Selectivity of mTOR-Phosphatidic Acid Interactions Is Driven by Acyl Chain Structure and Cholesterol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies, Chemicals, Reagents
2.2. DNA Constructs
2.3. Expression and Purification of Recombinant Proteins
2.4. Preparation of Large Unilamellar Vesicles (LUVs)
2.5. Flotation Assay
2.6. Preparation of Giant Unilamellar Vesicles (GUVs)
2.7. GUV Binding Assay and Image Quantification
2.8. BLI Measurements
2.9. BLI Data Analysis
3. Results
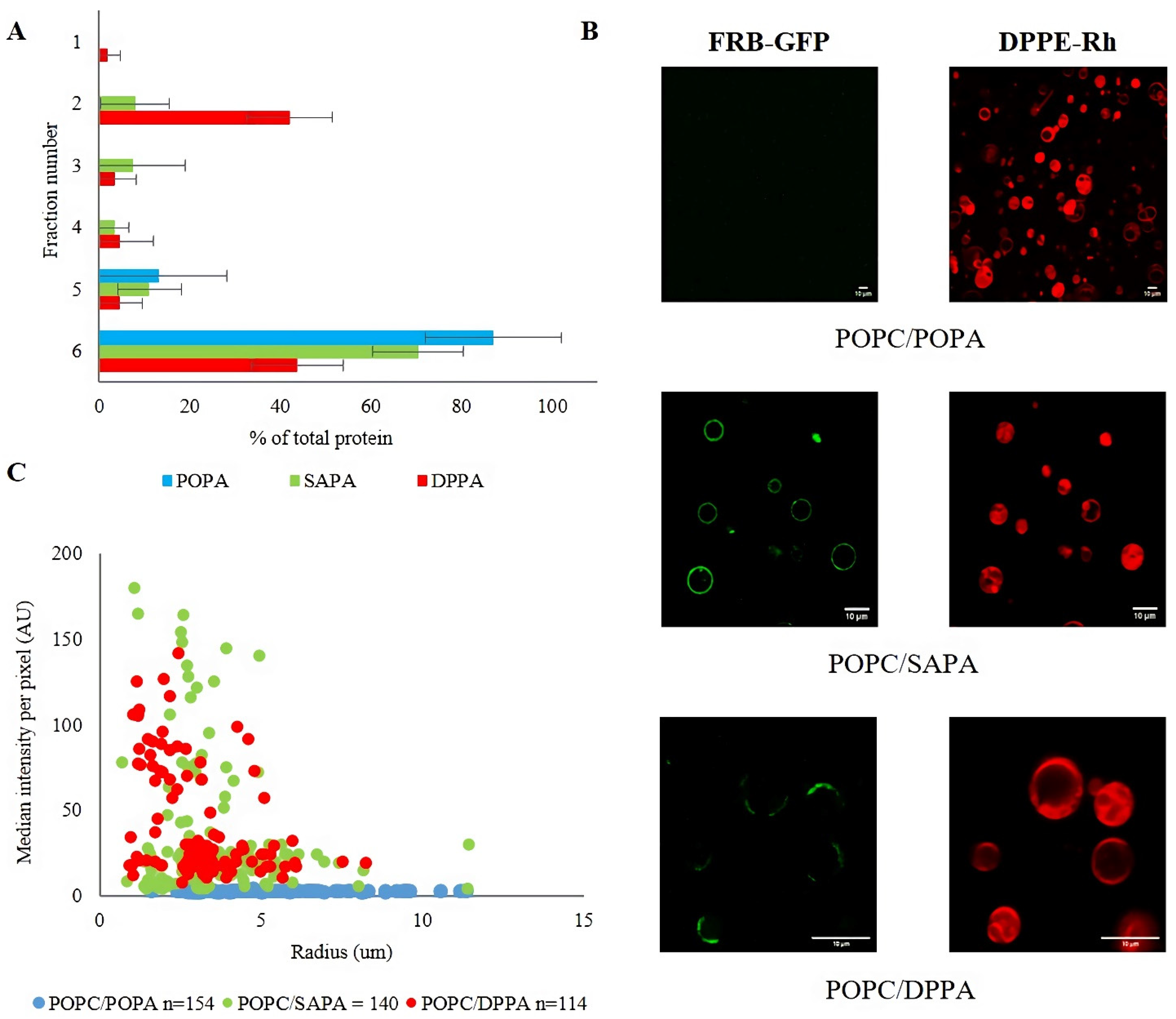
3.1. Structure of Acyl Chains of PA Determines Binding Specificity of mTOR FRB Domain
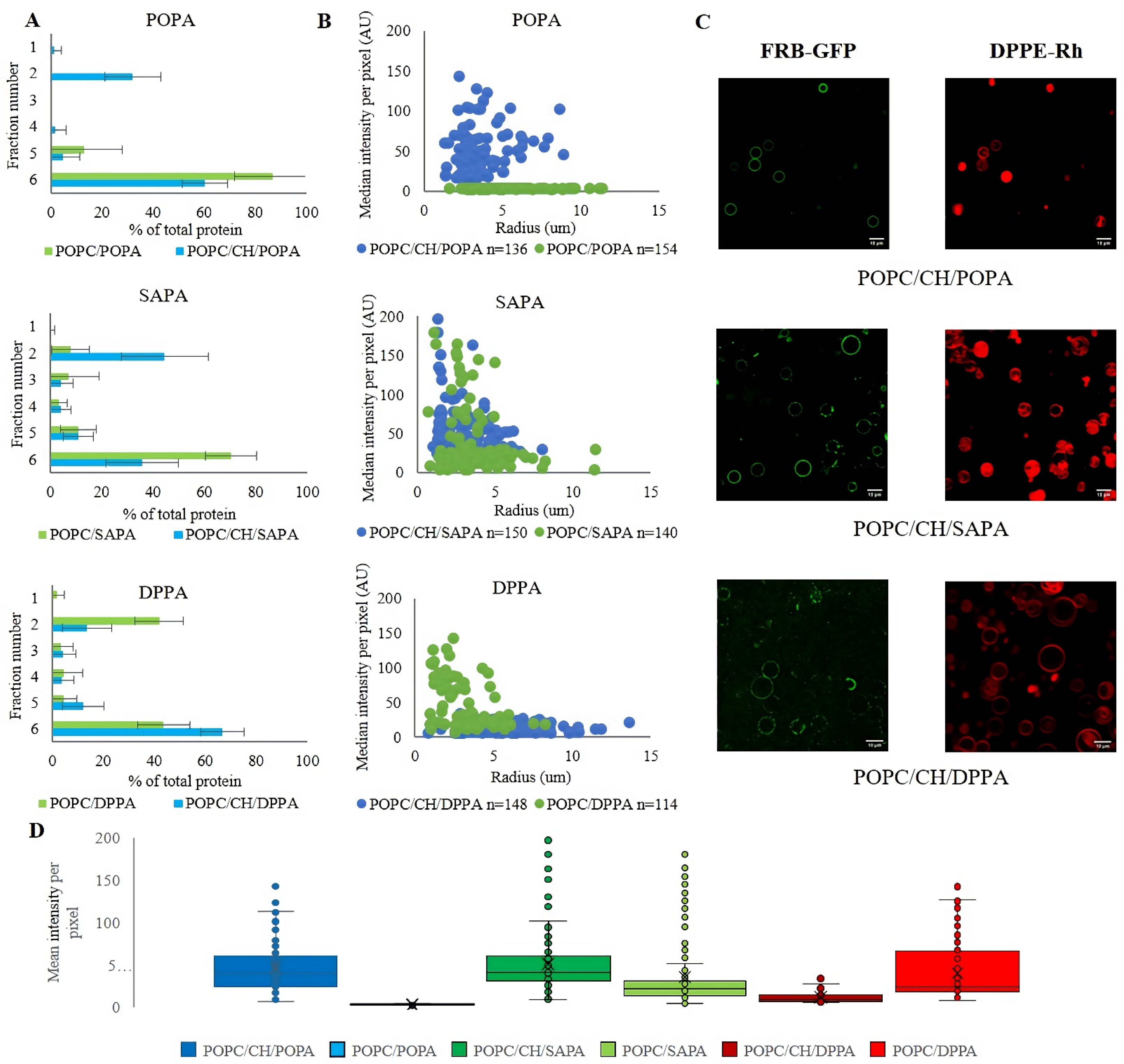
3.2. Effect of Cholesterol on FRB-PA Binding
3.3. Kinetic Analysis of FRB–PA Interaction by BLI Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Czogalla, A.; Grzybek, M.; Jones, W.; Coskun, Ü. Validity and applicability of membrane model systems for studying interactions of peripheral membrane proteins with lipids. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2014, 1841, 1049–1059. [Google Scholar] [CrossRef]
- Lingwood, D.; Binnington, B.; Róg, T.; Vattulainen, I.; Grzybek, M.; Coskun, Ü.; Lingwood, C.A.; Simons, K. Cholesterol modulates glycolipid conformation and receptor activity. Nat. Chem. Boil. 2011, 7, 5–7. [Google Scholar] [CrossRef]
- MPizzuto, M.; Lonez, C.; Baroja-Mazo, A.; Martínez-Banaclocha, H.; Tourlomousis, P.; Gangloff, M.; Pelegrin, P.; Ruysschaert, J.-M.; Gay, N.J.; Bryant, C.E. Saturation of acyl chains converts cardiolipin from an antagonist to an activator of Toll-like receptor-4. Cell. Mol. Life Sci. 2019, 76, 3667–3678. [Google Scholar] [CrossRef] [Green Version]
- Van Galen, J.; Van Balkom, B.W.M.; Serrano, R.L.; Kaloyanova, D.; Eerland, R.; Stüven, E.; Helms, J.B. Binding of GAPR-1 to negatively charged phospholipid membranes: Unusual binding characteristics to phosphatidylinositol. Mol. Membr. Biol. 2010, 27, 81–91. [Google Scholar] [CrossRef]
- Weise, C.F.; Login, F.H.; Ho, O.; Gröbner, G.; Wolf-Watz, H.; Wolf-Watz, M. Negatively charged lipid membranes promote a disorder-order transition in the Yersinia YscU protein. Biophys. J. 2014, 107, 1950–1961. [Google Scholar] [CrossRef] [Green Version]
- Ouberai, M.M.; Wang, J.; Swann, M.J.; Galvagnion, C.; Guilliams, T.; Dobson, C.M.; Welland, M.E. α-Synuclein senses lipid packing defects and induces lateral expansion of lipids leading to membrane remodeling. J. Biol. Chem. 2013, 288, 20883–20895. [Google Scholar] [CrossRef] [Green Version]
- Drin, G.; Antonny, B. Amphipathic helices and membrane curvature. FEBS Lett. 2010, 584, 1840–1847. [Google Scholar] [CrossRef]
- Antonny, B. Mechanisms of membrane curvature sensing. Annu. Rev. Biochem. 2011, 80, 101–123. [Google Scholar] [CrossRef]
- Balla, T. Inositol-lipid binding motifs: Signal integrators through protein-lipid and protein-protein interactions. J. Cell Sci. 2005, 118, 2093–2104. [Google Scholar] [CrossRef] [Green Version]
