in Cancer-A Review. Int. J. Mol. Sci. 2020, 21, 7624. [Google Scholar] [CrossRef]
- Labernadie, A.; Kato, T.; Brugués, A.; Serra-Picamal, X.; Derzsi, S.; Arwert, E.; Weston, A.; González-Tarragó, V.; Elosegui-Artola, A.; Albertazzi, L.; et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 2017, 19, 224–237. [Google Scholar] [CrossRef]
- Finkelstein, M.; Etkovitz, N.; Breitbart, H. Ca2+ signaling in mammalian spermatozoa. Mol. Cell Endocrinol. 2020, 516, 110953. [Google Scholar] [CrossRef]
- Marín-Briggiler, C.I.; Veiga, M.F.; Matos, M.L.; Echeverría, M.F.; Furlong, L.I.; Vazquez-Levin, M.H. Expression of epithelial cadherin in the human male reproductive tract and gametes and evidence of its participation in fertilization. Mol. Hum. Reprod. 2008, 14, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Marín-Briggiler, C.I.; Lapyckyj, L.; González Echeverría, M.F.; Rawe, V.Y.; Alvarez Sedó, C.; Vazquez-Levin, M.H. Neural cadherin is expressed in human gametes and participates in sperm-oocyte interaction events. Int. J. Androl. 2010, 33, e228–e239. [Google Scholar] [CrossRef]
- Vazquez-Levin, M.H.; Marín-Briggiler, C.I.; Caballero, J.N.; Veiga, M.F. Epithelial and neural cadherin expression in the mammalian reproductive tract and gametes and their participation in fertilization-related events. Dev. Biol. 2015, 401, 2–16. [Google Scholar] [CrossRef] [Green Version]
- Bergin, E.; Levine, J.S.; Koh, J.S.; Lieberthal, W. Mouse proximal tubular cell-cell adhesion inhibits apoptosis by a cadherin-dependent mechanism. Am. J. Physiol. Renal. Physiol. 2000, 278, F758–F768. [Google Scholar] [CrossRef]
- Devemy, E.; Blaschuk, O.W. Identification of a novel N-cadherin antagonist. Peptides 2008, 29, 1853–1861. [Google Scholar] [CrossRef]
- Devemy, E.; Blaschuk, O.W. Identification of a novel dual E- and N-cadherin antagonist. Peptides 2009, 30, 1539–1547. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals: Washington, DC, USA, 2011. [Google Scholar]
- Veaute, C.; Furlong, L.I.; Cameo, M.; Harris, J.D.; Vazquez-Levin, M.H. Antiacrosin antibodies and infertility. II. Gene immunization with human proacrosin to assess the effect of immunity toward proacrosin/acrosin upon protein activities and animal fertility. Fertil. Steril. 2009, 91, 1256–1268. [Google Scholar] [CrossRef]
- Fraser, L.R.; Drury, L.M. The relationship between sperm concentration and fertilization in vitro of mouse eggs. Biol. Reprod. 1975, 13, 513–518. [Google Scholar] [CrossRef]
