Genotoxicity Associated with 131I and 99mTc Exposure in Nuclear Medicine Staff: A Physical and Biological Monitoring Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Study Population
2.2. Iodine-131 and 99mTc Blood Activity Measurements and Concentrations in Room Air
2.3. Whole-Body Physical Dosimetry
2.4. Cytokinesis-Block Micronucleus Assay (CBMN)
2.5. Premature Chromosome Condensation (PCC) Test
2.6. DNA Damage Measurement by Comet Assay
2.7. Statistical Analysis
3. Results
3.1. Internal and Physical Exposure Monitoring and Characteristics of the 99mtc Group
3.2. Characteristics of the Examined Groups Stratified by Selected Confounding Factors
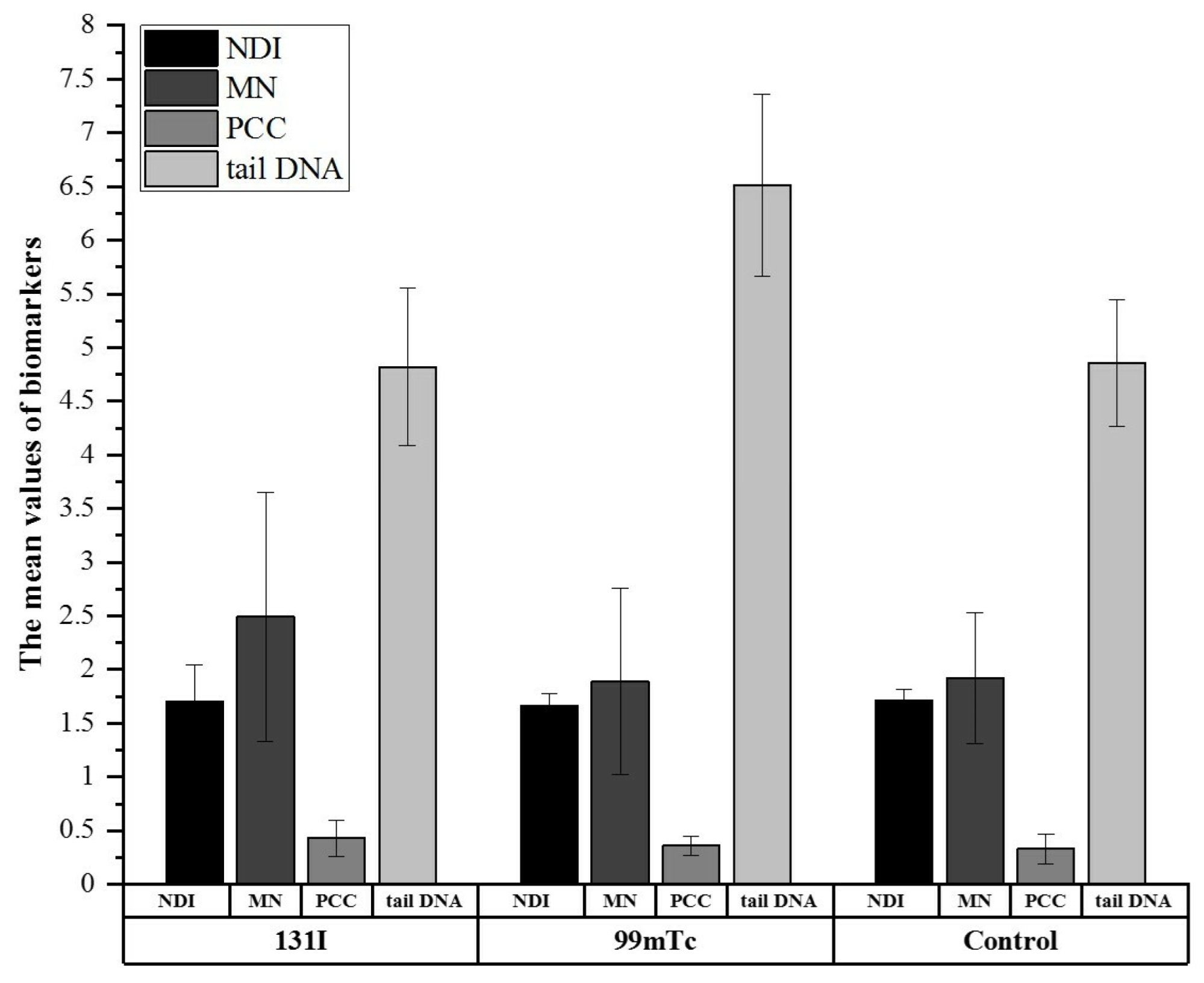
3.3. Biological Monitoring via CBMN, PCC, and Comet Assays Confirmed Differences in Cell Response between Exposed and Control Groups
3.4. Physical Activity and Smoking Status Differentiate Investigated Groups Based on DNA Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Dis. 2020, 19, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Beijst, C.; Kunnen, B.; Lam, M.G.; de Jong, H.W. Technical advances in image guidance of radionuclide therapy. J. Nucl. Med. Technol. 2017, 45, 272–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ersahin, D.; Doddamane, I.; Cheng, D. Targeted radionuclide therapy. Cancers 2011, 3, 3838–3855. [Google Scholar] [CrossRef] [PubMed]
- Chatal, J.F.; Hoefnagel, C.A. Radionuclide therapy. Lancet 1999, 354, 931–935. [Google Scholar] [CrossRef]
- Sebastiano, B.; Luana, P.; Serena, P. Thyroid pathologies in nuclear medicine. J. Adv. Health Care 2021, 3, 6–28. [Google Scholar] [CrossRef]
- Allahabadia, A.; Daykin, J.; Sheppard, M.C.; Gough, S.C.L.; Franklyn, J.A. Radioiodine treatment of hyperthyroidism—Prognostic factors for outcome. J. Clin. Endocrinol. Metab. 2001, 86, 3611–3617. [Google Scholar] [PubMed] [Green Version]
