Current Status and Future Perspectives on Machine Perfusion: A Treatment Platform to Restore and Regenerate Injured Lungs Using Cell and Cytokine Adsorption Therapy
Abstract
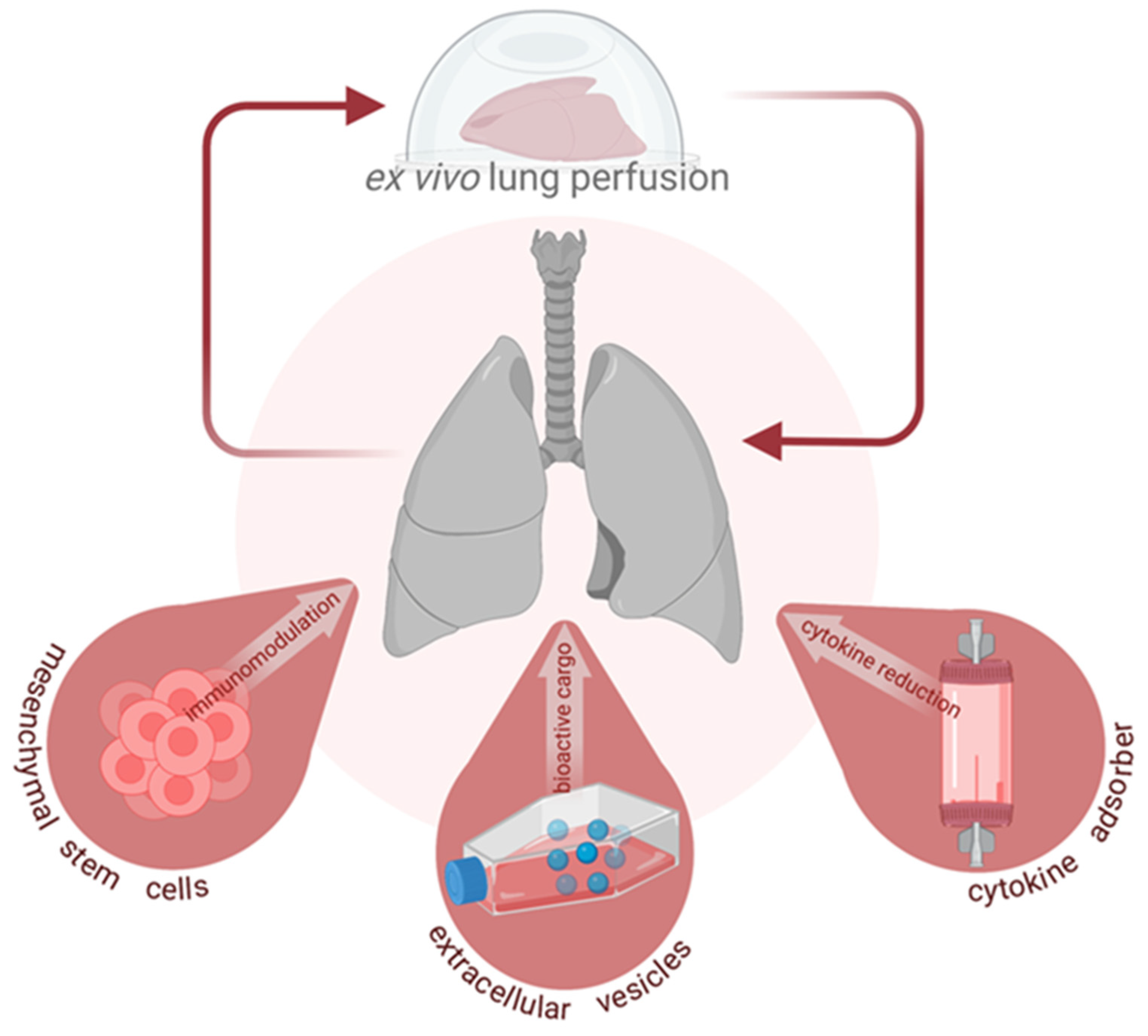
:1. Introduction
2. Mesenchymal Stromal (Stem) Cells
3. Extracellular Vesicles
4. Cytokine Adsorption
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Perrot, M.; Liu, M.; Waddell, T.K.; Keshavjee, S. Ischemia-reperfusion-induced lung injury. Am. J. Respir. Crit. Care Med. 2003, 167, 490–511. [Google Scholar] [CrossRef]
- Ghaidan, H.; Fakhro, M.; Andreasson, J.; Pierre, L.; Ingemansson, R.; Lindstedt, S. Ten year follow-up of lung transplantations using initially rejected donor lungs after reconditioning using ex vivo lung perfusion. J. Cardiothorac. Surg. 2019, 14, 125. [Google Scholar] [CrossRef] [Green Version]
- Ingemansson, R.; Eyjolfsson, A.; Mared, L.; Pierre, L.; Algotsson, L.; Ekmehag, B.; Gustafsson, R.; Johnsson, P.; Koul, B.; Lindstedt, S.; et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann. Thorac. Surg. 2009, 87, 255–260. [Google Scholar] [CrossRef]
- Lindstedt, S.; Hlebowicz, J.; Koul, B.; Wierup, P.; Sjogren, J.; Gustafsson, R.; Steen, S.; Ingemansson, R. Comparative outcome of double lung transplantation using conventional donor lungs and non-acceptable donor lungs reconditioned ex vivo. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 162–165. [Google Scholar] [CrossRef] [Green Version]
- Steen, S.; Ingemansson, R.; Eriksson, L.; Pierre, L.; Algotsson, L.; Wierup, P.; Liao, Q.; Eyjolfsson, A.; Gustafsson, R.; Sjoberg, T. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann. Thorac. Surg. 2007, 83, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Machuca, T.N.; Cypel, M. Ex vivo lung perfusion. J. Thorac. Dis. 2014, 6, 1054–1062. [Google Scholar] [CrossRef]
- Yeung, J.C.; Krueger, T.; Yasufuku, K.; de Perrot, M.; Pierre, A.F.; Waddell, T.K.; Singer, L.G.; Keshavjee, S.; Cypel, M. Outcomes after transplantation of lungs preserved for more than 12 h: A retrospective study. Lancet Respir. Med. 2017, 5, 119–124. [Google Scholar] [CrossRef]
- De Wolf, J.; Glorion, M.; Jouneau, L.; Estephan, J.; Leplat, J.J.; Blanc, F.; Richard, C.; Urien, C.; Roux, A.; Le Guen, M.; et al. Challenging the Ex Vivo Lung Perfusion Procedure With Continuous Dialysis in a Pig Model. Transplantation 2021, in press. [Google Scholar] [CrossRef]
- Wang, X.; Parapanov, R.; Debonneville, A.; Wang, Y.; Abdelnour-Berchtold, E.; Gonzalez, M.; Gronchi, F.; Perentes, J.Y.; Ris, H.B.; Eckert, P.; et al. Treatment with 3-aminobenzamide during ex vivo lung perfusion of damaged rat lungs reduces graft injury and dysfunction after transplantation. Am. J. Transpl. 2020, 20, 967–976. [Google Scholar] [CrossRef]
- Lonati, C.; Battistin, M.; Dondossola, D.E.; Bassani, G.A.; Brambilla, D.; Merighi, R.; Leonardi, P.; Carlin, A.; Meroni, M.; Zanella, A.; et al. NDP-MSH treatment recovers marginal lungs during ex vivo lung perfusion (EVLP). Peptides 2021, 141, 170552. [Google Scholar] [CrossRef]
- Van Raemdonck, D.; Neyrinck, A.; Rega, F.; Devos, T.; Pirenne, J. Machine perfusion in organ transplantation: A tool for ex-vivo graft conditioning with mesenchymal stem cells? Curr. Opin. Organ. Transpl. 2013, 18, 24–33. [Google Scholar] [CrossRef]
- Lange, C.; Togel, F.; Ittrich, H.; Clayton, F.; Nolte-Ernsting, C.; Zander, A.R.; Westenfelder, C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005, 68, 1613–1617. [Google Scholar] [CrossRef] [Green Version]