- Zahn, A.; Furlong, L.I.; Biancotti, J.C.; Ghiringhelli, P.D.; Marin-Briggiler, C.I.; Vazquez-Levin, M.H. Evaluation of the proacrosin/acrosin system and its mechanism of activation in human sperm extracts. J. Reprod. Immunol. 2002, 54, 43–63. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. 1979. Biotechnology 1992, 24, 145–149. [Google Scholar]
- Barraud-Lange, V.; Naud-Barriant, N.; Saffar, L.; Gattegno, L.; Ducot, B.; Drillet, A.S.; Bomsel, M.; Wolf, J.P.; Ziyyat, A. Alpha6beta1 integrin expressed by sperm is determinant in mouse fertilization. BMC Dev. Biol. 2007, 7, 102. [Google Scholar] [CrossRef] [Green Version]
- Battistone, M.A.; Alvau, A.; Salicioni, A.M.; Visconti, P.E.; Da Ros, V.G.; Cuasnicú, P.S. Evidence for the involvement of proline-rich tyrosine kinase 2 in tyrosine phosphorylation downstream of protein kinase A activation during human sperm capacitation. Mol. Hum. Reprod. 2014, 20, 1054–1066. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.B.; Mandal, A.; Digilio, L.C.; Coonrod, S.A.; Maier, B.; Herr, J.C. Mouse SLLP1, a sperm lysozyme-like protein involved in sperm-egg binding and fertilization. Dev. Biol. 2005, 284, 126–142. [Google Scholar] [CrossRef] [Green Version]
- Yoshida-Noro, C.; Suzuki, N.; Takeichi, M. Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev. Biol. 1984, 101, 19–27. [Google Scholar] [CrossRef]
- Shirayoshi, Y.; Okada, T.S.; Takeichi, M. The calcium-dependent cell-cell adhesion system regulates inner cell mass formation and cell surface polarization in early mouse development. Cell 1983, 35, 631–638. [Google Scholar] [CrossRef]
- Perrais, M.; Chen, X.; Perez-Moreno, M.; Gumbiner, B.M. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol. Biol. Cell 2007, 18, 2013–2025. [Google Scholar] [CrossRef] [Green Version]
- Gloushankova, N.A.; Rubtsova, S.N.; Zhitnyak, I.Y. Cadherin-mediated cell-cell interactions in normal and cancer cells. Tissue Barriers 2017, 5, e1356900. [Google Scholar] [CrossRef] [Green Version]
- Ziv, S.; Rufas, O.; Shalgi, R. Cadherins expression during gamete maturation and fertilization in the rat. Mol. Reprod. Dev. 2002, 62, 547–556. [Google Scholar] [CrossRef]
- Tsuiki, A.; Hoshiai, H.; Takahashi, K.; Suzuki, M.; Hoshi, K. Sperm-egg interactions observed by scanning electron microscopy. Arch. Androl. 1986, 16, 35–47. [Google Scholar] [CrossRef]
- Fléchon, J.E.; Pavlok, A. Ultrastructural study of the interactions and fusion of ram spermatozoa with zona-free hamster oocytes. Reprod. Nutr. Dev. 1986, 26, 999–1008. [Google Scholar] [CrossRef]