- Miszczyk, J.; Rawojć, K.; Panek, A.; Gałaś, A.; Kowalska, A.; Szczodry, A.; Brudecki, K. Assessment of the nuclear medicine personel occupational exposure to radioiodine. Eur. J. Radiol. 2019, 121, 108712. [Google Scholar] [CrossRef] [Green Version]
- Brudecki, K.; Borkowska, E.; Gorzkiewicz, K.; Kostkiewicz, M.; Mróz, T. 99mTc activity concentrations in room air and resulting internal contamination of medical personnel during ventilation–perfusion lung scans. Radiat. Environ. Biophys. 2019, 58, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Bajc, M.; Schümichen, C.; Grüning, T.; Lindqvist, A.; Le Roux, P.-Y.; Alatri, A.; Bauer, R.W.; Dilic, M.; Neilly, B.; Verberne, H.J.; et al. EANM guideline for ventilation/perfusion single-photon emission computed tomography (SPECT) for diagnosis of pulmonary embolism and beyond. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2429–2451. [Google Scholar] [CrossRef] [Green Version]
- Krajewska, G.; Pachocki, K.A. Assessment of exposure of workers to ionizing radiation from radioiodine and technetium in nuclear medicine departmental facilities. Med. Pr. 2013, 64, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Alramlawy, S.; Khalil, M.M. Effective radiation dose to staff members due to myocardial perfusion SPECT imaging: Tracking the exposure from preparation to patient release. Radiat. Prot. Dosim. 2018, 182, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Baudin, C.; Bernier, M.-O.; Klokov, D.; Andreassi, M.G. Biomarkers of genotoxicity in medical workers exposed to low-dose ionizing radiation: Systematic review and meta-analyses. Int. J. Mol. Sci. 2021, 22, 7504. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; De Marco, P.; Origgi, D.; Pedroli, G. SPECT/CT radiation dosimetry. Clin. Transl. Imaging 2014, 2, 557–569. [Google Scholar] [CrossRef] [Green Version]
- The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007, 37, 331–332.
- Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation; National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII, Phase II; National Academic Press: Washington, DC, USA, 2006.
