Regulation of Oxidative Phosphorylation of Liver Mitochondria in Sepsis
Abstract
1. Introduction
2. Mitochondrial Oxidative Phosphorylation
2.1. Mitochondrial Structure
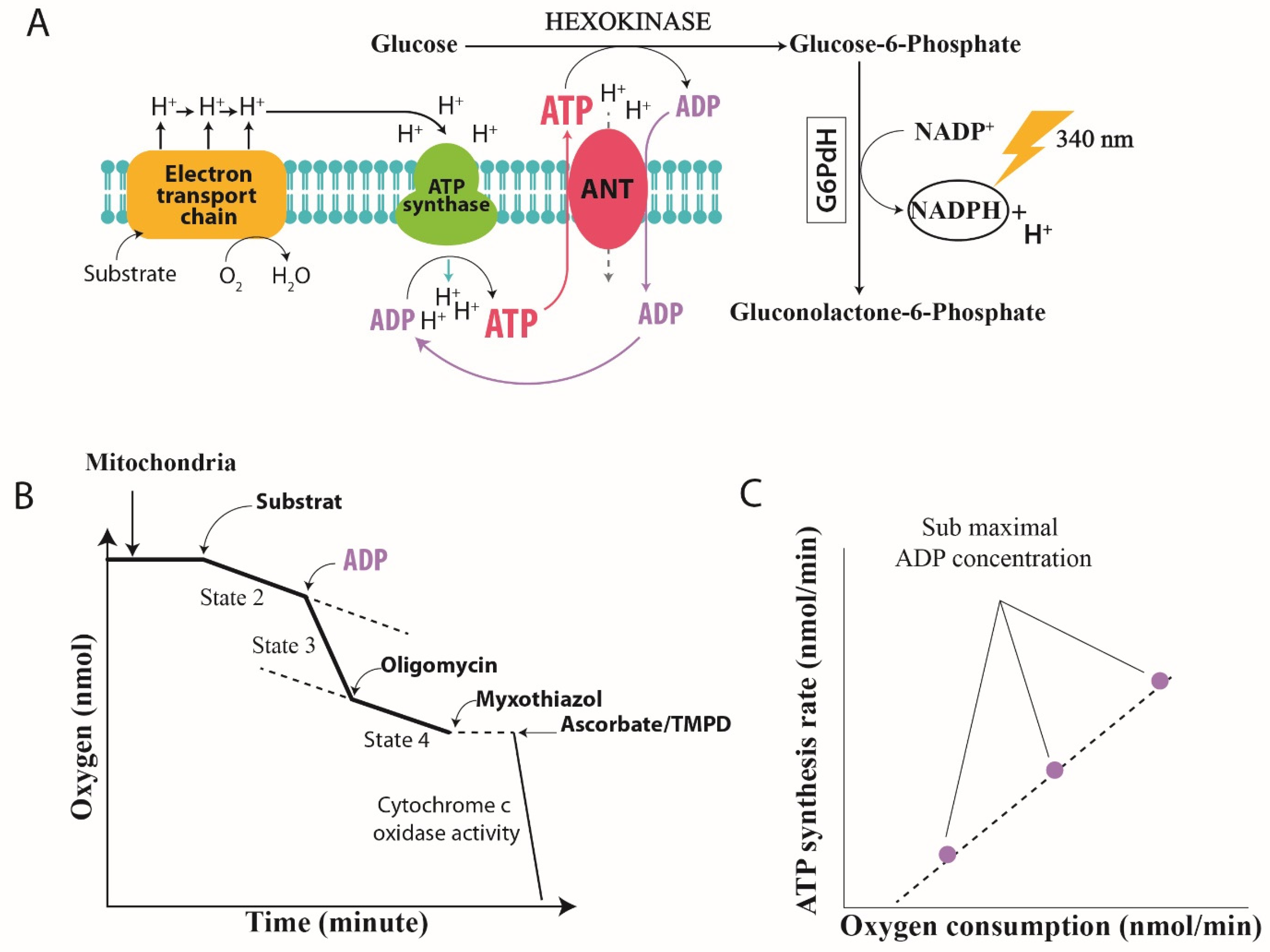
2.2. Oxidative Phosphorylation Efficiency
2.2.1. Quantification of Mitochondrial Efficiency
2.2.2. Regulation of Mitochondrial Oxidative Phosphorylation in Sepsis
A. Proton Conductance and Proton Leakage through the Inner Membrane
B. Changes in Proton Pumping Stoichiometry
B.1 Redox Slipping
B.2 Proton Slipping
3. Therapeutic Implications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.E.; Pearlstein, L.; Fulton, R.L.; Polk, H.C., Jr. Multiple system organ failure: The role of uncontrolled infection. Arch. Surg. 1980, 115, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Nesseler, N.; Launey, Y.; Aninat, C.; White, J.; Corlu, A.; Pieper, K.; Malledant, Y.; Seguin, P. Clinical review: The liver in sepsis. Crit. Care 2012, 16, 235. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ba, Z.F.; Chaudry, I.H. Mechanism of hepatocellular dysfunction during early sepsis: Key role of increased gene expression and release of pro inflammatory cytokines tumor necrosis factor and interleukin-6. Arch. Surg. 1991, 214, 141–148. [Google Scholar] [CrossRef]
- Wang, P.; Ba, Z.F.; Chaudry, I.H. Hepatic extraction of indocyanine green is depressed early in sepsis despite increased hepatic blood flow and cardiac output. Arch. Surg. 1991, 126, 219–224. [Google Scholar] [CrossRef]
- Eyenga, P.; Roussel, D.; Morel, J.; Rey, B.; Romestaing, C.; Teulier, L.; Sheu, S.S.; Goudable, J.; Negrier, C.; Viale, J.P. Early septic shock induces oxidative stress and loss of oxidative Phosphorylation yield plasticity in liver mitochondria. J. Physiol. Biochem. 2014, 70, 285–2967. [Google Scholar] [CrossRef]
- Carchman, E.H.; Whelan, S.; Lougran, P.; Mollen, K.; Stratamirovic, S.; Shiva, S.; Rosengart, M.R.; Zuckerbraun, B.S. Experimental sepsis-induced mitochondrial biogenesis is dependent on autophagy, TLR4, and TLR9 signaling in liver. FASEB J. 2013, 27, 4703–4711. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 2. [Google Scholar] [CrossRef]
- Singer, M.; De Santis, V.; Vitale, D. Multiorgan failure is an adaptative endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet 2004, 364, 545–548. [Google Scholar] [CrossRef]
- Conley, K.E. Mitochondria to motion: Optimizing oxidative phosphorylation to improve exercise performance. J. Exp. Biol. 2016, 219, 243–249. [Google Scholar] [CrossRef]