- Sakane, F.; Hoshino, F.; Murakami, C. New era of diacylglycerol kinase, phosphatidic acid and phosphatidic acid-binding protein. Int. J. Mol. Sci. 2020, 21, 6794. [Google Scholar] [CrossRef]
- Wang, X.; Devaiah, S.P.; Zhang, W.; Welti, R. Signaling functions of phosphatidic acid. Prog. Lipid Res. 2006, 45, 250–278. [Google Scholar] [CrossRef]
- Testerink, C.; Munnik, T. Phosphatidic acid: A multifunctional stress signaling lipid in plants. Trends Plant Sci. 2005, 10, 368–375. [Google Scholar] [CrossRef]
- Stace, C.L.; Ktistakis, N.T. Phosphatidic acid- and phosphatidylserine-binding proteins. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2006, 1761, 913–926. [Google Scholar] [CrossRef]
- Tanguy, E.; Kassas, N.; Vitale, N. Protein–Phospholipid Interaction Motifs: A Focus on Phosphatidic Acid. Biomolecules 2018, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Zegarlińska, J.; Piaścik, M.; Sikorski, A.F.; Czogalla, A. Phosphatidic acid—A simple phospholipid with multiple faces. Acta Biochim. Pol. 2018, 65, 163–171. [Google Scholar] [CrossRef]
- Buckland, A.G.; Wilton, D.C. Anionic phospholipids, interfacial binding and the regulation of cell functions. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2000, 1483, 199–216. [Google Scholar] [CrossRef]
- Carman, G.M.; Henry, S.A. Phosphatidic Acid Plays a Central Role in the Transcriptional Regulation of Glycerophospholipid Synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 2013, 282, 37293–37297. [Google Scholar] [CrossRef] [Green Version]
- Pleskot, R.; Li, J.; Žárský, V.; Potocký, M.; Staiger, C.J. Regulation of cytoskeletal dynamics by phospholipase D and phosphatidic acid. Trends Plant Sci. 2013, 18, 496–504. [Google Scholar] [CrossRef]
- Jang, J.-H.; Lee, C.S.; Hwang, D.; Ryu, S.H. Understanding of the roles of phospholipase D and phosphatidic acid through their binding partners. Prog. Lipid Res. 2012, 51, 71–81. [Google Scholar] [CrossRef]
- Burger, K.N.J.; Demel, R.A.; Schmid, S.L.; de Kruijff, B. Dynamin is membrane-active: Lipid insertion is induced by phosphoinositides and phosphatidic acid. Biochemistry 2000, 39, 12485–12493. [Google Scholar] [CrossRef]
- daCosta, C.J.B.; Wagg, I.D.; McKay, M.E.; Baenziger, J.E. Phosphatidic acid and phosphatidylserine have distinct structural and functional interactions with the nicotinic acetylcholine receptor. J. Biol. Chem. 2004, 279, 14967–14974. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Hirakawa, K.; Horiuchi, H.; Fukuda, R.; Ohta, A. Phosphatidic acid and phosphoinositides facilitate liposome association of Yas3p and potentiate derepression of ARE1 (alkane-responsive element one)-mediated transcription control. Fungal Genet. Biol. 2013, 61, 100–110. [Google Scholar] [CrossRef]