- Togel, F.E.; Westenfelder, C. Mesenchymal stem cells: A new therapeutic tool for AKI. Nat. Rev. Nephrol. 2010, 6, 179–183. [Google Scholar] [CrossRef]
- Tan, J.; Wu, W.; Xu, X.; Liao, L.; Zheng, F.; Messinger, S.; Sun, X.; Chen, J.; Yang, S.; Cai, J.; et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: A randomized controlled trial. JAMA 2012, 307, 1169–1177. [Google Scholar] [CrossRef] [Green Version]
- Pan, G.Z.; Yang, Y.; Zhang, J.; Liu, W.; Wang, G.Y.; Zhang, Y.C.; Yang, Q.; Zhai, F.X.; Tai, Y.; Liu, J.R.; et al. Bone marrow mesenchymal stem cells ameliorate hepatic ischemia/reperfusion injuries via inactivation of the MEK/ERK signaling pathway in rats. J. Surg. Res. 2012, 178, 935–948. [Google Scholar] [CrossRef]
- Popp, F.C.; Renner, P.; Eggenhofer, E.; Slowik, P.; Geissler, E.K.; Piso, P.; Schlitt, H.J.; Dahlke, M.H. Mesenchymal stem cells as immunomodulators after liver transplantation. Liver. Transpl. 2009, 15, 1192–1198. [Google Scholar] [CrossRef]
- Gregorini, M.; Corradetti, V.; Pattonieri, E.F.; Rocca, C.; Milanesi, S.; Peloso, A.; Canevari, S.; De Cecco, L.; Dugo, M.; Avanzini, M.A.; et al. Perfusion of isolated rat kidney with Mesenchymal Stromal Cells/Extracellular Vesicles prevents ischaemic injury. J. Cell Mol. Med. 2017, 21, 3381–3393. [Google Scholar] [CrossRef]
- De Perrot, M.; Sekine, Y.; Fischer, S.; Waddell, T.K.; McRae, K.; Liu, M.; Wigle, D.A.; Keshavjee, S. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am. J. Respir. Crit. Care Med. 2002, 165, 211–215. [Google Scholar] [CrossRef]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Krasnodembskaya, A.; McKenna, D.H.; Song, Y.; Abbott, J.; Matthay, M.A. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am. J. Respir. Crit. Care Med. 2013, 187, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2019, 7, 154–162. [Google Scholar] [CrossRef]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J.; Canadian Critical Care Trials, G. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef]
- Gorman, E.; Shankar-Hari, M.; Hopkins, P.; Tunnicliffe, W.S.; Perkins, G.D.; Silversides, J.; McGuigan, P.; Krasnodembskaya, A.; Jackson, C.; Boyle, R.; et al. Repair of acute respiratory distress syndrome by stromal cell administration (REALIST) trial: A phase 1 trial. EClinicalMedicine 2021, 41, 101167. [Google Scholar] [CrossRef]
- Lee, J.W.; Fang, X.; Gupta, N.; Serikov, V.; Matthay, M.A. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. USA 2009, 106, 16357–16362. [Google Scholar] [CrossRef] [Green Version]
- Viget, N.B.; Guery, B.P.; Ader, F.; Neviere, R.; Alfandari, S.; Creuzy, C.; Roussel-Delvallez, M.; Foucher, C.; Mason, C.M.; Beaucaire, G.; et al. Keratinocyte growth factor protects against Pseudomonas aeruginosa-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1199–L1209. [Google Scholar] [CrossRef] [Green Version]
- Welsh, D.A.; Summer, W.R.; Dobard, E.P.; Nelson, S.; Mason, C.M. Keratinocyte growth factor prevents ventilator-induced lung injury in an ex vivo rat model. Am. J. Respir. Crit. Care Med. 2000, 162, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Mason, C.M.; Guery, B.P.; Summer, W.R.; Nelson, S. Keratinocyte growth factor attenuates lung leak induced by alpha-naphthylthiourea in rats. Crit. Care Med. 1996, 24, 925–931. [Google Scholar] [CrossRef]