- Bahreyni-Toossi, M.T.; Vosoughi, H.; Azimian, H.; Rezaei, A.R.; Momennezhad, M. In vivo exposure effects of 99mTc-methoxyisobutylisonitrile on the FDXR and XPA genes expression in human peripheral blood lymphocytes. Asia Ocean. J. Nucl. Med. Biol. 2018, 6, 32–40. [Google Scholar]
- Bozkurt, G.; Yuksel, M.; Karabogaz, G.; Sut, N.; Savran, F.O.; Palanduz, S.; Yigitbasi, O.N.; Algunes, C. Sister chromatid exchanges in lymphocytes of nuclear medicine physicians. Mutat. Res. 2003, 535, 205–213. [Google Scholar] [CrossRef]
- Flux, G.; Bardies, M.; Monsieurs, M.; Savolainen, S.; Strands, S.-E.; Lassmann, M.; EANM Dosimetry Committee. The impact of PET and SPECT on dosimetry for targeted radionuclide therapy. Z. Med. Phys. 2006, 16, 47–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eken, A.; Aydin, A.; Erdem, O.; Akay, C.; Sanal, H.T.; Soykut, B.; Sayal, A.; Somuncu, I. Cytogenetic analysis of peripheral blood lymphocytes of hospital staff occupationally exposed to low doses of ionizing radiation. Toxicol. Ind. Health 2010, 26, 273–280. [Google Scholar] [CrossRef]
- Sahin, A.; Tatar, A.; Oztas, S.; Seven, B.; Varoglu, E.; Yesilyurt, A.; Ayan, A.K. Evaluation of the genotoxic effects of chronic low-dose ionizing radiation exposure on nuclear medicine workers. Nucl. Med. Biol. 2009, 36, 575–578. [Google Scholar] [CrossRef]
- Gilbert, E.S. Invited commentary: Studies of workers exposed to low doses of radiation. Am. J. Epid. 2001, 153, 319–322. [Google Scholar] [CrossRef]
- Borkowska, E.; Brudecki, K.; Kostkiewicz, M.; Gorzkiewicz, K.; Misiak, R.; Nalichowska, E.; Miszczyk, J.; Mróz, T. 99mTc internal contaminations measurements among nuclear medicine medical personnel during ventilation—Perfusion SPECT lung scans. Radiat. Environ. Biophys. 2021, 60, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Brudecki, K.; Kowalska, A.; Zagrodzki, P.; Szczodry, A.; Mróz, T.; Janowski, P.; Mietelski, J.W. Measurement of 131I activity in thyroid of nuclear medical staff and internal dose assessment in a Polish nuclear medical hospital. Radiat. Environ. Biophys. 2017, 56, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brudecki, K.; Szczodry, A.; Mróz, T.; Kowalska, A.; Mietelski, J.W. Measurement of 131I activity in air indoor Polish nuclear medical hospital as a tool for an internal dose assessment. Radiat. Environ. Biophys. 2018, 57, 77–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szumska, A.; Budzanowski, M.; Kopeć, R. Occupational exposure to the whole body, extremities and to the eye lens in interventional radiology in Poland, as based on personnel dosimetry records at IFJ PAN. Radiat. Phys. Chem. 2014, 104, 72–75. [Google Scholar] [CrossRef]
- Fenech, M. The in vitro micronucleus technique. Mutat. Res. 2000, 455, 81–95. [Google Scholar] [CrossRef]
- Rawojć, K.; Miszczyk, J.; Możdżeń, A.; Swakoń, J.; Sowa-Staszczak, A. Evaluation of the premature chromosome condensation scoring protocol after proton and X-ray irradiation of human peripheral blood lymphocytes at high doses range. Int. J. Radiat. Biol. 2018, 94, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Panek, A.; Miszczyk, J.; Swakoń, J. Biological effects and inter-individual variability in peripheral blood lymphocytes of healthy donors exposed to 60 MeV proton radiotherapeutic beam. Int. J. Radiat. Biol. 2018, 94, 1085–1094. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Statist. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Prince, S.A.; Rasmussen, C.L.; Biswas, A.; Holtermann, A.; Aulakh, T.; Merucci, K.; Coenen, P. The effect of leisure time physical activity and sedentary behaviour on the health of workers with different occupational physical activity demands: A systematic review. Int. J. Beh. Nutr. Phys. Act. 2021, 18, 100. [Google Scholar] [CrossRef]