- Horchani, H.; de Saint-Jean, M.; Barelli, H.L.H.L.; Antonny, B. Interaction of the Spo20 membrane-sensor motif with phosphatidic acid and other anionic lipids, and influence of the membrane environment. PLoS ONE 2014, 9, e113484. [Google Scholar] [CrossRef] [Green Version]
- Young, B.P.; Shin, J.J.H.; Orij, R.; Chao, J.T.; Li, S.C.; Guan, X.L.; Khong, A.; Jan, E.; Wenk, M.R.; Prinz, W.A.; et al. Phosphatidic acid is a pH biosensor that links membrane biogenesis to metabolism. Science 2010, 329, 1085–1088. [Google Scholar] [CrossRef]
- Eaton, J.M.; Mullins, G.R.; Brindley, D.N.; Harris, T.E. Phosphorylation of lipin 1 and charge on the phosphatidic acid head group control its phosphatidic acid phosphatase activity and membrane association. J. Biol. Chem. 2013, 288, 9933–9945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Bell, R.M. Regulation of raf-1 kinase by interaction with the lipid second messenger, phosphatidic acid. Biochem. Soc. Trans. 1997, 25, 561–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooijman, E.E.; Burger, K.N.J. Biophysics and function of phosphatidic acid: A molecular perspective. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2009, 1791, 881–888. [Google Scholar] [CrossRef]
- EKooijman, E.E.; Tieleman, D.P.; Testerink, C.; Munnik, T.; Rijkers, D.T.; Burger, K.N.; de Kruijff, B. An electrostatic/hydrogen bond switch as the basis for the specific interaction of phosphatidic acid with proteins. J. Biol. Chem. 2007, 282, 11356–11364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keith, C.T.; Schreiber, S.L. PIK-related kinases: DNA repair, recombination, and cell cycle checkpoints. Science 1995, 270, 50–51. [Google Scholar] [CrossRef]
- Betz, C.; Hall, M.N. Where is mTOR and what is it doing there? J. Cell Biol. 2013, 203, 563–574. [Google Scholar] [CrossRef] [Green Version]
- Laplante, M.; Sabatini, D.M. mTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [Green Version]
- Kim, L.C.; Cook, R.S.; Chen, J. mTORC1 and mTORC2 in cancer and the tumor microenvironment Laura. Oncogene 2017, 36, 2191–2201. [Google Scholar] [CrossRef] [Green Version]
- Oh, W.J.; Jacinto, E. mTOR complex 2 signaling and functions. Cell Cycle 2011, 10, 2305–2316. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Howell, J.J.; Asara, J.M.; Manning, B.D. Stimulation of de Novo Pyrimidine Synthesis by Growth Signaling Through mTOR and S6K1. Science 2013, 339, 1323–1328. [Google Scholar] [CrossRef] [Green Version]
- Hagiwara, A.; Cornu, M.; Cybulski, N.; Polak, P.; Betz, C.; Trapani, F.; Terracciano, L.; Heim, M.; Rüegg, M.A.; Hall, M.N. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 2012, 15, 725–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L.; Sabatini, D.M. The rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Peled, L.; Chantranupong, L.; Cherniack, A.D.; Chen, W.W.; Ottina, K.A.; Grabiner, B.C.; Spear, E.D.; Carter, S.L.; Meyerson, M.; Sabatini, D.M. A Tumor Suppressor Complex with GAP Activity for the Rag GTPases That Signal Amino Acid Sufficiency to mTORC1. Science 2013, 340, 1100–1106. [Google Scholar] [CrossRef] [Green Version]