- Hyttel, P.; Xu, K.P.; Greve, T. Scanning electron microscopy of in vitro fertilization in cattle. Anat. Embryol. 1988, 178, 41–46. [Google Scholar] [CrossRef]
- Kadam, A.L.; Fateh, M.; Naz, R.K. Fertilization antigen (FA-1) completely blocks human sperm binding to human zona pellucida: FA-1 antigen may be a sperm receptor for zona pellucida in humans. J. Reprod. Immunol. 1995, 29, 19–30. [Google Scholar] [CrossRef]
- Frolikova, M.; Manaskova-Postlerova, P.; Cerny, J.; Jankovicova, J.; Simonik, O.; Pohlova, A.; Secova, P.; Antalikova, J.; Dvorakova-Hortova, K. CD9 and CD81 Interactions and Their Structural Modelling in Sperm Prior to Fertilization. Int. J. Mol. Sci. 2018, 19, 1236. [Google Scholar] [CrossRef] [Green Version]
- Erikson, D.W.; Way, A.L.; Chapman, D.A.; Killian, G.J. Detection of osteopontin on Holstein bull spermatozoa, in cauda epididymal fluid and testis homogenates, and its potential role in bovine fertilization. Reproduction 2007, 133, 909–917. [Google Scholar] [CrossRef]
- Ying, X.; Liu, Y.; Guo, Q.; Qu, F.; Guo, W.; Zhu, Y.; Ding, Z. Endoplasmic reticulum protein 29 (ERp29), a protein related to sperm maturation is involved in sperm-oocyte fusion in mouse. Reprod. Biol. Endocrinol. 2010, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.; Kashir, J.; Thanassoulas, A.; Safieh-Garabedian, B.; Lai, F.A.; Nomikos, M. Essential Role of Sperm-Specific PLC-Zeta in Egg Activation and Male Factor Infertility: An Update. Front. Cell Dev. Biol. 2020, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Gianzo, M.; Urizar-Arenaza, I.; Muñoa-Hoyos, I.; Larreategui, Z.; Garrido, N.; Irazusta, J.; Subirán, N. (Pro)renin Receptor Is Present in Human Sperm and It Adversely Affects Sperm Fertility Ability. Int. J. Mol. Sci. 2021, 22, 3215. [Google Scholar] [CrossRef]
- Miro-Moran, A.; Jardin, I.; Ortega-Ferrusola, C.; Salido, G.M.; Peña, F.J.; Tapia, J.A.; Aparicio, I.M. Identification and function of exchange proteins activated directly by cyclic AMP (Epac) in mammalian spermatozoa. PLoS ONE 2012, 7, e37713. [Google Scholar]
- Satouh, Y.; Inoue, N.; Ikawa, M.; Okabe, M. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J. Cell Sci. 2012, 125 Pt 21, 4985–4990. [Google Scholar] [CrossRef] [Green Version]
- Yamatoya, K.; Kousaka, M.; Ito, C.; Nakata, K.; Hatano, M.; Araki, Y.; Toshimori, K. Cleavage of SPACA1 regulates assembly of sperm-egg membrane fusion machinery in mature spermatozoa. Biol. Reprod. 2020, 102, 750–757. [Google Scholar] [CrossRef]