- Al-Mohammed, H.I.; Sulieman, A.; Mayhoub, F.H.; Salah, H.; Legarde, C.; Alkhorayef, M.; Aldhebaib, A.; Kappas, C.; Bradley, D.A. Occupational exposure and radiobiological risk from thyroid risk from thyroid radioiodine therapy in Saudi Arabia. Sci. Rep. 2021, 11, 14557. [Google Scholar] [CrossRef]
- Gricienė, B.; Slapšytė, G.; Mierauskienė, J. Cytogenetic monitoring of nuclear workers occupationally exposed to ionising radiation. Radiat. Prot. Dosim. 2014, 159, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Oh, E.; Lee, J.; Sul, D.; Lee, J. Use of the tail moment of the lymphocytes to evaluate DNA damage in human biomonitoring studies. Toxicol. Sci. 2004, 81, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Jakl, L.; Marková, E.; Koláriková, L.; Belyaev, I. Biodosimetry of low dose ionizing radiation using DNA repair foci in human lymphocytes. Genes 2020, 11, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatzi, V.I.; Terzoudi, G.I.; Paraskevopoulou, C.; Makropoulos, V.; Matthopoulos, D.P.; Pantelias, G.E. The use of premature chromosome condensation to study in interphase cells the influence of environmental factors on human genetic material. Sci. World J. 2006, 6, 1174–1190. [Google Scholar] [CrossRef] [PubMed]
- Biegała, M.; Jakubowska, T. Levels of exposure to ionizing radiation among the personnel engaged in cyclotron operation and the personnel engaged in the production of radiopharmaceuticals, based on radiation monitoring system. Radiat. Prot. Dosim. 2020, 189, 56–62. [Google Scholar] [CrossRef]
- Morgan, W.F.; Bair, W.J. Issues in low dose radiation biology: The controversy continues. A perspective. Radiat. Res. 2013, 179, 501–510. [Google Scholar] [CrossRef]
- Burtt, J.J.; Thompson, P.A.; Lafrenie, R.M. Non-targeted effects and radiation-induced carcinogenesis: A review. J. Radiat. Prot. 2016, 36, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Hieu, T.T.; Russell, A.W.; Cuneo, R.; Clark, J.; Kron, T.; Hall, P.; Doi, A.A.R. Cancer risk after medical exposure to radioactive iodine in benign thyroid diseases: A meta-analysis. Endocr.-Relat. Cancer 2012, 19, 645–655. [Google Scholar] [CrossRef] [Green Version]
- Mavragani, I.V.; Nikitaki, Z.; Souli, M.P.; Aziz, A.; Nowsheen, S.; Aziz, K.; Rogakou, E.; Georgakilas, A.G. Complex DNA damage: A route to radiation-induced genomic instability and carcinogenesis. Cancers 2017, 9, 91. [Google Scholar] [CrossRef]
- Zakeri, F.; Hirobe, T.; Noghabi, K.A. Biological effects of low-dose ionizing radiation exposure on interventional cardiologists. Occup. Med. 2010, 60, 464–469. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.L.; Lu, X.; Cai, T.J.; Lyu, Y.M.; Tian, M.; Liu, Q.J. Cytogenetic monitoring of peripheral blood lymphocytes from medical radiation professionals occupationally exposed to low-dose ionizing radiation. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2021, 867, 503370. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, R.; Kashino, I.; Kasai, H.; Kawai, K.; Li, Y.S.; Nanri, A.; Higuchi, M.; Mizoue, T. Leisure-time physical activity and DNA damage among Japanese workers. PLoS ONE 2019, 4, e0212499. [Google Scholar] [CrossRef]