- Ebner, M.; Sinkovics, B.; Szczygieł, M.; Ribeiro, D.W.; Yudushkin, I. Localization of mTORC2 activity inside cells. J. Cell Biol. 2017, 216, 343–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, D.A. Phosphatidic acid signaling to mTOR: Signals for the survival of human cancer cells. Biochim. Biophys. Acta 2009, 1791, 949–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila-Flores, A.; Santos, T.; Rincón, E.; Mérida, I. Modulation of the mammalian target of rapamycin pathway by diacylglycerol kinase-produced phosphatidic acid. J. Biol. Chem. 2005, 280, 10091–10099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gingras, A.; Raught, B.; Sonenberg, N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001, 15, 807–826. [Google Scholar] [CrossRef] [Green Version]
- Foster, D.A. Regulation of mTOR by phosphatidic acid? Cancer Res. 2007, 67, 1–4. [Google Scholar] [CrossRef] [Green Version]
- You, J.S.; Frey, J.W.; Hornberger, T.A. Mechanical Stimulation Induces mTOR Signaling via an ERK-Independent Mechanism: Implications for a Direct Activation of mTOR by Phosphatidic Acid. PLoS ONE 2012, 7, e47258. [Google Scholar]
- O’neil, T.K.; Duffy, L.R.; Frey, J.W.; Hornberger, T.A. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J. Physiol. 2009, 587, 3691–3701. [Google Scholar] [CrossRef]
- Fang, Y.; Vilella-Bach, M.; Bachmann, R.; Flanigan, A.; Chen, J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 2001, 294, 1942–1945. [Google Scholar] [CrossRef]
- Ballou, L.M.; Jiang, Y.P.; Du, G.; Frohman, M.A.; Lin, R.Z. Ca2+- and phospholipase D-dependent and -independent pathways activate mTOR signaling. FEBS Lett. 2003, 550, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.H.; Kim, D.-H.; Kim, I.-S.; Kim, J.H.; Lee, M.N.; Lee, H.J.; Kim, J.H.; Jang, S.K.; Suh, P.-G.; Ryu, S.H. PLD2 forms a functional complex with mTOR/raptor to transduce mitogenic signals. Cell. Signal. 2006, 18, 2283–2291. [Google Scholar] [CrossRef]
- Takahara, T.; Hara, K.; Yonezawa, K.; Sorimachi, H.; Maeda, T. Nutrient-dependent multimerization of the mammalian target of rapamycin through the N-terminal HEAT repeat region. J. Biol. Chem. 2006, 281, 28605–28614. [Google Scholar] [CrossRef] [Green Version]
- Veverka, V.; Crabbe, T.; Bird, I.; Lennie, G.; Muskett, F.W.; Taylor, R.J.; Carr, M.D. Structural characterization of the interaction of mTOR with phosphatidic acid and a novel class of inhibitor: Compelling evidence for a central role of the FRB domain in small molecule-mediated regulation of mTOR. Oncogene 2008, 27, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Toschi, A.; Lee, E.; Xu, L.; Garcia, A.; Gadir, N.; Foster, D.A. Regulation of mTORC1 and mTORC2 Complex Assembly by Phosphatidic Acid: Competition with Rapamycin. Mol. Cell. Biol. 2009, 29, 1411–1420. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zheng, Y.; Foster, D.A. Phospholipase D confers rapamycin resistance in human breast cancer cells. Oncogene 2003, 22, 3937–3942. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, G.M.; Frohman, M.A. Phospholipase D: A lipid centric review. Cell. Mol. Life Sci. 2005, 62, 2305–2316. [Google Scholar] [CrossRef]