- Lamas-Toranzo, I.; Hamze, J.G.; Bianchi, E.; Fernández-Fuertes, B.; Pérez-Cerezales, S.; Laguna-Barraza, R.; Fernández-González, R.; Lonergan, P.; Gutiérrez-Adán, A.; Wright, G.J.; et al. TMEM95 is a sperm membrane protein essential for mammalian fertilization. eLife 2020, 9, e53913. [Google Scholar] [CrossRef]
- Fujihara, Y.; Lu, Y.; Noda, T.; Oji, A.; Larasati, T.; Kojima-Kita, K.; Yu, Z.; Matzuk, R.M.; Matzuk, M.M.; Ikawa, M. Spermatozoa lacking Fertilization Influencing Membrane Protein (FIMP) fail to fuse with oocytes in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 9393–9400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, T.; Lu, Y.; Fujihara, Y.; Oura, S.; Koyano, T.; Kobayashi, S.; Matzuk, M.M.; Ikawa, M. Sperm proteins SOF1, TMEM95, and SPACA6 are required for sperm-oocyte fusion in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 11493–11502. [Google Scholar] [CrossRef]
- Larue, L.; Ohsugi, M.; Hirchenhain, J.; Kemler, R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc. Natl. Acad. Sci. USA 1994, 91, 8263–8267. [Google Scholar] [CrossRef] [Green Version]
- Radice, G.L.; Rayburn, H.; Matsunami, H.; Knudsen, K.A.; Takeichi, M.; Hynes, R.O. Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol. 1997, 181, 64–78. [Google Scholar] [CrossRef]
- Brener, E.; Rubinstein, S.; Cohen, G.; Shternall, K.; Rivlin, J.; Breitbart, H. Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol. Reprod. 2003, 68, 837–845. [Google Scholar] [CrossRef]
- Breitbart, H.; Finkelstein, M. Actin cytoskeleton and sperm function. Biochem. Biophys. Res. Commun. 2018, 506, 372–377. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, L.; Shi, D.S.; Jiang, J.R. Effects of latrunculin A on the relocation of sperm IZUMO1 during gamete interaction in mouse. Mol. Reprod. Dev. 2017, 84, 1183–1190. [Google Scholar] [CrossRef]
- Kumakiri, J.; Oda, S.; Kinoshita, K.; Miyazaki, S. Involvement of Rho family G protein in the cell signaling for sperm incorporation during fertilization of mouse eggs: Inhibition by Clostridium difficile toxin B. Dev. Biol. 2003, 260, 522–535. [Google Scholar] [CrossRef] [Green Version]
- Steinbacher, T.; Ebnet, K. The regulation of junctional actin dynamics by cell adhesion receptors. Histochem. Cell Biol. 2018, 150, 341–350. [Google Scholar] [CrossRef]
