Characterization of Oxygen Levels in an Uninfected and Infected Human Blood-Cerebrospinal-Fluid-Barrier Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Streptococcus suis Cultivation
2.2. Purification of Human Neutrophils
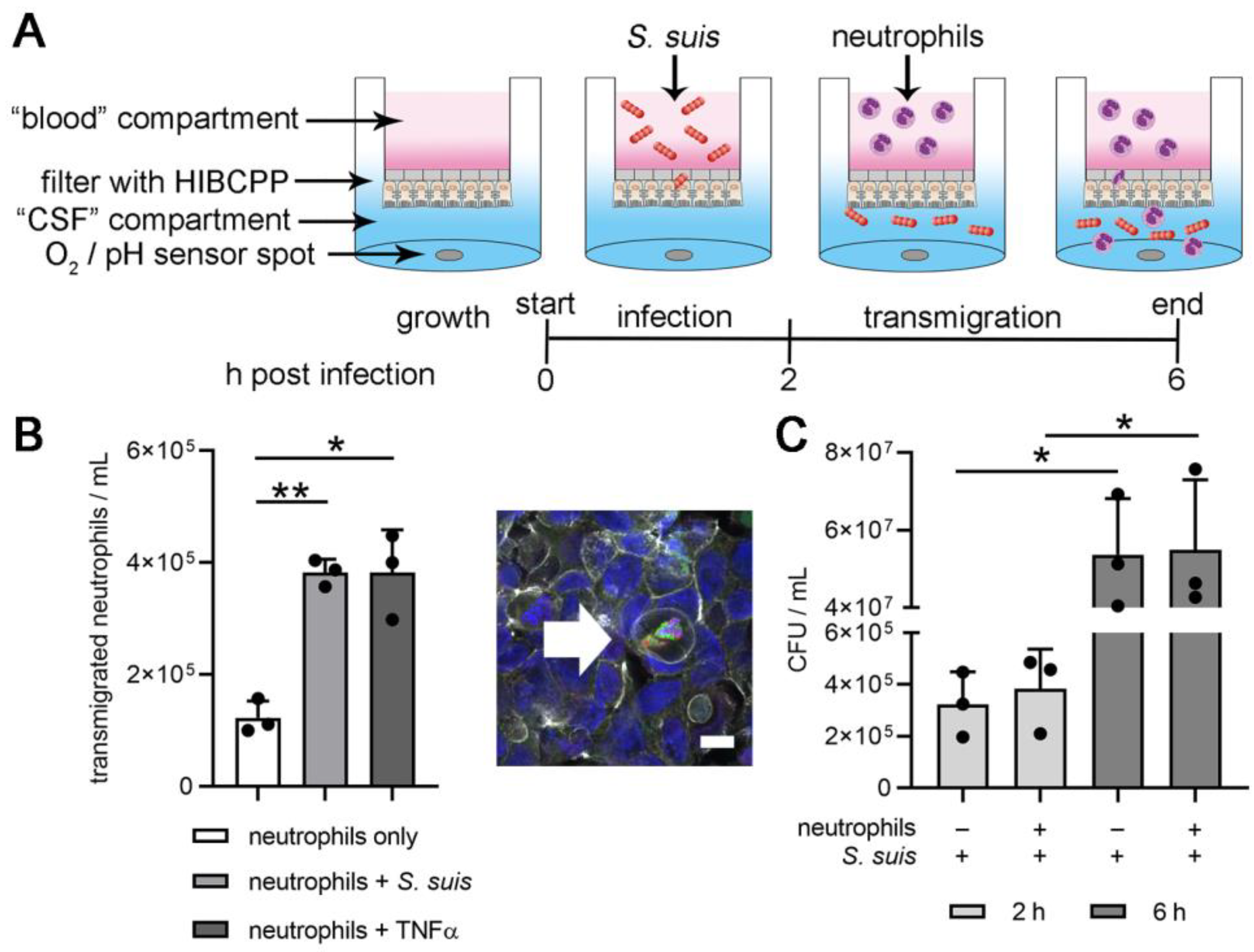
2.3. Two-D Model of the BCSFB with S. suis Infection and Neutrophil Transmigration
2.4. Profile of Bacterial Growth in the 2D-Infection Model
2.5. Read Out: S. suis and Neutrophils in Lower (“CSF”) Compartment
2.6. Read Out: Oxygen and pH Value in the Lower (“CSF”) Compartment
2.7. Filter Immunostaining of LL-37 and Myeloperoxidase
2.8. Statistical Analyses
3. Results
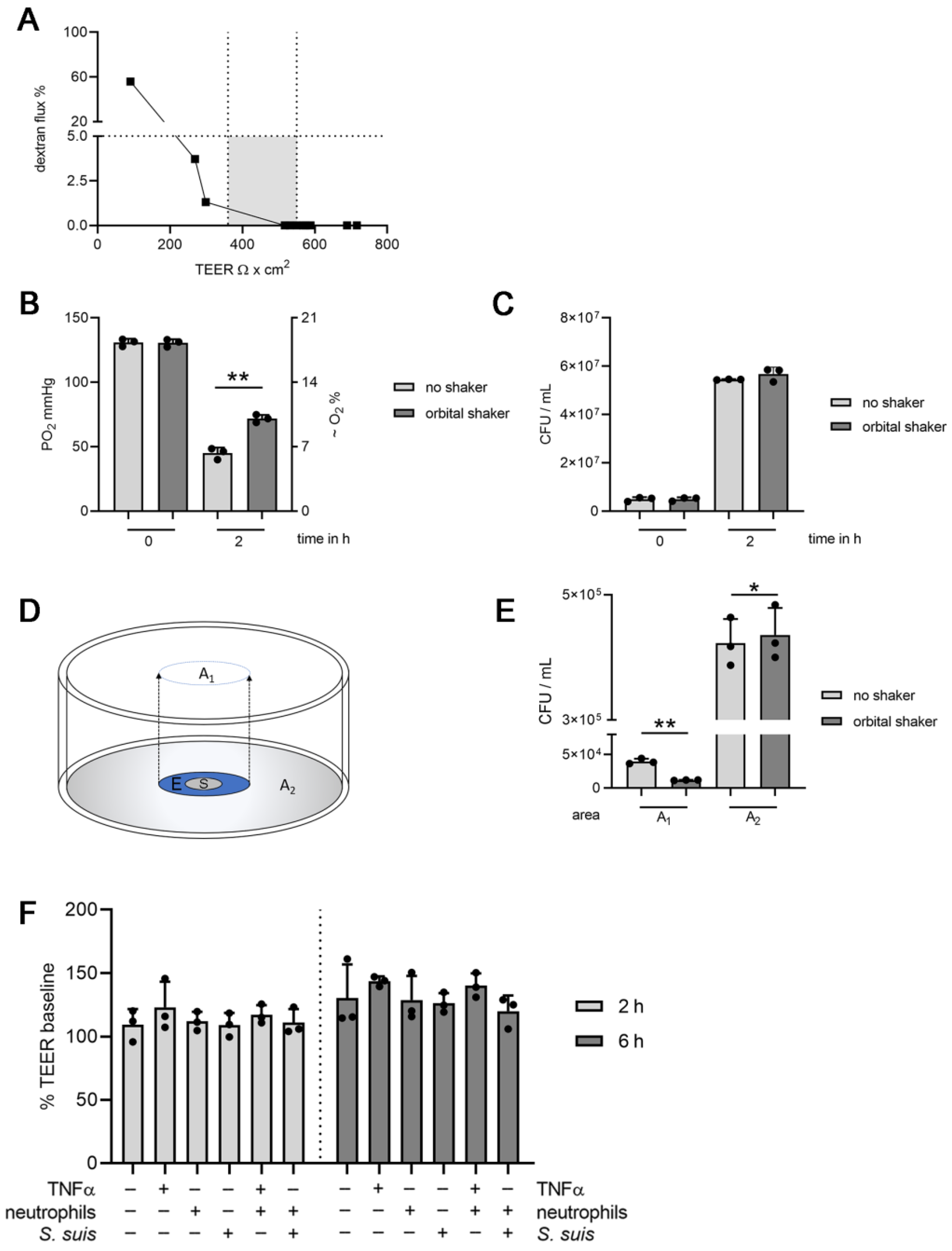
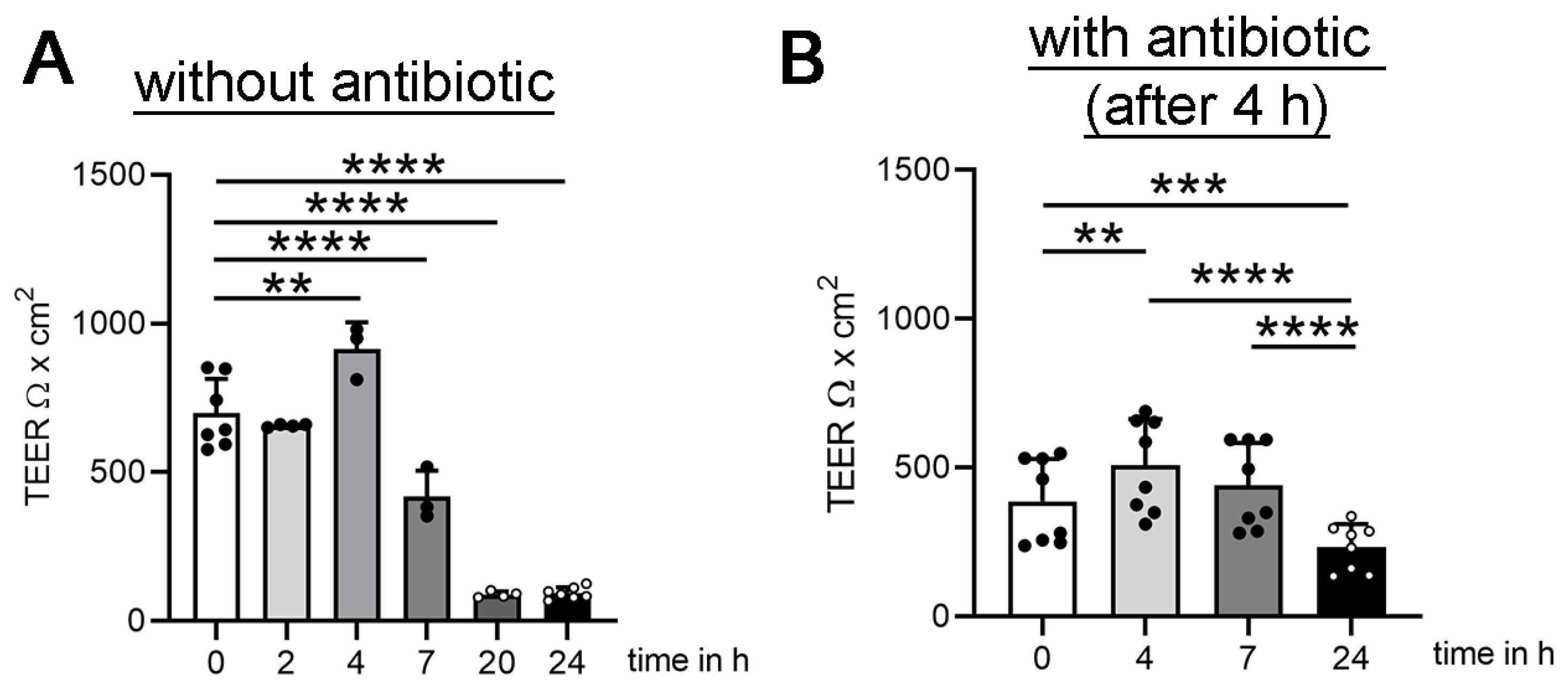
3.1. Establishing Oxygen Measurement Inside the CSF Compartment of the BCSFB Model during S. suis Infection under Shaking Conditions
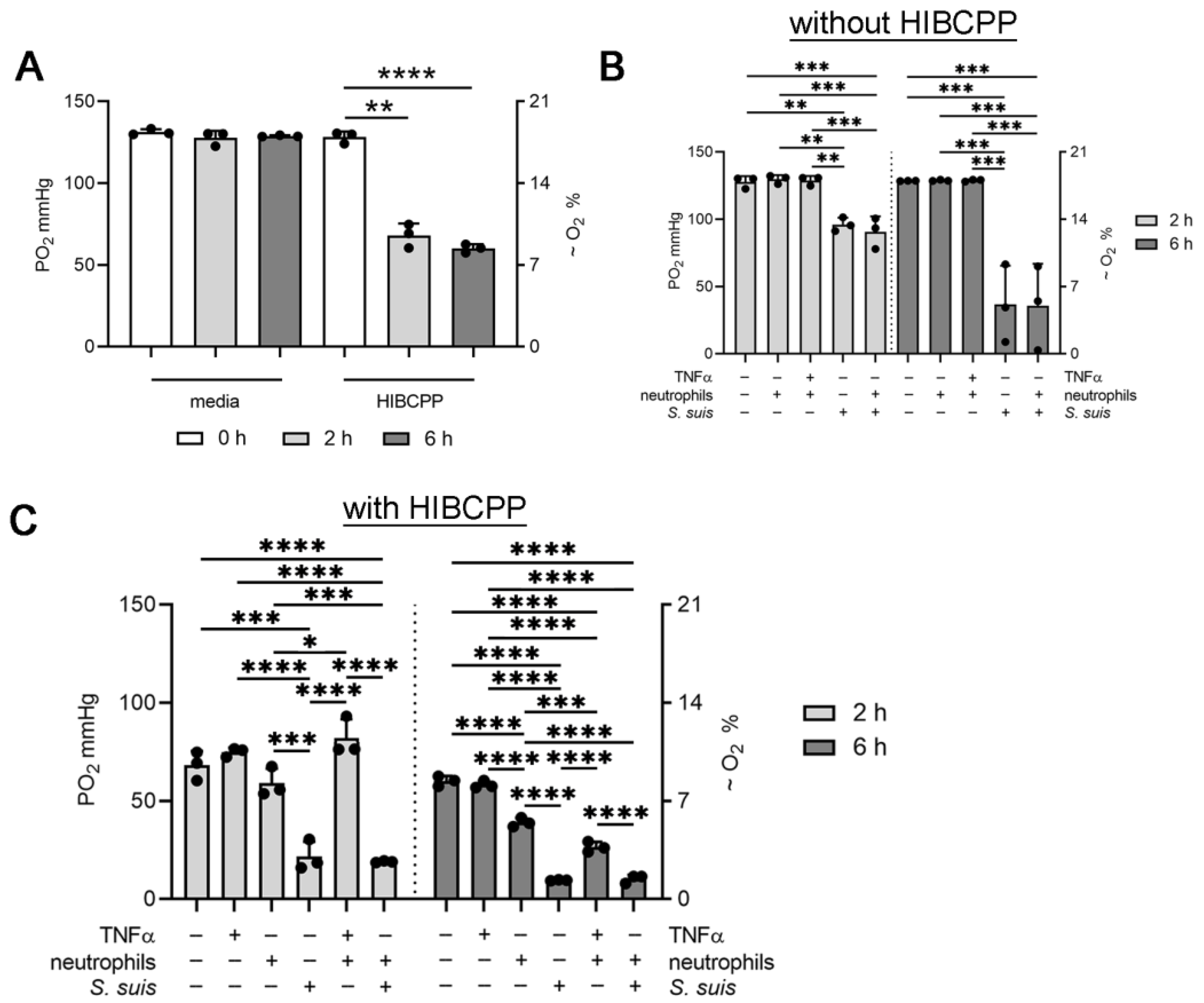
3.2. Choroid plexus Epithelial Cells (HIBCPP), Neutrophils and S. suis Consume Oxygen in the BCSFB Model
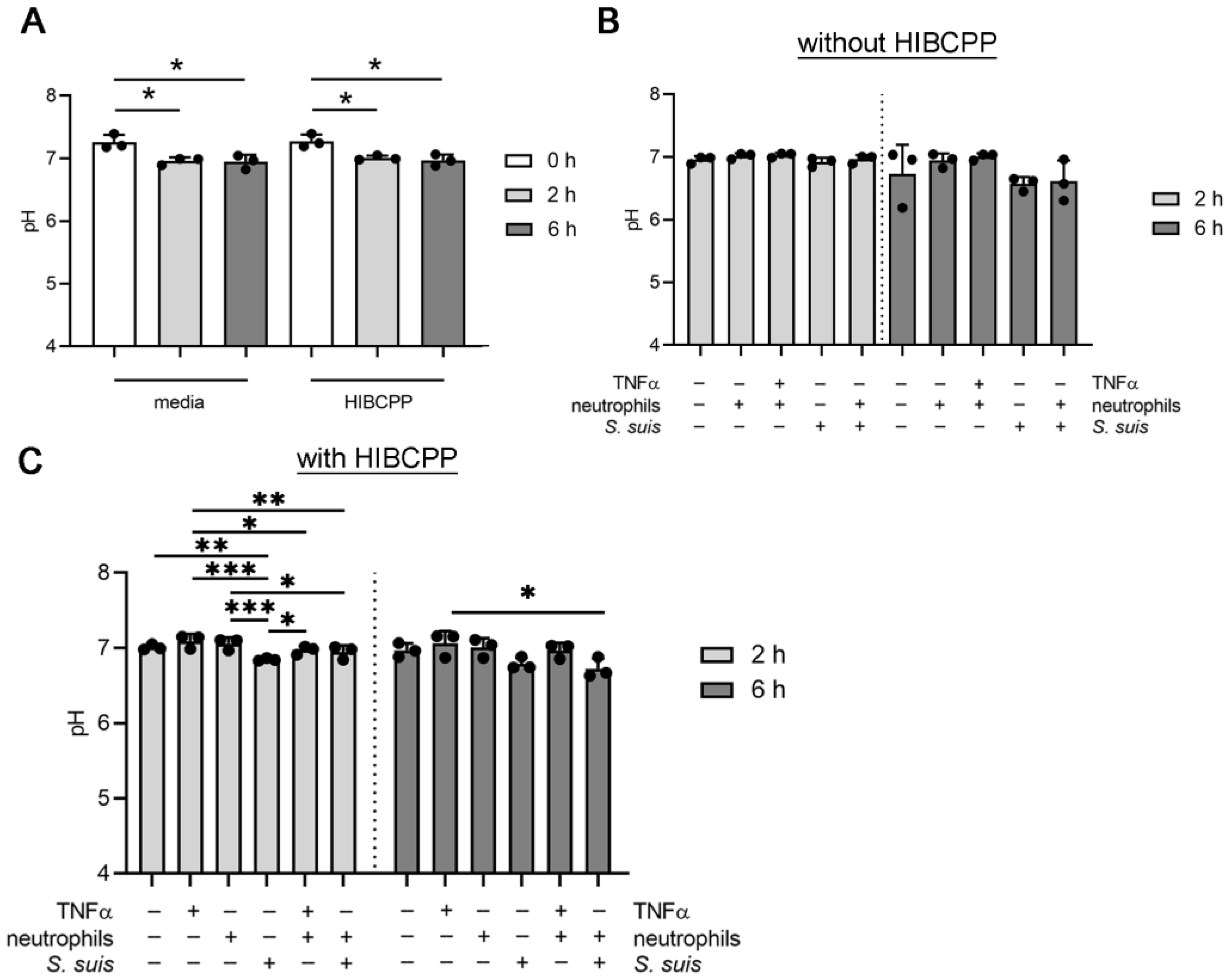
3.3. S. suis and Neutrophils Slightly Decrease the pH in the BCSFB Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Alanis, A.J. Resistance to Antibiotics: Are We in the Post-Antibiotic Era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Ziółkowska-Suchanek, I. Mimicking Tumor Hypoxia in Non-Small Cell Lung Cancer Employing Three-Dimensional In Vitro Models. Cells 2021, 10, 141. [Google Scholar] [CrossRef]