- McAuley, D.F.; Curley, G.F.; Hamid, U.I.; Laffey, J.G.; Abbott, J.; McKenna, D.H.; Fang, X.; Matthay, M.A.; Lee, J.W. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L809–L815. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Abbott, J.; Cheng, L.; Colby, J.K.; Lee, J.W.; Levy, B.D.; Matthay, M.A. Human Mesenchymal Stem (Stromal) Cells Promote the Resolution of Acute Lung Injury in Part through Lipoxin A4. J. Immunol 2015, 195, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Mordant, P.; Nakajima, D.; Kalaf, R.; Iskender, I.; Maahs, L.; Behrens, P.; Coutinho, R.; Iyer, R.K.; Davies, J.E.; Cypel, M.; et al. Mesenchymal stem cell treatment is associated with decreased perfusate concentration of interleukin-8 during ex vivo perfusion of donor lungs after 18-hour preservation. J. Heart Lung Transpl. 2016, 35, 1245–1254. [Google Scholar] [CrossRef]
- Nakajima, D.; Watanabe, Y.; Ohsumi, A.; Pipkin, M.; Chen, M.; Mordant, P.; Kanou, T.; Saito, T.; Lam, R.; Coutinho, R.; et al. Mesenchymal stromal cell therapy during ex vivo lung perfusion ameliorates ischemia-reperfusion injury in lung transplantation. J. Heart Lung Transpl. 2019, 38, 1214–1223. [Google Scholar] [CrossRef]
- Pacienza, N.; Santa-Cruz, D.; Malvicini, R.; Robledo, O.; Lemus-Larralde, G.; Bertolotti, A.; Marcos, M.; Yannarelli, G. Mesenchymal Stem Cell Therapy Facilitates Donor Lung Preservation by Reducing Oxidative Damage during Ischemia. Stem Cells Int. 2019, 2019, 8089215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Francesca, S.; Ting, A.E.; Sakamoto, J.; Rhudy, J.; Bonenfant, N.R.; Borg, Z.D.; Cruz, F.F.; Goodwin, M.; Lehman, N.A.; Taggart, J.M.; et al. Multipotent adult progenitor cells decrease cold ischemic injury in ex vivo perfused human lungs: An initial pilot and feasibility study. Transpl. Res. 2014, 3, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martens, A.; Ordies, S.; Vanaudenaerde, B.M.; Verleden, S.E.; Vos, R.; Van Raemdonck, D.E.; Verleden, G.M.; Roobrouck, V.D.; Claes, S.; Schols, D.; et al. Immunoregulatory effects of multipotent adult progenitor cells in a porcine ex vivo lung perfusion model. Stem. Cell Res. Ther. 2017, 8, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nykanen, A.I.; Mariscal, A.; Duong, A.; Estrada, C.; Ali, A.; Hough, O.; Sage, A.; Chao, B.T.; Chen, M.; Gokhale, H.; et al. Engineered mesenchymal stromal cell therapy during human lung ex vivo lung perfusion is compromised by acidic lung microenvironment. Mol. Ther. Methods Clin. Dev. 2021, 23, 184–197. [Google Scholar] [CrossRef]
- Mohamed, M.S. Mesenchymal stem cells transplantation during ex vivo lung perfusion. J. Heart Lung Transpl. 2017, 36, 243. [Google Scholar] [CrossRef] [PubMed]
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007, 25, 1384–1392. [Google Scholar] [CrossRef] [Green Version]
- Laffey, J.G.; Matthay, M.A. Fifty Years of Research in ARDS. Cell-based Therapy for Acute Respiratory Distress Syndrome. Biology and Potential Therapeutic Value. Am. J. Respir Crit Care Med. 2017, 196, 266–273. [Google Scholar] [CrossRef]
- Wick, K.D.; Leligdowicz, A.; Zhuo, H.; Ware, L.B.; Matthay, M.A. Mesenchymal stromal cells reduce evidence of lung injury in patients with ARDS. JCI Insight 2021, 6. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Kronstadt, S.M.; Pottash, A.E.; Levy, D.; Wang, S.; Chao, W.; Jay, S.M. Therapeutic Potential of Extracellular Vesicles for Sepsis Treatment. Adv. Ther. 2021, 4, 2000259. [Google Scholar] [CrossRef]
- Stone, M.L.; Zhao, Y.; Robert Smith, J.; Weiss, M.L.; Kron, I.L.; Laubach, V.E.; Sharma, A.K. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir. Res. 2017, 18, 212. [Google Scholar] [CrossRef]