- Kassas, N.; Tanguy, E.; Thahouly, T.; Fouillen, L.; Heintz, D.; Chasserot-Golaz, S.; Bader, M.F.; Grant, N.J.; Vitale, N. Comparative characterization of phosphatidic acid sensors and their localization during frustrated phagocytosis. J. Biol. Chem. 2017, 292, 4266–4279. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, R.A.; Yang, Y.C. Interleukin-11 induces phosphatidic acid formation and activates map kinase in mouse 3T3-L1 cells. Cell. Signal. 1995, 7, 247–259. [Google Scholar] [CrossRef]
- Zhang, C.; Wendel, A.A.; Keogh, M.R.; Harris, T.E.; Chen, J.; Coleman, R.A. Glycerolipid signals alter mTOR complex 2 (mTORC2) to diminish insulin signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 1667–1672. [Google Scholar] [CrossRef] [Green Version]
- Menon, D.; Salloum, D.; Bernfeld, E.; Gorodetsky, E.; Akselrod, A.; Frias, M.A.; Sudderth, J.; Chen, P.-H.; DeBerardinis, R.; Foster, D.A. Lipid sensing by mTOR complexes via de novo synthesis of phosphatidic acid. J. Biol. Chem. 2017, 292, 6303–6311. [Google Scholar] [CrossRef] [Green Version]
- Foster, D.A. Phosphatidic acid and lipid-sensing by mTOR. Trends Endocrinol. Metab. 2013, 24, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Jellqvist, B.; Hughes, G.J.; Pasquali, C.; Paquet, N.; Ravier, F.; Sanchez, J.-C.; Frutiger, S.; Hochstrasser, D. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 1993, 14, 1023–1031. [Google Scholar] [CrossRef]
- Rouser, G.; Fleischer, S.; Yamamoto, A. Two Dimensional Thin Layer Chromatographic Separation of Polar Lipids and Determination of Phospholipids by Phosphorus Analysis of Spots. Lipids 1970, 5, 494–496. [Google Scholar] [CrossRef]
- Dimitrov, D.S.; Angelova, M.I. Lipid swelling and liposome formation mediated by electric fields. J. Electroanal. Chem. 1988, 253, 323–336. [Google Scholar] [CrossRef]
- Méléard, P.; Bagatolli, L.A.; Pott, T. Giant Unilamellar Vesicle Electroformation. From Lipid Mixtures to Native Membranes Under Physiological Conditions. Methods Enzymol. 2009, 465, 161–176. [Google Scholar]
- Reeves, J.P.; Dowben, R.M. Formation and properties of thin-walled phospholipid vesicles. J. Cell. Physiol. 1969, 73, 49–60. [Google Scholar] [CrossRef]
- Tsumoto, K.; Matsuo, H.; Tomita, M.; Yoshimura, T. Efficient formation of giant liposomes through the gentle hydration of phosphatidylcholine films doped with sugar. Colloids Surf. B Biointerfaces 2009, 68, 98–105. [Google Scholar] [CrossRef]