- Irimia, D.; Wang, X. Inflammation-on-a-Chip: Probing the Immune System Ex Vivo. Trends Biotechnol. 2018, 36, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wion, D.; Christen, T.; Barbier, E.L.; Coles, J.A. PO2 Matters in Stem Cell Culture. Cell Stem Cell 2009, 5, 242–243. [Google Scholar] [CrossRef]
- Kaneko, S.; Takamatsu, K. Cell Handling and Culture Under Controlled Oxygen Concentration. In Biomedical Tissue Culture; InTech: Nappanee, IN, USA, 2012; ISBN 978-953-51-0788-0. [Google Scholar]
- Branitzki-Heinemann, K.; Möllerherm, H.; Völlger, L.; Husein, D.M.; de Buhr, N.; Blodkamp, S.; Reuner, F.; Brogden, G.; Naim, H.Y.; von Köckritz-Blickwede, M. Formation of Neutrophil Extracellular Traps under Low Oxygen Level. Front. Immunol. 2016, 7, 518. [Google Scholar] [CrossRef] [Green Version]
- Möllerherm, H.; Branitzki-Heinemann, K.; Brogden, G.; Elamin, A.A.; Oehlmann, W.; Fuhrmann, H.; Singh, M.; Naim, H.Y.; von Köckritz-Blickwede, M. Hypoxia Modulates the Response of Mast Cells to Staphylococcus aureus Infection. Front. Immunol. 2017, 8, 541. [Google Scholar] [CrossRef] [Green Version]
- Möllerherm, H.; von Köckritz-Blickwede, M.; Branitzki-Heinemann, K. Antimicrobial Activity of Mast Cells: Role and Relevance of Extracellular DNA Traps. Front. Immunol. 2016, 7, 265. [Google Scholar] [CrossRef] [Green Version]
- Bordt, E.A. The importance of controlling in vitro oxygen tension to accurately model in vivo neurophysiology. Neurotoxicology 2018, 66, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Lun, Z.-R.; Wang, Q.-P.; Chen, X.-G.; Li, A.-X.; Zhu, X.-Q. Streptococcus suis: An emerging zoonotic pathogen. Lancet Infect. Dis. 2007, 7, 201–209. [Google Scholar] [CrossRef]