- Gennai, S.; Monsel, A.; Hao, Q.; Park, J.; Matthay, M.A.; Lee, J.W. Microvesicles Derived From Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. Am. J. Transpl. 2015, 15, 2404–2412. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.G.; Feng, X.M.; Abbott, J.; Fang, X.H.; Hao, Q.; Monsel, A.; Qu, J.M.; Matthay, M.A.; Lee, J.W. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Lonati, C.; Bassani, G.A.; Brambilla, D.; Leonardi, P.; Carlin, A.; Maggioni, M.; Zanella, A.; Dondossola, D.; Fonsato, V.; Grange, C.; et al. Mesenchymal stem cell-derived extracellular vesicles improve the molecular phenotype of isolated rat lungs during ischemia/reperfusion injury. J. Heart Lung Transpl.. 2019, 38, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Varkouhi, A.K.; Jerkic, M.; Ormesher, L.; Gagnon, S.; Goyal, S.; Rabani, R.; Masterson, C.; Spring, C.; Chen, P.Z.; Gu, F.X.; et al. Extracellular Vesicles from Interferon-gamma-primed Human Umbilical Cord Mesenchymal Stromal Cells Reduce Escherichia coli-induced Acute Lung Injury in Rats. Anesthesiology 2019, 130, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, S.; Lim, H.; Liu, A.; Hu, S.; Lee, J.; Zhuo, H.; Hao, Q.; Matthay, M.A.; Lee, J.W. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax 2019, 74, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miceli, V.; Bertani, A.; Chinnici, C.M.; Bulati, M.; Pampalone, M.; Amico, G.; Carcione, C.; Schmelzer, E.; Gerlach, J.C.; Conaldi, P.G. Conditioned Medium from Human Amnion-Derived Mesenchymal Stromal/Stem Cells Attenuating the Effects of Cold Ischemia-Reperfusion Injury in an In Vitro Model Using Human Alveolar Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 510. [Google Scholar] [CrossRef]
- Huang, R.; Qin, C.; Wang, J.; Hu, Y.; Zheng, G.; Qiu, G.; Ge, M.; Tao, H.; Shu, Q.; Xu, J. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging 2019, 11, 7996–8014. [Google Scholar] [CrossRef] [PubMed]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosyula, P.; Korutla, L.; Habertheuer, A.; Reddy, S.; Schaufler, C.; Lasky, J.; Diamond, J.; Cantu, E., 3rd. Ex Vivo Lung Perfusion Model to Study Pulmonary Tissue Extracellular Microvesicle Profiles. Ann. Thorac. Surg. 2017, 103, 1758–1766. [Google Scholar] [CrossRef] [Green Version]
- Gruda, M.C.; Ruggeberg, K.G.; O’Sullivan, P.; Guliashvili, T.; Scheirer, A.R.; Golobish, T.D.; Capponi, V.J.; Chan, P.P. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb(R) sorbent porous polymer beads. PLoS ONE 2018, 13, e0191676. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Kovacs, E.; Racz, K.; Soltesz, A.; Szigeti, S.; Kiss, N.; Csikos, G.; Koritsanszky, K.B.; Berzsenyi, V.; Trembickij, G.; et al. Impact of intraoperative cytokine adsorption on outcome of patients undergoing orthotopic heart transplantation-an observational study. Clin. Transpl. 2018, 32, e13211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferdinand, J.R.; Hosgood, S.A.; Moore, T.; Ferro, A.; Ward, C.J.; Castro-Dopico, T.; Nicholson, M.L.; Clatworthy, M.R. Cytokine absorption during human kidney perfusion reduces delayed graft function-associated inflammatory gene signature. Am. J. Transpl. 2021, 21, 2188–2199. [Google Scholar] [CrossRef]
- Schadler, D.; Pausch, C.; Heise, D.; Meier-Hellmann, A.; Brederlau, J.; Weiler, N.; Marx, G.; Putensen, C.; Spies, C.; Jorres, A.; et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS ONE 2017, 12, e0187015. [Google Scholar] [CrossRef] [Green Version]
- Rieder, M.; Wengenmayer, T.; Staudacher, D.; Duerschmied, D.; Supady, A. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation. Crit. Care 2020, 24, 435. [Google Scholar] [CrossRef]