- Haden, D.W.; Suliman, H.B.; Carraway, M.S.; Welty-Wolf, K.E.; Ali, A.S.; Shitara, H.; Yonekawa, H.; Piantadosi, C.A. Mitochondrial biogenesis restores oxidative metabolism during Staphylococcus aureus sepsis. Am. J. Respir. Crit. Care Med. 2007, 176, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Eyenga, P.; Roussel, D.; Morel, J.; Rey, B.; Romestaing, C.; Gueguen-Chaignon, V.; Sheu, S.S.; Viale, J.P. Time course of liver mitochondrial function and intrinsic changes in oxidative phosphorylation in a rat model of sepsis. Intensive Care Med. Exp. 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zong, S.; Wu, M.; Gu, J.; Yang, M. Architecture of human mitochondrial respiratory megacomplex I2III2IV2. Cell 2017, 170, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. The efficiency and plasticity of mitochondrial energy transduction. Biochem. Soc. Trans. 2005, 33, 897–904. [Google Scholar] [CrossRef]
- Goo, S.; Pham, T.; Han, J.C.; Nielsen, P.; Taberner, A.; Hickey, A.; Loiselle, D. Multiscale measurement of cardiac energetics. Clin. Exp. Pharmacol. Physiol. 2013, 40, 671–681. [Google Scholar] [CrossRef]
- Fontaine, E.M.; Moussa, M.; Devin, A.; Garcia, J.; Ghisolfi, J.; Rigoulet, M.; Leverve, X.M. Effect of polyunsaturated fatty acids deficiency on oxidative phosphorylation in rat liver mitochondria. Biochim. Biophys. Acta 1996, 1276, 181–187. [Google Scholar] [CrossRef][Green Version]
- Passarella, S.; Ostuni, A.; Atlante, A.; Quagliariello, E. Increase in the ADP/ATP exchange in rat liver mitochondria irradiated in vitro by helium-neon laser. Biochem. Biophys. Res. Commun. 1988, 156, 978–986. [Google Scholar] [CrossRef]
- Gouspillou, G.; Rouland, R.; Calmettes, G.; Deschodt-Arsac, V.; Franconi, J.M.; Bourdel-Marchasson, I.; Diolez, P. Accurate determination of oxidative phosphorylation affinity for ADP in isolated mitochondria. PLoS ONE 2011, 6, e20709. [Google Scholar] [CrossRef]
- Herminghaus, A.; Papenbrock, H.; Eberhardt, R.; Vollmer, C.; Truse, R.; Schulz, J.; Bauer, I.; Weidinger, A.; Kozlov, A.V.; Stiban, J.; et al. Time-related changes in hepatic and colonic mitochondrial oxygen consumption after abdominal infection in rats. Intensive Care Med. Exp. 2019, 7, 4. [Google Scholar] [CrossRef]
- Kantrow, S.P.; Taylor, D.E.; Carraway, M.S.; Piantadosi, C.A. Oxidative metabolism in rat hepatocytes and mitochondria during sepsis. Arch. Biochem. Biophys. 1997, 245, 278–288. [Google Scholar] [CrossRef]
- Geller, E.R.; Jankauskas, S.; Kirkpatrick, J. Mitochondrial death in sepsis: A failed concept. J. Surg. Res. 1986, 40, 514–517. [Google Scholar] [CrossRef]
- Dejager, L.; Pinheiro, I.; Dejonckheere, E.; Libert, C. Cecal ligation and puncture: The gold standard model for polymicrobial sepsis? Trends Microbiol. 2011, 19, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Chaudry, I.H. Cellular energetics and ATP-MgCl2 therapy in sepsis. Am. J. Emerg. Med. 1984, 360, 219–223. [Google Scholar] [CrossRef]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.E.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. [Google Scholar] [CrossRef]
- Fontaine, E.M.; Devin, A.; Rigoulet, M.; Leverve, X.M. The yield of oxidative phosphorylation is controlled both by force and flux. Biochem. Biophys. Res. Commun. 1997, 232, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Tsou, C.S.; Van Dam, K. Dependence of the efficiency of uncouplers on respiratory rate. Biochim. Biophys. Acta 1969, 172, 174–176. [Google Scholar] [CrossRef]