- Gottschalk, M. Streptococcosis. In Diseases of Swine; Locke, A.K., Zimmerman, J., Ramirez, A., Schwartz, K.J., Stevenson, G., Eds.; Wiley-Blackwell: Ames, IA, USA, 2012; pp. 841–855. ISBN 978-0-8138-2267-9. [Google Scholar]
- Cloutier, G.; Surprenant, C.; Lacouture, S.; D’Allaire, S.; Gottschalk, M.; Martinez, G. Epidemiology of Streptococcus suis serotype 5 infection in a pig herd with and without clinical disease. Vet. Microbiol. 2003, 97, 135–151. [Google Scholar] [CrossRef]
- Gottschalk, M.; Xu, J.; Calzas, C.; Segura, M. Streptococcus suis: A new emerging or an old neglected zoonotic pathogen? Future Microbiol. 2010, 5, 371–391. [Google Scholar] [CrossRef]
- Wertheim, H.F.L.; Nghia, H.D.T.; Taylor, W.; Schultsz, C. Streptococcus suis: An emerging human pathogen. Clin. Infect. Dis. 2009, 48, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Zhang, H.; Wu, Z.; Wang, S.; Cao, M.; Hu, D.; Wang, C. Streptococcus suis infection. Virulence 2014, 5, 477–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beineke, A.; Bennecke, K.; Neis, C.; Schröder, C.; Waldmann, K.-H.; Baumgärtner, W.; Valentin-Weigand, P.; Baums, C.G. Comparative evaluation of virulence and pathology of Streptococcus suis serotypes 2 and 9 in experimentally infected growers. Vet. Microbiol. 2008, 128, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.E.; Blakemore, W.F. Pathogenesis of meningitis caused by Streptococcus suis type 2. J. Infect. Dis. 1990, 162, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Shechter, R.; London, A.; Schwartz, M. Orchestrated leukocyte recruitment to immune-privileged sites: Absolute barriers versus educational gates. Nat. Rev. Immunol. 2013, 13, 206–218. [Google Scholar] [CrossRef]