- Popescu, M.; Dima, S.; David, C.; Tudor, A.; Simionescu, M.; Tomescu, D. Standard renal replacement therapy combined with hemoadsorption in the treatment of critically ill septic patients. Ther. Apher. Dial. 2021, 25, 663–670. [Google Scholar] [CrossRef]
- Schittek, G.A.; Zoidl, P.; Eichinger, M.; Orlob, S.; Simonis, H.; Rief, M.; Metnitz, P.; Fellinger, T.; Soukup, J. Adsorption therapy in critically ill with septic shock and acute kidney injury: A retrospective and prospective cohort study. Ann. Intensive Care 2020, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Friesecke, S.; Stecher, S.S.; Gross, S.; Felix, S.B.; Nierhaus, A. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: A prospective single-center study. J. Artif. Organs 2017, 20, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute Lung Injury: A Clinical and Molecular Review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meduri, G.U.; Headley, S.; Kohler, G.; Stentz, F.; Tolley, E.; Umberger, R.; Leeper, K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 1995, 107, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Zhuo, H.; Brady, S.; Levitt, J.; Steingrub, J.; Siegel, M.D.; Soto, G.; Peterson, M.W.; Chesnutt, M.S.; Matthay, M.A.; et al. Pathogenetic and predictive value of biomarkers in patients with ALI and lower severity of illness: Results from two clinical trials. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L634–L639. [Google Scholar] [CrossRef] [PubMed]
- Kakishita, T.; Oto, T.; Hori, S.; Miyoshi, K.; Otani, S.; Yamamoto, S.; Waki, N.; Yoshida, O.; Okazaki, M.; Yamane, M.; et al. Suppression of inflammatory cytokines during ex vivo lung perfusion with an adsorbent membrane. Ann. Thorac. Surg. 2010, 89, 1773–1779. [Google Scholar] [CrossRef] [Green Version]
- Iskender, I.; Arni, S.; Maeyashiki, T.; Citak, N.; Sauer, M.; Rodriguez, J.M.; Frauenfelder, T.; Opitz, I.; Weder, W.; Inci, I. Perfusate adsorption during ex vivo lung perfusion improves early post-transplant lung function. J. Thorac. Cardiovasc. Surg. 2021, 161, e109–e121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iskender, I.; Cosgun, T.; Arni, S.; Trinkwitz, M.; Fehlings, S.; Yamada, Y.; Cesarovic, N.; Yu, K.; Frauenfelder, T.; Jungraithmayr, W.; et al. Cytokine filtration modulates pulmonary metabolism and edema formation during ex vivo lung perfusion. J. Heart Lung Transpl. 2017. [Google Scholar] [CrossRef]
| Author | Year | Model, Subject Number | Experimental Groups | Cell Type | Cell Characteristics | Cell Dose (Total Cells) | Lung Injury Model | EVLP Length | Treatment Levels of IL-8 | Treatment Levels of IL-10 | Treatment Levels of TNF-α | Pulmonary Function Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone marrow-derived cells | ||||||||||||
| Martens et al. [35] | 2017 | Pig, 6/group | MAPCs vs. perfusate (control) | MAPC | Obtained from Athersys/Regenesys (Cleveland, OH, USA) Tested qPCR and flow for negative and positive markers, tube formation assay, CFSE assay | 150 × 106 | 1.5 h warm ischemia, 1 h cold ischemia | 6 h | Below detection limit for both groups in BAL | Below detection limit for both groups in BAL | Decreased in BAL | No differences in compliance, oxygenation, or PVR |
| Fang et al. [30] | 2015 | Mouse, 12/group | MSCs vs. PBS | MSC | Obtained from Institute for Regenerative Medicine at Texas A&M | 5 × 105 | In vivo ALI with 5 mg/kg IT LPS | No EVLP | - | - | Decreased in in vivo mice and in coculture of MSCs with ATII cells | Increased 48 h survival rate |