- Vary, T.C. Sepsis-induced alterations in pyruvate dehydrogenase complex activity in rat skeletal muscle: Effects on plasma lactate. Shock 1996, 6, 89–94. [Google Scholar] [CrossRef]
- Khan, A.U.; Delude, R.L.; Han, Y.Y.; Sappington, P.L.; Han, X.; Carcillo, J.A.; Fink, M.P. Liposomal NAD(C) prevents diminished O(2) consumption by immunostimulated Caco-2 cells. Am. J. Physiol. Lung Mol. Physiol. 2002, 282, L1082–L1091. [Google Scholar] [CrossRef]
- Cimolai, M.C.; Alvarez, S.; Bode, C.; Bugger, H. Mitochondrial mechanisms in septic cardiomyopathy. Int. J. Mol. Sci. 2015, 16, 17763–17778. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, Y.; Zhang, Z. Sepsis-induced myocardial dysfunction (SIMD): The pathophysiological mechanisms and therapeutic strategies targeting mitochondria. Inflammation 2020, 43, 1184–1200. [Google Scholar] [CrossRef]
- Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by chemi-osmotic type of mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, H.; Mela, H. Alteration of mitochondrial metabolism and protein concentrations in subacute septicemia. Infect. Immun. 1982, 38, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Divakaruni, A.S.; Brand, M.D. The regulation and physiology of mitochondrial proton leak. Physiology 2011, 26, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat liver mitochondria as determined by ion distribution. Eur. J. Biochem. 1974, 50, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S.; Rolfe, D.F.; Brand, M.D. The proton permeability of liposomes made from mitochondrial inner membrane phospholipids: Comparison with isolated mitochondria. J. Membr. Biol. 1997, 155, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Julian, M.W.; Huff, J.E.; Struck, J.; Cook, C.H. Carbamoyl phosphate synthase-1: A marker of mitochondrial damage and depletion in liver during sepsis. Crit. Care Med. 2006, 34, 2439–2446. [Google Scholar] [CrossRef]
- Brand, M.D.; Pakay, J.L.; Ocloo, A. The basal conductance of mitochondria depends on adenine nucleotide translocase content. Biochem. J. 2005, 392, 353–362. [Google Scholar] [CrossRef]
- Hong, Y.; Fink, B.D.; Dillon, J.S.; Sivitz, W.I. Effect of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. Endocrinology 2001, 142, 249–256. [Google Scholar] [CrossRef][Green Version]
- Cortez-Pinto, H.; Yang, S.Q.; Lin, H.Z.; Costa, S.; Hwang, C.S.; Lane, M.D.; Bagby, G.; Diehl, A.M. Bacterial lipopolysaccharide induces uncoupling protein-2 expression in hepatocytes by a tumor necrosis factor-alpha-dependent mechanism. Biochem. Biophys. Res. Commun. 1998, 251, 313–319. [Google Scholar] [CrossRef]
- Yu, X.X.; Barger, J.L.; Boyer, B.B.; Brand, M.D.; Pan, C.; Adams, S.H. Impacts of endotoxin on UCP homolog mRNA abundance, thermoregulation and mitochondrial proton leak kinetics. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E433–E444. [Google Scholar] [CrossRef]