- Engelhardt, B.; Coisne, C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS 2011, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johanson, C. Choroid Plexus Blood-CSF Barrier: Major Player in Brain Disease Modeling and Neuromedicine. J. Neurol. Neuromed. 2018, 3, 39–58. [Google Scholar] [CrossRef]
- Erb, U.; Schwerk, C.; Schroten, H.; Karremann, M. Review of functional in vitro models of the blood-cerebrospinal fluid barrier in leukaemia research. J. Neurosci. Methods 2020, 329, 108478. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, I.; Ishiwata, C.; Ishiwata, E.; Sato, Y.; Kiguchi, K.; Tachibana, T.; Hashimoto, H.; Ishikawa, H. Establishment and characterization of a human malignant choroids plexus papilloma cell line (HIBCPP). Hum. Cell 2005, 18, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Schwerk, C.; Papandreou, T.; Schuhmann, D.; Nickol, L.; Borkowski, J.; Steinmann, U.; Quednau, N.; Stump, C.; Weiss, C.; Berger, J.; et al. Polar invasion and translocation of neisseria meningitidis and streptococcus suis in a novel human model of the blood-cerebrospinal fluid barrier. PLoS ONE 2012, 7, e30069. [Google Scholar] [CrossRef]
- de Buhr, N.; Reuner, F.; Neumann, A.; Stump-Guthier, C.; Tenenbaum, T.; Schroten, H.; Ishikawa, H.; Müller, K.; Beineke, A.; Hennig-Pauka, I.; et al. Neutrophil extracellular trap formation in the Streptococcus suis-infected cerebrospinal fluid compartment. Cell. Microbiol. 