| McAuley et al. [29] | 2014 | Human, 3–4/group | MSC vs. perfusate (control) | MSC | Obtained from GMP facility at University of Minnesota, (+) markers: CD73, CD90, CD105 (−) markers: CD14, CD19, CD34, CD45, HLA-DR. Tested for trilineage differentiation | 5 × 106 | 31 +/− 6 h (control) 33 +/− 31 h (MSC) Cold ischemia | 4 h | - | - | - | No differences in pulmonary arterial pressures, perfusate oxygenation, AFC restored |
| Lee et al. [20] | 2013 | Human, 3–4/group | MSC IV vs. MSC IB vs. normal lung fibroblasts (PromoCell, control) | MSC | Obtained from GMP facility at University of Minnesota; met criteria defined by ISCT | 5 × 106 | <48 h ischemic time; followed by induction of ALI in EVLP either with 6 mg E. coli endotoxin or 109 or 109 CFU E. coli bacteria | 6–10 h | Decrease after MSC instillation | In vitro increase in co-culture of MSC with monocytes | In vitro decrease in co-culture of MSC with monocytes | AFC restored |
| Lee et al. [25] | 2009 | Human, 3–6/group | MSC vs. conditioned medium vs. normal lung fibroblasts (PromoCell, control) | MSC | Obtained from NIH repository, Tulane Center for Gene Therapy; met criteria defined by ISCT | 5 × 106 | 21 +/− 13 h ischemic time; induced ALI in EVLP with 0.1 mg/kg E. coli endotoxin | 4 h | MSC not different from injured control | MSC not different from injured control | MSC not different from injured control | AFC restored |
| Human umbilical cord perivascular cells | ||||||||||||
| Nykänen et al. [36] | 2021 | Human 4–5/group | MSCs in one lung vs. perfusate in matched pair lung | MSC modified to produce IL-10 | (+) markers: CD73, CD90, CD105, CD10, CD166, CD140b, CD146, MHC I; (−) markers: CD34, CD45, MHC-II; transgene expression of FLAG tag for IL-10 transduction | 40 × 106 | cold ischemia of 9 h (7.6–12.3) in control, 8.9 h (7.9–11.6) in MSC | 12 h | MSC not different from injured control | Increased in tissue and perfusate | - | No difference in PVR, oxygenation, compliance, airway pressure |
| Pacienza et al. [33] | 2019 | Rat 8–10/group | MSCs vs. vehicle control of Krebs-Henseleit solution | MSC | Obtained from Laboratory of Gene Therapy at Universidad Austral, met ISCT guidelines, (+) markers: CD44, CD90, CD105 (−) markers: CD11b, CD34, CD45 | 1 × 106 | 2 h warm ischemia, 90 min cold ischemia | 1 h | - | - | - | Compliance decreased by less from baseline in MSC group |
| Nakajima et al. [32] | 2019 | Pig, 6/group | MSCs vs. perfusate (control) | MSC | Obtained from Tissue Regeneration Therapeutics, (+) marker: CD73 | 5 × 106/kg | 24 h cold ischemia | 12 h | MSC not different from control in EVLP or post-transplant | - | MSC not different from control in EVLP, decreased post- transplant | Peak airway pressure reduced in EVLP, no change in oxygenation, PVR, compliance in EVLP or post-transplant |
| Stone et al. [41] | 2017 | Mouse 6–8/group | MSCs vs. EVs vs. Steen Solution vs. Krebs Henseleit buffer | MSCs | (+) markers: CD73, CD90, CD105, CD44 (−) markers: CD45, CD34, CD11b, CD19, HLA-DR Tested for trilineage differentiation | 1 × 106 before ischemia, 3 × 106 in EVLP | In vivo 1 h hilar occlusion followed by 2 h reperfusion Or 1 h warm ischemia, 1 h cold ischemia followed by EVLP | 1 h | - | Increased in in vivo model | Decreased in in vivo model | Increasing compliance, decreased PA pressure in both in vivo and EVLP models |