- Hoffmann, H.; Högel, J.; Speit, G. The effect of smoking on DNA effects in the comet assay: A meta-analysis. Mutagenesis 2005, 20, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Jovičić, D.; Pajić, J.; Radivojević, L.; Rakić, B.; Sarić-Krsmanović, M. Micronucleus frequencies in peripheral blood lymphocytes in a Serbian human population exposed to pesticides. Pestic. Phytomed. 2015, 30, 51–60. [Google Scholar] [CrossRef] [Green Version]



| Subject Code | Age (y) | Profession | Gender | DoE (y) | DI | PI | DI | PI | DI | PI | DI | PI | DI | PI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | ||||||||||
| 1 | 58 | Nurse | F | 15 | 1.60 | 7.35 | 1.08 | 1.80 | 1.28 | 1.98 | 0.97 | 2.29 | 0.00 | 0.53 |
| 2 | 30 | Technician | F | 8 | 1.10 | 68.51 | 0.84 | 116.56 | 0.45 | 46.81 | 0.83 | 65.04 | 0.06 | 21.99 |
| 3 | 68 | Medical doctor | F | 40 | 0.01 | - | 0.00 | - | 0.00 | - | 0.00 | - | 0.00 | - |
| 4 | 55 | Technician | F | 34 | 1.89 | 52.88 | 1.74 | 48.90 | 1.24 | 50.32 | 0.72 | 26.49 | 0.25 | 6.68 |
| 5 | 49 | Medical doctor | M | 24 | 0.08 | - | 0.05 | - | 0.00 | - | 0.00 | - | 0.00 | - |
| 6 | 55 | Nurse | F | 16 | 1.07 | 2.93 | 0.57 | 0.81 | 0.24 | 1.24 | 0.50 | 0.80 | 0.02 | 0.29 |
| 7 | 55 | Technician | F | 34 | 0.46 | 27.00 | 0.26 | 29.56 | 0.09 | 20.01 | 0.02 | 16.21 | 0.06 | 5.92 |
| 8 | 57 | Nurse | F | 19 | 0.73 | 5.81 | 0.67 | 0.04 | 0.82 | 0.36 | 1.04 | 0.44 | 0.07 | 0.00 |
| 9 | 35 | Technician | M | 4 | - | - | 0.01 | - | 0.00 | - | 0.00 | 2.30 | 0.00 | - |
| Confounding Factors | 131I | 99mTc | Controls |
|---|---|---|---|
| [n = 29] | [n = 9] | [n = 32] | |
| Age | 46.9 ± 4.5 | 51.3 ± 11.8 | 48.0 ± 8.6 |
| Mean (S.D.) | |||
| Sex (%) | 93.1 | 77.8 | 96.9 |
| Women | 6.9 | 22.2 | 3.1 |
| Men | |||
| Smoking status (%) | 10.3 | 0 | 15.6 |
| Current smoker | 24.1 | 22.2 | 28.1 |
| Past regular smoker | 3.5 | 22.2 | 3.1 |
| Past occasional smoker | 62.1 | 55.6 | 53.1 |
| Non-smoker | |||
| Leisure-time physical activity (%) | 53.8 | 55.6 | 74.1 |
| Normal | |||
| (<Q3: 25.04 MET-h/week) | 46.2 | 44.4 | 25.9 |
| High | |||
| (>Q3: 25.04 MET-h/week) | |||
| Work-related physical effort (%) | 58.6 | 44.4 | 75 |
| (<100 MET-h/week) | 41.4 | 55.6 | 25 |
| (≥100 MET-h/week) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miszczyk, J.; Gałaś, A.; Panek, A.; Kowalska, A.; Kostkiewicz, M.; Borkowska, E.; Brudecki, K. Genotoxicity Associated with 131I and 99mTc Exposure in Nuclear Medicine Staff: A Physical and Biological Monitoring Study. Cells 2022, 11, 1655. https://doi.org/10.3390/cells11101655
Miszczyk J, Gałaś A, Panek A, Kowalska A, Kostkiewicz M, Borkowska E, Brudecki K. Genotoxicity Associated with 131I and 99mTc Exposure in Nuclear Medicine Staff: A Physical and Biological Monitoring Study. Cells. 2022; 11(10):1655. https://doi.org/10.3390/cells11101655
Chicago/Turabian StyleMiszczyk, Justyna, Aleksander Gałaś, Agnieszka Panek, Aldona Kowalska, Magdalena Kostkiewicz, Eliza Borkowska, and Kamil Brudecki. 2022. "Genotoxicity Associated with 131I and 99mTc Exposure in Nuclear Medicine Staff: A Physical and Biological Monitoring Study" Cells 11, no. 10: 1655. https://doi.org/10.3390/cells11101655