- Wallner, J.; Lhota, G.; Jeschek, D.; Mader, A.; Vorauer-uhl, K. Application of Bio-Layer Interferometry for the analysis of protein/liposome interactions. J. Pharm. Biomed. Anal. 2013, 72, 150–154. [Google Scholar] [CrossRef]
- Katsamba, P.; Navratilova, I.; Calderon-Cacia, M.; Fan, L.; Thornton, K.; Zhu, M.; Bos, T.V.; Forte, C.; Friend, D.; Laird-Offringa, I.; et al. Kinetic analysis of a high-affinity antibody/antigen interaction performed by multiple Biacore users. Anal. Biochem. 2006, 352, 208–221. [Google Scholar] [CrossRef]
- Contreras, F.-X.; Ernst, A.; Haberkant, P.; Björkholm, P.; Lindahl, E.; Gönen, B.; Tischer, C.; Elofsson, A.; von Heijne, G.; Thiele, C.; et al. Molecular recognition of a single sphingolipid species by a protein’s transmembrane domain. Nature 2012, 481, 525–529. [Google Scholar] [CrossRef] [Green Version]
- Shulga, Y.V.; Topham, M.K.; Epand, R.M. Regulation and functions of diacylglycerol kinases. Chem. Rev. 2011, 111, 6186–6208. [Google Scholar] [CrossRef]
- Drabik, D.; Czogalla, A. Simple Does Not Mean Trivial: Behavior of Phosphatidic Acid in Lipid Mono- and Bilayers. Int. J. Mol. Sci. 2021, 22, 11523. [Google Scholar] [CrossRef]
- SAntollini, S.; Soto, M.A.; de Romanelli, I.B.; Gutiérrez-Merino, C.; Sotomayor, P.; Barrantes, F.J. Physical state of bulk and protein-associated lipid in nicotinic acetylcholine receptor-rich membrane studied by laurdan generalized polarization and fluorescence energy transfer. Biophys. J. 1996, 70, 1275–1284. [Google Scholar] [CrossRef] [Green Version]
- Tattrie, N.H.; Bennett, J.R.; Cyr, R. Maximum and minimum values for lecithin classes from various biological sources. Can. J. Biochem. 1968, 46, 819–824. [Google Scholar] [CrossRef]
- Sezgin, E.; Schwille, P. Model membrane platforms to study protein-membrane interactions. Mol. Membr. Biol. 2012, 29, 144–154. [Google Scholar] [CrossRef]
- Macháň, R.; Hof, M. Lipid diffusion in planar membranes investigated by fluorescence correlation spectroscopy. Biochim. Biophys. Acta-Biomembr. 2010, 1798, 1377–1391. [Google Scholar] [CrossRef] [Green Version]
- Kulig, W.; Korolainen, H.; Zatorska, M.; Kwolek, U.; Kepczynski, M. Complex Behavior of Phosphatidylcholine—Phosphatidic Acid Bilayers and Monolayers: Effect of Acyl Chain Unsaturation. Langmuir 2019, 35, 5944–5956. [Google Scholar] [CrossRef]
- Cambrea, L.R.; Haque, F.; Schieler, J.L.; Rochet, J.-C.; Hovis, J.S. Effect of Ions on the Organization of Phosphatidylcholine/Phosphatidic Acid Bilayers. Biophys. J. 2007, 93, 1630–1638. [Google Scholar] [CrossRef] [Green Version]
- Incardona, J.P.; Eaton, S. Cholesterol in signal transduction. Curr. Opin. Cell Biol. 2000, 12, 193–203. [Google Scholar] [CrossRef]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef]
- Ouweneel, A.B.; Thomas, M.J.; Sorci-Thomas, M.G. The ins and outs of lipid rafts: Functions in intracellular cholesterol homeostasis, microparticles, and cell membranes. J. Lipid Res. 2020, 61, 676–686. [Google Scholar] [CrossRef] [Green Version]