| Mordant et al. [31] | 2016 | Pig, 3–5/group | IB MSC vs. IV MSC vs. no cells | MSC | Obtained from Tissue Regeneration Therapeutics, (+) marker: CD73 | IB: 50 × 106 IV: 50 × 106 150 × 106 300 × 106 | 18 h cold ischemia | 12 h | Decreased in IV MSC | IV MSC not different from control | - | No change in PVR in IV MSC, transient increase in IB, Increased oxygenation, compliance with 150 × 106 IV dose. |
| La Francesca et al. [34] | 2014 | Human, 4 | MAPC or sterile saline (control) | MAPC | (+) markers: CD49c, CD90 (−) markers: MHC class II, CD45 | 1 × 107 | 8 h cold ischemia | 4 h | - | No significant difference in tissue or BAL | - | Reduced injury on histology scoring, reduced neutrophils and eosinophils |
| Author | Year | Model, Subject Number | EV Type | Characteristics | Dose | Reported Size | Isolation Method | Origin of MSC | EVLP | Pulmonary Function Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Whole media or whole fraction of EVs | ||||||||||
| Miceli et al. [51] | 2021 | Human cell line | Unmanipulated conditioned medium | No characterization of EVs | Each mL of collected medium was conditioned by 106 cells, Media from 2 days of cell growth after second passage | Not Applicable | Centrifugation, unspecified | Amnion of human term placenta | Modification adapted for cultured A549 cells | - |
| Lonati et al. [48] | 2019 | Rat, 5/group | EVs | NanoSightfor distribution Reported using further FACS, western blot, and EM | 0.5 mL aliquot with 24.56 ± 5.53 × 1010 EVs/mL diluted into 5 mL | Average diameter of 100 nm | Supernatant after overnight culture from 1 × 106 cells that was centrifuged at 3000× g for 20 min and then 100,000× g for 120 min at 4 °C | Unspecified | 3 h | Decreased TPVR, NO metabolites and peak pressure. No difference in compliance or oxygenation. |
| Varkouhi et al. [49] | 2019 | Rat, 8–18/group | EVs (from naïve or IFN-γ primed MSCs) | Flow cytometry with small particle detection modifications, TEM detection | 100 × 106 EVs/kg derived from 35–40 × 106 MSCs | 71.8 nm ± 15.7 nm (naïve) and 47.7 ± 25.2 nm (IFN-γ primed) | Centrifuged at 300× g and 2000× g for 10 min and then 100,000 g for 90 min at 4 °C | Human umbilical cord | No | Enhanced survival after E. coli pneunonia |
| Stone et al. [41] | 2017 | Mouse 6–8/group | EVs | Nanosight for size and concentration, imaging flow cytometry for CD90, CD44, CD73 and lipohilic dye, quantified protein and RNA content | 1 × 106 prior to ischemia and 3 × 106 in EVLP | 164 ± 10.4 nm | Supernatant from cells overnight was centrifuged at 10,000× g for 20 min and then 100,000× g for 1 h at 4 °C twice | Human Umbilical cord | 1 h | Improved pulmonary compliance and pulmonary artery pressure |
| Microvesicles | ||||||||||
| Park et al. [50] | 2019 | Human lungs, 5–9/group | Microvesicles | Nanosight, Labeled to separate from debris and did flow cytometry (CD9, CD44), SEM | 1 × or 2 × 200uL, 10 uL is release of 106 cells over 2 days | Mean size of 180 ± 14 nm | Conditioned medium collected after 48 h, centrifuged 3000 rpm for 20 min and then 100,000 for 1 h twice at 4 °C | Human bone marrow | 6 h | Improved AFC, no significant difference in PAP, PVR, compliance, or oxygenation |
| Vallabhajosyula et al. [52] | 2017 | Human lungs, 6 | Microvesicles | Nanosight fluorescence analysis (MHC I, MHC II, VE-cadherin, CD14, Flotillin-1, CD63, PECAM-1, cytochromeC, β-actin), RNA analysis of cargo, protein and western blot analysis proteomic profiling | EVs from the lung, isolated from perfusate | Median size 212 nm (195–240) and 165 nm (161–190) across groups | Perfusate first centrifuged at 500× g 10 min, then passed through Sepharose exclusion column and eluant was pooled and ultrafiltered (100-kDa cutoff) and ultracentrifugated 120,000× g for 4 h at 4 °C | Vesicles released by perfused human lung | up to 4 h | Larger vesicle size in lungs not transplanted |