- Jaburek, M.; Varecha, M.; Gimeno, R.E.; Dembski, M.; Jezek, P.; Zhang, M.; Burn, P.; Tartaglia, L.A.; Garlid, K.D. Transport function and regulation of mitochondrial uncoupling protein 2 and 3. J. Biol. Chem. 1999, 274, 26003–26007. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Luo, S.; Xie, P.; Hou, T.; Yu, T.; Fu, X. Over expression of UCP 2 regulates mitochondrial flashes and reverses lipopolysaccharides -induces cardiomyocytes injury. Am. J. Transl. Res. 2018, 10, 1347–1356. [Google Scholar] [PubMed]
- Pan, S.; Wang, N.; Bisetto, S.; Bing, Y.; Sheu, S.S. Downregulation of adenine nucleotide translocator 1 exacerbates tumor necrosis factor -α-mediated cardiac inflammatory responses. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H39–H48. [Google Scholar] [CrossRef] [PubMed]
- Pietrobon, D.; Azzone, G.F.; Walz, D. Effect of funiculosin and Antimycin A on the redox-driven H+-pumps in mitochondria: On the nature of “leaks”. Eur. J. Biochem. 1981, 117, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Borutaite, V. Nitric oxide and mitochondrial respiration in heart. Cardiovasc. Res. 2007, 75, 283–290. [Google Scholar] [CrossRef]
- Eyenga, P.; Lhuillier, F.; Morel, J.; Roussel, D.; Sibille, B.; Letexier, D.; Cespuglio, R.; Duchamp, C.; Goudable, J.; Bricca, G.; et al. Time course of liver nitric oxide concentration in early septic shock by cecal ligation and puncture in rats. Nitric Oxide 2010, 23, 194–198. [Google Scholar] [CrossRef]
- Brealey, D.; Karyampudi, S.; Jacques, T.S.; Novelli, M.; Stidwill, R.; Taylor, V.; Smolenski, R.T.; Singer, M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 286, E491–E497. [Google Scholar] [CrossRef]
- Zapelini, P.H.; Rezin, G.T.; Cardoso, M.R.; Ritter, C.; Klamt, F.; Moreira, J.C.; Streck, E.L.; Dal-Pizzol, F. Antioxidant treatment reverses mitochondrial dysfunction in a sepsis animal model. Mitochondrion 2008, 8, 211–218. [Google Scholar] [CrossRef]
- Papa, S.; Guerrieri, F.; Capitanio, N. A possible role of slips in cytochrome c oxidase in antioxygen defense system of cell. Biosci. Rep. 1997, 17, 23–31. [Google Scholar] [CrossRef][Green Version]
- Piquet, M.A.; Nogueira, V.; Devin, A.; Sibille, M.; Filippi, C.; Fontaine, E.; Roulet, M.; Rigoulet, M.; Leverve, X.M. Chronic ethanol ingestion increases efficiency of oxidative phosphorylation in rat liver mitochondria. FEBS Lett. 2000, 468, 239–242. [Google Scholar] [CrossRef][Green Version]