2017, 19, e12649. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- de Buhr, N.; Martens, A.; Meurer, M.; Bonilla, M.C.; Söbbeler, F.; Twele, L.; Neudeck, S.; Wendt, M.; Beineke, A.; Kästner, S.; et al. In vivo oxygen measurement in cerebrospinal fluid of pigs to determine physiologic and pathophysiologic oxygen values during CNS infections. BMC Neurosci. 2021, 22, 45. [Google Scholar] [CrossRef]
- Vecht, U.; Wisselink, H.J.; Stockhofe-Zurwieden, N.; Smith, H.E. Characterization of virulence of the Sreptococcus suis serotype 2 reference strain Henrichsen S 735 in newborn gnotobiotic pigs. Vet. Microbiol. 1996, 51, 125–136. [Google Scholar] [CrossRef]
- Smith, H.E.; Damman, M.; Van Der Velde, J.; Wagenaar, F.; Wisselink, H.J.; Stockhofe-Zurwieden, N.; Smits, M.A. Identification and characterization of the cps locus of Streptococcus suis serotype 2: The capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 1999, 67, 1750–1756. [Google Scholar] [CrossRef]
- Meurer, M.; Öhlmann, S.; Bonilla, M.C.; Valentin-Weigand, P.; Beineke, A.; Hennig-Pauka, I.; Schwerk, C.; Schroten, H.; Baums, C.G.; von Köckritz-Blickwede, M.; et al. Role of Bacterial and Host DNases on Host-Pathogen Interaction during Streptococcus suis Meningitis. Int. J. Mol. Sci. 2020, 21, 5289. [Google Scholar] [CrossRef]
- de Buhr, N.; von Köckritz-Blickwede, M. Detection, Visualization, and Quantification of Neutrophil Extracellular Traps (NETs) and NET Markers. Methods Mol. Biol. 2020, 2087, 425–442. [Google Scholar] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Place, T.L.; Domann, F.E.; Case, A.J. Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free Radic. Biol. Med. 2017, 113, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.L.; Bruyninckx, W.J.; Kelly, C.J.; Glover, L.E.; McNamee, E.N.; Bowers, B.E.; Bayless, A.J.; Scully, M.; Saeedi, B.J.; Golden-Mason, L.; et al. Transmigrating Neutrophils Shape the Mucosal Microenvironment through Localized Oxygen Depletion to Influence Resolution of Inflammation. Immunity 2014, 40, 66–77. [Google Scholar] [CrossRef] [Green Version]
- Stuart, J.A.; Fonseca, J.; Moradi, F.; Cunningham, C.; Seliman, B.; Worsfold, C.R.; Dolan, S.; Abando, J.; Maddalena, L.A. How Supraphysiological Oxygen Levels in Standard Cell Culture Affect Oxygen-Consuming Reactions. Oxid. Med. Cell. Longev. 2018, 2018, 8238459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Zhu, L.; Fan, M. Oxygen, a Key Factor Regulating Cell Behavior during Neurogenesis and Cerebral Diseases. Front. Mol. Neurosci. 2011, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, F.G.; Panchalingam, K.M.; Anjo, S.I.; Manadas, B.; Pereira, R.; Sousa, N.; Salgado, A.J.; Behie, L.A. Do hypoxia/normoxia culturing conditions change the neuroregulatory profile of Wharton Jelly mesenchymal stem cell secretome? Stem Cell Res. Ther. 2015, 6, 133. [Google Scholar] [CrossRef] [Green Version]
- Chadwick, W.; Boyle, J.P.; Zhou, Y.; Wang, L.; Park, S.-S.; Martin, B.; Wang, R.; Becker, K.G.; Wood, W.H.; Zhang, Y.; et al. Multiple Oxygen Tension Environments Reveal Diverse Patterns of Transcriptional Regulation in Primary Astrocytes. PLoS ONE 2011, 6, e21638. [Google Scholar] [CrossRef]