| Gennai et al. [44] | 2015 | Human lungs 4–6/group | Microvesicles | TEM, protein content, Ang1 expression, western blot (CD44), PCR for Ang1 | 100 or 200 uL doses; (10 uL per 1 × 106 cells) | 50 to 200 nm | Media from 48 h was centrifuged at 300× g for 20 min and then 100,000× g for 1 h at 4 °C twice | Human bone marrow | 8 h | Improved AFC, restored tracheal pressure, increased compliance relative to baseline. Reduced PAP or PVR. No significant different in oxygenation. |
| Zhu et al. [45] | 2014 | Mouse 14–20/group | Microvesicles | TEM, total protein, RT-PCR (Ang1), KGF/FGF7, CO1 & CO2) | 15 and 30 uL (10 uL per 1 × 106 cells) | Approx 200 nm | Media from 48 h was centrifuged at 3000 rpm for 20 min and then 100,000× g for 1 h at 4 °C twice | Human bone marrow | No | Increased protein permeability in primary cultures of ATII cells |
| Author | Year | Model, Subject Number | Lung Injury Model | EVLP Lenght | Cytokine Filtration Type | Treatment Levels of IL-8 | Treatment Levels of TNF-a | Oxygenation | Histology |
|---|---|---|---|---|---|---|---|---|---|
| Kakishita et al. [66] | 2010 | Porcine 5–6/group | Not applicable | 12 h | Lixelle S35 | Significantly lower in treatment group | Significantly lower in treatment group | No significant differences between groups | Similar levels of edema formation between groups. |
| Iskender et al. [68] | 2017 | Porcine, 5/group | 24 h cold ischemia | 12 h | CytoSorb adsorber | Significantly lower plasma levels of all cytokines in treatment group during EVLP. | Significantly lower in treatment group | Not studied | Significantly lower lung injury scores in treatement group. |
| Iskender et al. [67] | 2021 | Porcine, 5/group | 24 h cold ischemia | 6 h | CytoSorb adsorber | Significantly lower plasma levels of all cytokines in treatment group after 6 h of EVLP, however no differences found at 8 h post transplantation. | Not studied | Significantly better venoareterial oxygen pressure gradient in adsorption group after 6 h of EVLP as well as post transplantation. | Comparable microscopic lung injury scoring between the groups. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niroomand, A.; Hirdman, G.; Olm, F.; Lindstedt, S. Current Status and Future Perspectives on Machine Perfusion: A Treatment Platform to Restore and Regenerate Injured Lungs Using Cell and Cytokine Adsorption Therapy. Cells 2022, 11, 91. https://doi.org/10.3390/cells11010091
Niroomand A, Hirdman G, Olm F, Lindstedt S. Current Status and Future Perspectives on Machine Perfusion: A Treatment Platform to Restore and Regenerate Injured Lungs Using Cell and Cytokine Adsorption Therapy. Cells. 2022; 11(1):91. https://doi.org/10.3390/cells11010091
Chicago/Turabian StyleNiroomand, Anna, Gabriel Hirdman, Franziska Olm, and Sandra Lindstedt. 2022. "Current Status and Future Perspectives on Machine Perfusion: A Treatment Platform to Restore and Regenerate Injured Lungs Using Cell and Cytokine Adsorption Therapy" Cells 11, no. 1: 91. https://doi.org/10.3390/cells11010091
APA StyleNiroomand, A., Hirdman, G., Olm, F., & Lindstedt, S. (2022). Current Status and Future Perspectives on Machine Perfusion: A Treatment Platform to Restore and Regenerate Injured Lungs Using Cell and Cytokine Adsorption Therapy. Cells, 11(1), 91. https://doi.org/10.3390/cells11010091