- Hryniewicz-Jankowska, A.; Augoff, K.; Sikorski, A.F. Highlight article: The role of cholesterol and cholesterol-driven membrane raft domains in prostate cancer. Exp. Biol. Med. 2019, 244, 1053–1061. [Google Scholar] [CrossRef]
- Drabik, D.; Gavutis, M.; Valiokas, R.N.; Ulčinas, A.R. Determination of the Mechanical Properties of Model Lipid Bilayers Using Atomic Force Microscopy Indentation. Langmuir 2020, 36, 13251–13262. [Google Scholar] [CrossRef]
- Barros, M.; Heinrich, F.; Datta, S.A.K.; Rein, A.; Karageorgos, I.; Nanda, H.; Lösche, M. Membrane Binding of HIV-1 Matrix Protein: Dependence on Bilayer Composition and Protein Lipidation. J. Virol. 2016, 90, 4544–4555. [Google Scholar] [CrossRef] [Green Version]
- Henderson, J.; Iyengar, N.S.; Lam, K.L.H.; Maldonado, E.; Suwatthee, T.; Roy, I.; Waring, A.J.; Lee, K.Y.C. Beyond electrostatics: Antimicrobial peptide selectivity and the influence of cholesterol-mediated fluidity and lipid chain length on protegrin-1 activity. Biochim. Biophys. Acta-Biomembr. 2019, 1861, 182977. [Google Scholar] [CrossRef] [PubMed]
- Heiner, A.L.; Gibbons, E.; Fairbourn, J.L.; Gonzalez, L.J.; McLemore, C.O.; Brueseke, T.J.; Judd, A.M.; Bell, J.D. Effects of cholesterol on physical properties of human erythrocyte membranes: Impact on susceptibility to hydrolysis by secretory phospholipase A2. Biophys. J. 2008, 94, 3084–3093. [Google Scholar] [CrossRef] [Green Version]
- Warnock, D.E.; Roberts, C.; Lutz, M.S.; Blackburn, W.A.; Young, W.W.; Baenziger, J.U. Determination of plasma membrane lipid mass and composition in cultured Chinese hamster ovary cells using high gradient magnetic affinity chromatography. J. Biol. Chem. 1993, 268, 10145–10153. [Google Scholar] [CrossRef]
- Maxfield, F.R.; Menon, A.K. Intracellular sterol transport and distribution. Curr. Opin. Cell Biol. 2006, 18, 379–385. [Google Scholar] [CrossRef]
- Martello, A.; Platt, F.M.; Eden, E.R. Staying in touch with the endocytic network: The importance of contacts for cholesterol transport. Traffic 2020, 21, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Litvinov, D.Y.; Savushkin, E.V.; Dergunov, A.D. Intracellular and Plasma Membrane Events in Cholesterol Transport and Homeostasis. J. Lipids. 2018, 2018, 3965054. [Google Scholar] [CrossRef] [Green Version]
- Camargo, D.C.R.; Link, N.M.; Dames, S.A. The FKBP-rapamycin binding domain of human tor undergoes strong conformational changes in the presence of membrane mimetics with and without the regulator phosphatidic acid. Biochemistry. 2012, 51, 4909–4921. [Google Scholar] [CrossRef]
- Capelluto, D.G.; Zhao, X.; Lucas, A.; Lemkul, J.A.; Xiao, S.; Fu, X.; Sun, F.; Bevan, D.R.; Finkielstein, C.V. Biophysical and molecular-dynamics studies of phosphatidic acid binding by the Dvl-2 DEP domain. Biophys. J. 2014, 106, 1101–1111. [Google Scholar] [CrossRef] [Green Version]