- Al-Ani, A.; Toms, D.; Kondro, D.; Thundathil, J.; Yu, Y.; Ungrin, M. Oxygenation in cell culture: Critical parameters for reproducibility are routinely not reported. PLoS ONE 2018, 13, e0204269. [Google Scholar] [CrossRef] [Green Version]
- Tiede, L.M.; Cook, E.A.; Morsey, B.; Fox, H.S. Oxygen matters: Tissue culture oxygen levels affect mitochondrial function and structure as well as responses to HIV viroproteins. Cell Death Dis. 2011, 2, e246. [Google Scholar] [CrossRef]
- Krzywinska, E.; Stockmann, C. Hypoxia, Metabolism and Immune Cell Function. Biomedicines 2018, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Steinmann, U.; Borkowski, J.; Wolburg, H.; Schröppel, B.; Findeisen, P.; Weiss, C.; Ishikawa, H.; Schwerk, C.; Schroten, H.; Tenenbaum, T. Transmigration of polymorphnuclear neutrophils and monocytes through the human blood-cerebrospinal fluid barrier after bacterial infection in vitro. J. Neuroinflamm. 2013, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, A.A.; Loeffen, P.L.; van den Berg, A.J.; Storm, P.K. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect. Immun. 1994, 62, 1742–1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenenbaum, T.; Adam, R.; Eggelnpöhler, I.; Matalon, D.; Seibt, A.; Novotny, G.E.K.; Galla, H.J.; Schroten, H. Strain-dependent disruption of blood-cerebrospinal fluid barrier by Streptoccocus suis in vitro. FEMS Immunol. Med. Microbiol. 2005, 44, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonilla, M.C.; Fingerhut, L.; Alfonso-Castro, A.; Mergani, A.; Schwennen, C.; von Köckritz-Blickwede, M.; de Buhr, N. How Long Does a Neutrophil Live?—The Effect of 24 h Whole Blood Storage on Neutrophil Functions in Pigs. Biomedicines 2020, 8, 278. [Google Scholar] [CrossRef]