- Papa, S.; Lorusso, M.; Capitanio, N. Mechanistic and phenomelogical features of proton pumps in respiratory chain of mitochondria. J. Bioenerg. Biomembr. 1994, 26, 559–608. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Liu, J.; Svahn, E.; Fergusson-Miller, S.; Brzezinski, P. Structural changes at the surface of cytochrome c oxidase alter the proton-pumping stoichiometry. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148116. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.J.; Vijayasarathy, C.; Raj, N.R.; Avadhani, N.G.; Deutschman, C.S. Competitive and non competitive inhibition of myocardial cytochrome C oxidase in sepsis. Shock 2004, 21, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Converso, D.P.; Taillé, C.; Carreras, M.C.; Jaitovich, A.; Poderoso, J.J.; Boczkowski, J. HO-1 is located in liver mitochondria and modulates mitochondria heme content and metabolism. FASEB J. 2006, 20, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Guo, H.; Kuo, P.C. Endotoxin-stimulated nitric oxide production inhibits expression of cytochrome c oxidase in ANA-1 murine macrophage. J. Immunol. 2002, 168, E110–E119. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Huttemann, M. Energy crisis: The role of oxidative phosphorylation in acute inflammation and sepsis. Biochim. Biophys. Acta 2014, 1842, 1579–1586. [Google Scholar] [CrossRef]
- Boczkowski, J.; Lisdero, C.; Lanone, S.; Samb, A.; Carreras, M.C.; Boveris, A.; Aubier, M.; Poderoso, J.J. Endogenous peroxynitrite mediate mitochondrial dysfunction in rat diaphragm during endotoxemia. FASEB J. 1999, 13, 1637–1646. [Google Scholar] [CrossRef]
- Callahan, L.A.; Supinski, G.S. Sepsis induces diaphragm electron transport chain dysfunction and protein depletion. Am. J. Respir. Crit. Care Med. 2005, 172, 861–868. [Google Scholar] [CrossRef]
- Clerc, P.; Rigoulet, M.; Leverve, X.; Fontaine, E. Nitric oxide increases oxidative phosphorylation efficiency. J. Bioenerg. Biomembr. 2007, 39, 158–166. [Google Scholar] [CrossRef]
- Lizasoain, L.; Moro, M.A.; Knowles, R.G.; Darley-Usmar, V.; Moncada, S. Nitric oxide and peroxynitrite exert distinct effect on mitochondrial respiration which are differentially blocked by glutathione or glucose. Biochem. J. 1996, 314, 877–880. [Google Scholar] [CrossRef]
- Samavati, L.; Lee, I.; Mathes, I.; Lottspeich, F.; Huttemann, M. Tumor necrosis factor α inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit 1 of cytochrome c oxidase. Intensive Care Med. 1996, 22, 849–855. [Google Scholar] [CrossRef]
- Berek, K.; Margreite, J.; Willeit, J.; Berek, A.; Schmutzhard, E.; Mutz, N.J. Polyneuropathies in critically ill patients: A prospective evaluation. Intensive Care Med. 1996, 22, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Rigoulet, M.; Leverve, X.; Fontaine, E.; Ouhabi, R.; Guerin, B. Quantitative analysis of some mechanisms affecting the yield of oxidative phosphorylation: Dependence upon both fluxes and forces. Mol. Cell Biochem. 1998, 184, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Jiapassu, A.M.; Santiago, A.P.; D’Avila, J.C.; Garcia-Souza, L.F.; Galina, A.; Castro Faria-Neto, H.C.; Bozza, F.A.; Oliveira, M.F. Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1F0 adenosine-5-triphophate synthase activity. Crit. Care Med. 2011, 39, 1056–1063. [Google Scholar] [CrossRef]