- Rufas, O.; Fisch, B.; Ziv, S.; Shalgi, R. Expression of cadherin adhesion molecules on human gametes. Mol. Hum. Reprod. 2000, 6, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Roy, S.K. Expression of E-cadherin and N-cadherin in perinatal hamster ovary: Possible involvement in primordial follicle formation and regulation by follicle-stimulating hormone. Endocrinology 2010, 151, 2319–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertoldo, M.J.; Guibert, E.; Faure, M.; Ramé, C.; Foretz, M.; Viollet, B.; Dupont, J.; Froment, P. Specific deletion of AMP-activated protein kinase (α1AMPK) in murine oocytes alters junctional protein expression and mitochondrial physiology. PLoS ONE 2015, 10, e0119680. [Google Scholar] [CrossRef] [Green Version]
- Nose, A.; Tsuji, K.; Takeichi, M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell 1990, 61, 147–155. [Google Scholar] [CrossRef]
- McClure, M.J.; Ramey, A.N.; Rashid, M.; Boyan, B.D.; Schwartz, Z. Integrin-α7 signaling regulates connexin 43, M-cadherin, and myoblast fusion. Am. J. Physiol. Cell Physiol. 2019, 316, C876–C887. [Google Scholar] [CrossRef]
- Manibog, K.; Sankar, K.; Kim, S.A.; Zhang, Y.; Jernigan, R.L.; Sivasankar, S. Molecular determinants of cadherin ideal bond formation: Conformation-dependent unbinding on a multidimensional landscape. Proc. Natl. Acad. Sci. USA 2016, 113, E5711–E5720. [Google Scholar] [CrossRef] [Green Version]
- Miron, R.J.; Hedbom, E.; Ruggiero, S.; Bosshardt, D.D.; Zhang, Y.; Mauth, C.; Gemperli, A.C.; Iizuka, T.; Buser, D.; Sculean, A. Premature osteoblast clustering by enamel matrix proteins induces osteoblast differentiation through up-regulation of connexin 43 and N-cadherin. PLoS ONE 2011, 6, e23375. [Google Scholar]
- Iwahashi, N.; Ikezaki, M.; Matsuzaki, I.; Yamamoto, M.; Toujima, S.; Murata, S.I.; Ihara, Y.; Ino, K. Calreticulin Regulates Syncytialization Through Control of the Synthesis and Transportation of E-Cadherin in BeWo Cells. Endocrinology 2019, 160, 359–374. [Google Scholar] [CrossRef] [Green Version]
- Aghababaei, M.; Hogg, K.; Perdu, S.; Robinson, W.P.; Beristain, A.G. ADAM12-directed ectodomain shedding of E-cadherin potentiates trophoblast fusion. Cell Death Differ. 2015, 22, 1970–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pey, R.; Vial, C.; Schatten, G.; Hafner, M. Increase of intracellular Ca2+ and relocation of E-cadherin during experimental decompaction of mouse embryos. Proc. Natl. Acad. Sci. USA 1998, 95, 12977–12982. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Okamoto, I.; Araki, N.; Kawano, Y.; Nakao, M.; Fujiyama, S.; Tomita, K.; Mimori, T.; Saya, H. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of beta-catenin from cell-cell contacts. Oncogene 1999, 18, 7080–7090. [Google Scholar] [CrossRef] [Green Version]