- Eaton, J.M.; Takkellapati, S.; Lawrence, R.T.; McQueeney, K.E.; Boroda, S.; Mullins, G.R.; Sherwood, S.G.; Finck, B.N.; Villen, J.; Harris, T.E. Lipin 2 binds phosphatidic acid by the electrostatic hydrogen bond switch mechanism independent of phosphorylation. J. Biol. Chem. 2014, 289, 18055–18066. [Google Scholar] [CrossRef] [Green Version]
- Boughter, C.T.; Monje-Galvan, V.; Im, W.; Klauda, J.B. Influence of Cholesterol on Phospholipid Bilayer Structure and Dynamics. J. Phys. Chem. B 2016, 120, 11761–11772. [Google Scholar] [CrossRef]
- Barbour, R.; Bova, M.P. Combining label-free technologies: Discovery in strength. Bioanalysis 2012, 4, 619–622. [Google Scholar] [CrossRef] [Green Version]
- DMyszka, G.; Jonsen, M.D.; Graves, B.J. Equilibrium analysis of high affinity interactions using BIACORE. Anal. Biochem. 1998, 265, 326–330. [Google Scholar] [CrossRef]
- Abdiche, Y.; Malashock, D.; Pinkerton, A.; Pons, J. Determining kinetics and affinities of protein interactions using a parallel real-time label-free biosensor, the Octet. Anal. Biochem. 2008, 377, 209–217. [Google Scholar] [CrossRef]
- Rich, R.L.; Myszka, D.G. Extracting kinetic rate constants from binding responses. Label-Free Biosens. Tech. Appl. 2009, 85–109. [Google Scholar]
- Wallner, J.; Lhota, G.; Schosserer, M.; Vorauer-Uhl, K. An approach for liposome immobilization using sterically stabilized micelles (SSMs) as a precursor for bio-layer interferometry-based interaction studies. Colloids Surf. B Biointerfaces 2017, 154, 186–194. [Google Scholar] [CrossRef]

| Liposomes | PA Concetration Range (µM) | Ka (1/Ms) | Kd (1/s) | KD (M) | KD2 (M) |
|---|---|---|---|---|---|
| POPC/CH/POPA | 12–36 | 7.38 × 101 | 2.53 × 10−4 | 3.43 × 10−6 | |
| POPC/CH/SAPA | 4–30 | 1.13 × 102 | 3.92 × 10−4 | 3.46 × 10−6 | |
| POPC/SAPA | 8–36 | 7.26 × 101 | 3.74 × 10−4 | 5.16 × 10−6 | |
| POPC/CH/DPPA | 12–36 | 1.96 × 101 | 1.67 × 10−4 | 8.53 × 10−6 | |
| POPC/DPPA | 2–24 | 8.82 × 101 | 3.06 × 10−4 | 3.47 × 10−6 | 4.4 × 10−6 |
| 1.15 × 104 | 5.07 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żelasko, J.; Czogalla, A. Selectivity of mTOR-Phosphatidic Acid Interactions Is Driven by Acyl Chain Structure and Cholesterol. Cells 2022, 11, 119. https://doi.org/10.3390/cells11010119
Żelasko J, Czogalla A. Selectivity of mTOR-Phosphatidic Acid Interactions Is Driven by Acyl Chain Structure and Cholesterol. Cells. 2022; 11(1):119. https://doi.org/10.3390/cells11010119
Chicago/Turabian StyleŻelasko, Jolanta, and Aleksander Czogalla. 2022. "Selectivity of mTOR-Phosphatidic Acid Interactions Is Driven by Acyl Chain Structure and Cholesterol" Cells 11, no. 1: 119. https://doi.org/10.3390/cells11010119
APA StyleŻelasko, J., & Czogalla, A. (2022). Selectivity of mTOR-Phosphatidic Acid Interactions Is Driven by Acyl Chain Structure and Cholesterol. Cells, 11(1), 119. https://doi.org/10.3390/cells11010119