- Lahoz-Beneytez, J.; Elemans, M.; Zhang, Y.; Ahmed, R.; Salam, A.; Block, M.; Niederalt, C.; Asquith, B.; Macallan, D. Human neutrophil kinetics: Modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood 2016, 127, 3431–3438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, A.; de Buhr, N.; Ishikawa, H.; Schroten, H.; von Köckritz-Blickwede, M. Characterization of Oxygen Levels in an Uninfected and Infected Human Blood-Cerebrospinal-Fluid-Barrier Model. Cells 2022, 11, 151. https://doi.org/10.3390/cells11010151
Martens A, de Buhr N, Ishikawa H, Schroten H, von Köckritz-Blickwede M. Characterization of Oxygen Levels in an Uninfected and Infected Human Blood-Cerebrospinal-Fluid-Barrier Model. Cells. 2022; 11(1):151. https://doi.org/10.3390/cells11010151
Chicago/Turabian StyleMartens, Alexander, Nicole de Buhr, Hiroshi Ishikawa, Horst Schroten, and Maren von Köckritz-Blickwede. 2022. "Characterization of Oxygen Levels in an Uninfected and Infected Human Blood-Cerebrospinal-Fluid-Barrier Model" Cells 11, no. 1: 151. https://doi.org/10.3390/cells11010151
APA StyleMartens, A., de Buhr, N., Ishikawa, H., Schroten, H., & von Köckritz-Blickwede, M. (2022). Characterization of Oxygen Levels in an Uninfected and Infected Human Blood-Cerebrospinal-Fluid-Barrier Model. Cells, 11(1), 151. https://doi.org/10.3390/cells11010151