- Ichinose, S.; Ogawa, T.; Hirokawa, N. Mechanism of Activity-Dependent Cargo Loading via the Phosphorylation of KIF3A by PKA and CaMKIIa. Neuron 2015, 87, 1022–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takezawa, Y.; Yoshida, K.; Miyado, K.; Sato, M.; Nakamura, A.; Kawano, N.; Sakakibara, K.; Kondo, T.; Harada, Y.; Ohnami, N.; et al. β-catenin is a molecular switch that regulates transition of cell-cell adhesion to fusion. Sci Rep 2011, 1, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Savagner, P. Epithelial-mesenchymal transitions: From cell plasticity to concept elasticity. Curr. Top. Dev. Biol. 2015, 112, 273–300. [Google Scholar] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yi, B.R.; Kim, N.H.; Choi, K.C. Role of the epithelial–mesenchymal transition and its effects on embryonic stem cells. Exp. Mol. Med. 2014, 46, e108. [Google Scholar] [CrossRef] [Green Version]
- Ismagulov, G.; Hamidi, S.; Sheng, G. Epithelial-Mesenchymal Transition Drives Three-Dimensional Morphogenesis in Mammalian Early Development. Front. Cell Dev. Biol. 2021, 9, 639244. [Google Scholar] [CrossRef]
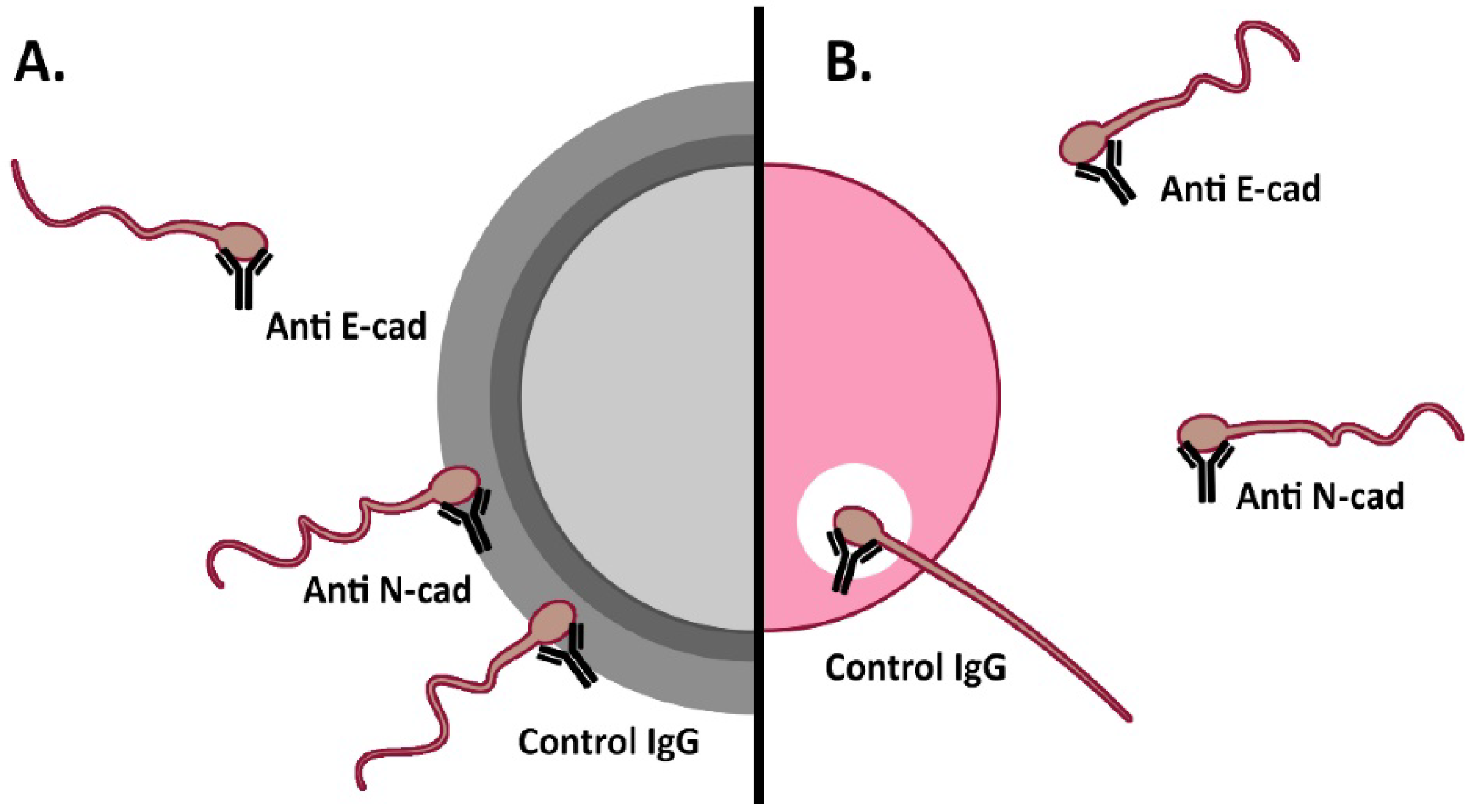

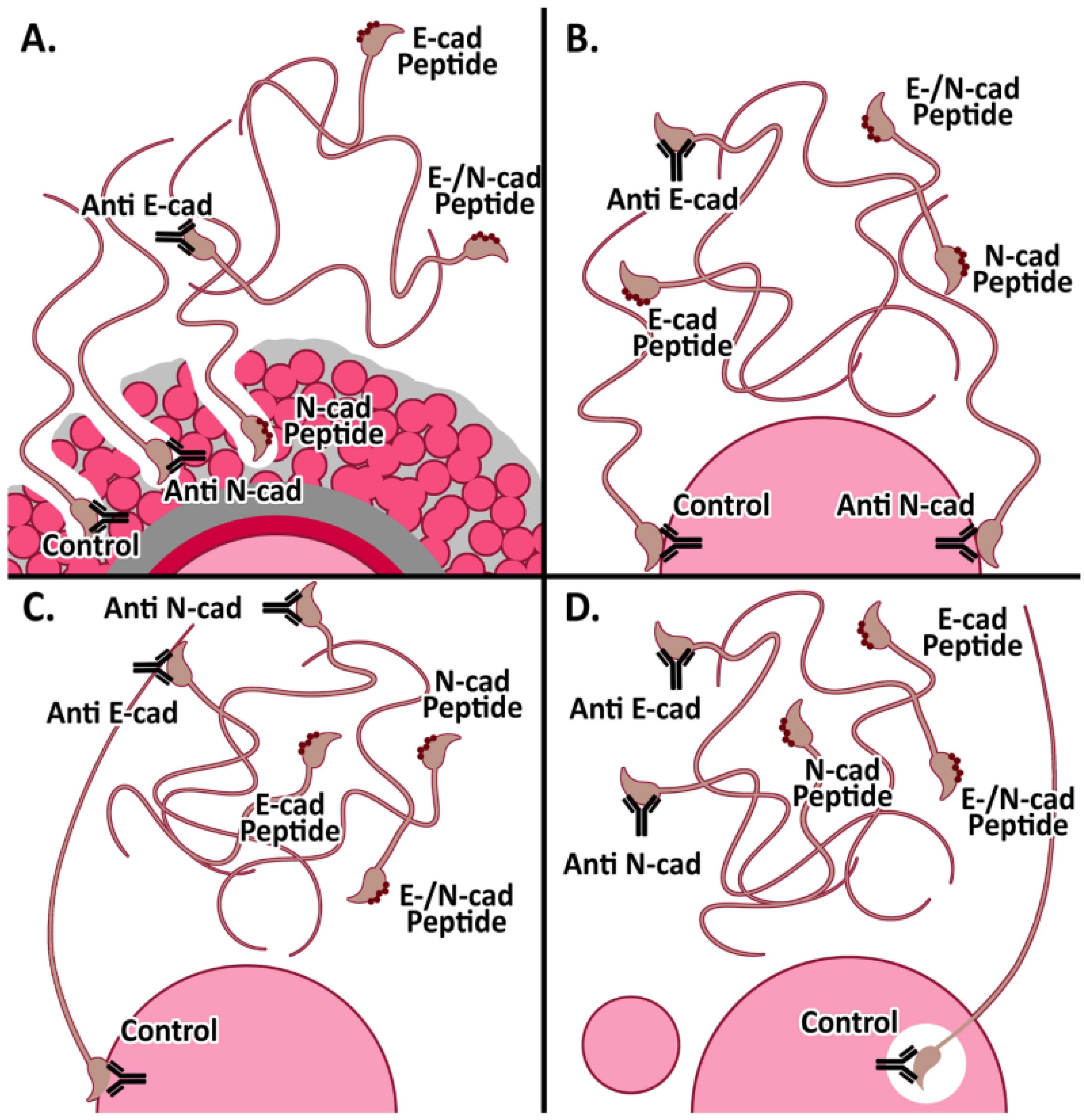
| A. | |||
| E-cad | A + PA | PA | N |
| Intact Non-Capacitated | 90.3 ± 5.7 | 8.0 ± 4.6 | 1.7 ± 1.2 |
| Intact Capacitated | 69.0 ± 6.0 | 22.7 ± 6.7 | 8.3 ± 1.5 |
| A23187 Ca2+-ionophore acrosome-reacted | 20.0 ± 2.7 | 75.3 ± 7.4 | 4.7 ± 4.7 |
| B. | |||
| N-cad | A | A + ES | ES |
| Intact Non-Capacitated | 39.8 ± 19.8 | 51.8 ± 15.6 | 8.4 ±6.0 |
| Intact Capacitated | 40.1 ± 17.9 | 40.6 ± 12.1 | 19.3 ± 6.0 |
| A23187 Ca2+-ionophore acrosome-reacted | 15.9 ± 5.2 | 17.2 ± 7.7 | 66.9 ± 10.0 |
| IVF | Control (%) | Pre-Treated (%) |
|---|---|---|
| COC E-cad Antibody | 66.6 ± 4.4 (n = 117) | 31.8 ± 7.2 * (n = 123) |
| N-cadAntibody | 43.8 ± 9.1 (n = 29) | 14.3 ± 8.3 ** (n = 40) |
| Cumulus-free Oocytes | ||
| E-cad Antibody | 57.5 ± 36.5 (n = 93) | 11.0 ± 1.6 * (n = 95) |
| N-cad Antibody | 56.4 ± 6.1 (n = 30) | 13.9 ± 2.8 ** (n = 30) |
| ZP-Free Oocytes IVF | Control (%) | Pre-Treated (%) |
|---|---|---|
| E-cad Antibody | 58.1 ± 1.0 (n = 24) | 24.5 ± 2.5 * (n = 25) |
| N-cadAntibody | 72.6 ± 6.3 (n = 29) | 27.8 ± 5.6 ** (n = 30) |