- Lu, S.M.; Song, S.M.; Liu, J.C.; Yang, H.M.; Li, P.; Wang, Z.G. Changes of proton transportation across the inner mitochondrial membrane and H (+) ATPase in endotoxic shock rats. Chin. J. Traumatol. 2003, 6, 292–296. [Google Scholar] [PubMed]
- Kadenbach, B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim. Biophys. Acta 2003, 1604, 77–94. [Google Scholar] [CrossRef]
- Wittig, I.; Carrozzo, R.; Santorelli, F.M.; Schagger, H. Supercomplexes and subcomplexes of mitochondrial oxidative phosphorylation. Biochim. Biophys. Acta-Bioenerg. 2006, 1757, 1066–1072. [Google Scholar] [CrossRef]
- Weissert, V.; Rieger, B.; Morris, S.; Arroun, T.; Psathaki, O.E.; Zobel, T.; Perkins, G.; Busch, K.B. Inhibition of the mitochondrial ATPase function by IF1 changes the spatiotemporal organization of ATP synthase. Biochim. Biophys. Acta-Bioenerg. 2021, 1862, 148322. [Google Scholar] [CrossRef]
- Lefebvre-Legendre, L.; Salin, B.; Schaeffer, J.; Brethes, D.; Dautant, A.; Ackerman, S.H.; Di Rago, J.P. Failure of assembly the alpha 3beta 3 subcomplex of ATP synthase lead to accumulation of alpha and beta sub units with inclusion bodies and the loss of mitochondrial cristae in saccharomyces cerivisae. J. Biol. Chem. 2005, 280, 18386–18392. [Google Scholar] [CrossRef]
- Lapaille, M.; Thiry, M.; Perez, E.; Gonzalez-Halphen, D.; Remacle, C.; Cardol, P. Loss of mitochondrial ATP synthase sub unit beta (ATP2) alters mitochondrial chloroplastic function and morphology in chlamydomonans. Biochim. Biophys. Acta 2010, 1797, 1533–1539. [Google Scholar] [CrossRef]
- Zheng, G.; Lyu, J.; Huang, J.; Xiang, D.; Xie, M.; Zeng, Q. Experimental treatments for mitochondrial dysfunction in sepsis: A narrative review. J. Res. Med. Sci. 2015, 20, 185–195. [Google Scholar] [PubMed]
- Dare, A.J.; Phillips, A.R.; Hickey, A.J.; Mittal, A.; Loveday, B.; Thompson, N.; Windsor, J.A. A systematic review of experimental treatments for mitochondrial dysfunction in sepsis and multiple organ dysfunction syndrome. Free Radic. Biol. Med. 2009, 47, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F. Bench-to-bedside review: Targeting antioxidants to mitochondria in sepsis. Crit. Care 2010, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Piel, D.A.; Gruber, P.J.; Weinheimer, C.J.; Courtois, M.R.; Robertson, C.M.; Coopersmith, C.M.; Deutschman, C.S.; Levy, R.J. Mitochondrial resuscitation with exogenous cytochrome c in the septic heart. Crit. Care Med. 2007, 35, 2120–2127. [Google Scholar] [CrossRef]
- Piel, D.A.; Deutschman, C.S.; Levy, R.J. Exogenous cytochrome C restores myocardial cytochrome c activity into the late phase of sepsis. Shock 2008, 29, 612–616. [Google Scholar] [CrossRef]
- Groening, P.; Huang, Z.; La Gamma, E.F.; Levy, R.J. Glutamine restores myocardial cytochrome c oxidase activity and improves cardiac function during experimental sepsis. J. Parenter. Enter. Nutr. 2011, 35, 249–254. [Google Scholar] [CrossRef]
- Verma, R.; Huang, Z.; Deutschman, C.S.; Levy, R.J. Caffeine restores myocardial cytochrome c oxidase activity and improves cardiac function during sepsis. Crit. Care Med. 2009, 37, 1397–1402. [Google Scholar] [CrossRef]
- Yang, X.; Lu, G.P.; Cai, X.D.; Lu, Z.J.; Kissoon, N. Alterations of complex IV in the tissues of a septic mouse model. Mitochondrion 2019, 49, 89–96. [Google Scholar] [CrossRef]
- Eyenga, P.; Roussel, D.; Rey, B.; Ndille, P.; Teulier, L.; Eyenga, F.; Romestaing, C.; Morel, J.; Gueguen-Chaignon, V.; Sheu, S.S. Mechanical ventilation preserves diaphragm mitochondrial function in a rat sepsis model. Intensive Care Med. Exp. 2021, 9, 19. [Google Scholar] [CrossRef]
- Choi, H.M.; Jo, S.K.; Kim, S.H.; Lee, J.W.; Cho, E.; Hyun, Y.Y.; Cha, J.J.; Kang, Y.S.; Cha, D.R.; Cho, W.Y.; et al. Glucocorticoids attenuate septic acute kidney injury. Biochem. Biophys. Res. Commun. 2013, 435, 678–684. [Google Scholar] [CrossRef]
- Arvier, M.; Lagoutte, L.; Johnson, G.; Dumas, J.F.; Sion, B.; Grizard, G.; Malthiery, Y.; Simard, G.; Ritz, P. Adenine nucleotide translocator promotes oxidative phosphorylation and mild uncoupling in mitochondria after dexamethasone treatment. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1320–E1324. [Google Scholar] [CrossRef][Green Version]
- Annane, D.; Bellissant, E.; Bollaert, P.E.; Briegel, J.; Keh, D.; Kupfer, Y. Corticosteroids for treating sepsis. Cochrane Database Syst. 2015, 12, CD002243. [Google Scholar] [CrossRef] [PubMed]
- Ogbi, M.; Chew, C.S.; Pohl, J.; Stuchlik, O.; Ogbi, S.; Johnson, J.A. Cytochrome c oxidase subunit IV as a marker of protein kinase Cε function in neonatal cardiac myocytes: Implications for cytochrome c oxidase activity. Biochem. J. 2004, 382, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Malaisse, W.J.; Nadi, A.B.; Ladriere, L.; Zhang, T.M. Protective effects of succinic acid dimethyl ester infusion in experimental endotoxemia. Nutrition 1997, 13, 330–341. [Google Scholar] [CrossRef]
- Chapela, S.P.; Burgos, I.; Congost, C.; Canzonieri, R.; Muryan, A.; Alonzo, M.; Stella, C.A. Parenteral succinate reduces systemic ros production in septic rats, but it does not reduce creatinine levels. Oxidative Med. Cell. Longev. 2018, 2018, 1928945. [Google Scholar] [CrossRef]
- Xin, T.; Lu, C. SirT3 activates AMPK-related mitochondrial biogenesis and ameliorates sepsis-induced myocardial injury. Aging 2020, 12, 16224–16237. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.F.; Liu, X.T.; Li, Y.C.; Zhu, H.M.; Sun, M.R.; Li, P.; Lui, B.; Yang, H. Songorine promotes cardiac mitochondrial biogenesis via Nrf2 induction during sepsis. Redox Biol. 2021, 38, 101771. [Google Scholar] [CrossRef]

 ) indicate increase, arrow (
) indicate increase, arrow ( ) indicate decrease, arrow (
) indicate decrease, arrow ( ) indicate no changes.
) indicate no changes.
 ) indicate increase, arrow (
) indicate increase, arrow ( ) indicate decrease, arrow (
) indicate decrease, arrow ( ) indicate no changes.