| E-cad Peptide | 50.9 ± 4.8 (n = 49) | 18.1 ± 5.9 *** (n = 55) |
| N-cadPeptide | 57.5 ± 8.5 (n = 48) | 24.3 ± 8.2 ** (n = 46) |
| E-/N-cad Dual Peptide | 69.1 ± 6.4 (n = 51) | 22.8 ± 8.6 *** (n = 45) |
| Cumulus Penetration Assay | Control (# Spermatozoa) | Pre-Treated (# Spermatozoa) |
|---|---|---|
| E-cad Antibody | 32.9 ± 2.0 (n = 16) | 16.8 ± 1.0 *** (n = 13) |
| N-cad Antibody | 32.9 ± 2.0 (n = 16) | 29.8 ± 2.3 (n = 17) |
| E-cad Peptide | 38.3 ± 2.0 (n = 32) | 27.3 ± 2.9 ** (n = 29) |
| N-cad Peptide | 32.1 ± 7.0 (n = 27) | 31.9 ± 14.5 (n = 30) |
| E-cad/N-cad Dual Peptide | 43.3 ± 2.4 (n = 28) | 33.6 ± 2.2 * (n = 25) |
| Oolemma Binding | Control (# Spermatozoa) | Pre-treated (# Spermatozoa) |
|---|---|---|
| E-cad Antibody | 25.8 ± 1.1 (n = 70) | 11.5 ± 1.0 *** (n = 60) |
| N-cad Antibody | 18.9 ± 1.6 (n = 54) | 15.8 ± 1.3 (n = 63) |
| E-cad Peptide | 9.0 ± 0.8 (n = 50) | 2.4 ± 0.6*** (n = 52) |
| N-cad Peptide | 14.2 ± 1.3 (n = 59) | 6.8 ± 1.7*** (n = 54) |
| E-cad/N-cad Dual Peptide | 14.5 ± 1.2 (n = 56) | 3.6 ± 0.6*** (n = 56) |
| Sperm-oocyte Fusion | Control (%) | Pre-Treated (%) |
|---|---|---|
| E-cad Antibody | 91.3 ± 4.2 (n = 52) | 24.3 ± 14.6 *** (n = 40) |
| N-cad Antibody | 73.7 ± 3.5 (n = 44) | 44.3 ± 9.9 * (n = 63) |
| E-cad Peptide | 76.5 ± 7.6 (n = 50) | 33.9 ± 7.5 *** (n = 52) |
| N-cad Peptide | 81.0 ± 6.6 (n = 59) | 28.3 ± 4.8 *** (n = 54) |
| E-cad/N-cad Dual Peptide | 75.9 ± 2.2 (n = 56) | 25.2 ± 10.3 *** (n = 56) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verón, G.L.; Veiga, M.F.; Cameo, M.; Marín-Briggiler, C.I.; Vazquez-Levin, M.H. Epithelial and Neural Cadherin in Mammalian Fertilization: Studies in the Mouse Model. Cells 2022, 11, 102. https://doi.org/10.3390/cells11010102
Verón GL, Veiga MF, Cameo M, Marín-Briggiler CI, Vazquez-Levin MH. Epithelial and Neural Cadherin in Mammalian Fertilization: Studies in the Mouse Model. Cells. 2022; 11(1):102. https://doi.org/10.3390/cells11010102
Chicago/Turabian StyleVerón, Gustavo Luis, María Florencia Veiga, Mónica Cameo, Clara Isabel Marín-Briggiler, and Mónica Hebe Vazquez-Levin. 2022. "Epithelial and Neural Cadherin in Mammalian Fertilization: Studies in the Mouse Model" Cells 11, no. 1: 102. https://doi.org/10.3390/cells11010102
APA StyleVerón, G. L., Veiga, M. F., Cameo, M., Marín-Briggiler, C. I., & Vazquez-Levin, M. H. (2022). Epithelial and Neural Cadherin in Mammalian Fertilization: Studies in the Mouse Model. Cells, 11(1), 102. https://doi.org/10.3390/cells11010102