) indicate no changes.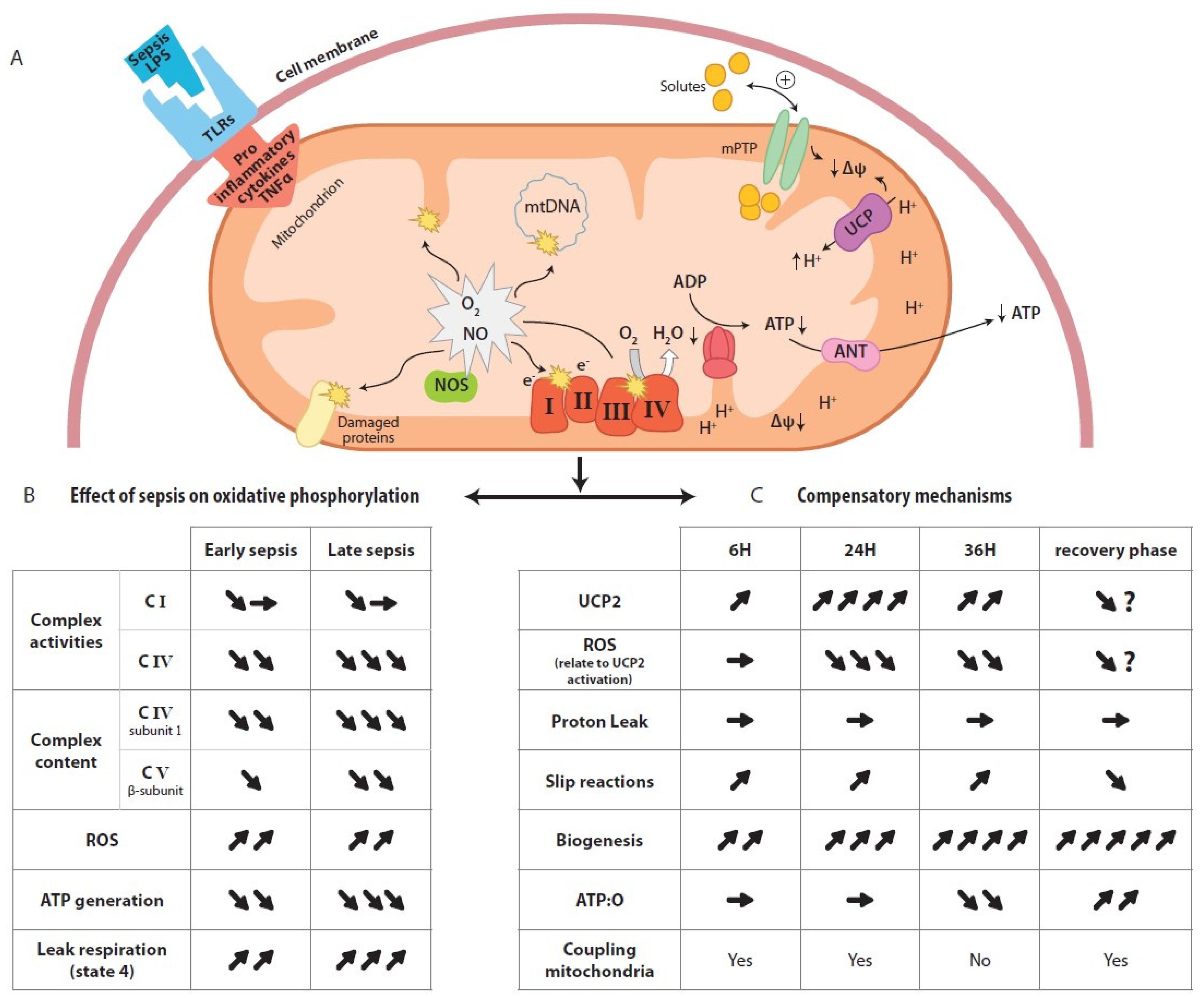
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eyenga, P.; Rey, B.; Eyenga, L.; Sheu, S.-S. Regulation of Oxidative Phosphorylation of Liver Mitochondria in Sepsis. Cells 2022, 11, 1598. https://doi.org/10.3390/cells11101598
Eyenga P, Rey B, Eyenga L, Sheu S-S. Regulation of Oxidative Phosphorylation of Liver Mitochondria in Sepsis. Cells. 2022; 11(10):1598. https://doi.org/10.3390/cells11101598
Chicago/Turabian StyleEyenga, Pierre, Benjamin Rey, Lilia Eyenga, and Shey-Shing Sheu. 2022. "Regulation of Oxidative Phosphorylation of Liver Mitochondria in Sepsis" Cells 11, no. 10: 1598. https://doi.org/10.3390/cells11101598
APA StyleEyenga, P., Rey, B., Eyenga, L., & Sheu, S.-S. (2022). Regulation of Oxidative Phosphorylation of Liver Mitochondria in Sepsis. Cells, 11(10), 1598. https://doi.org/10.3390/cells11101598






